August 2015 | VOL. 14, NO. 8 | www.McGowan.pitt.edu
Computer Simulation Predicts Development, Progress of Pressure Sores
 Researchers at the University of Pittsburgh School of Medicine have devised a computational model that could enhance understanding, diagnosis, and treatment of pressure ulcers related to spinal cord injury. In a report published online in PLOS Computational Biology, the team also described results of virtual clinical trials that showed that for effective treatment of the lesions, anti-inflammatory measures had to be applied well before the earliest clinical signs of ulcer formation.
Researchers at the University of Pittsburgh School of Medicine have devised a computational model that could enhance understanding, diagnosis, and treatment of pressure ulcers related to spinal cord injury. In a report published online in PLOS Computational Biology, the team also described results of virtual clinical trials that showed that for effective treatment of the lesions, anti-inflammatory measures had to be applied well before the earliest clinical signs of ulcer formation.
Pressure ulcers affect more than 2.5 million Americans annually and patients who have spinal cord injuries that impair movement are more vulnerable to developing them, said McGowan Institute for Regenerative Medicine faculty member and senior investigator Yoram Vodovotz, PhD, professor of surgery and director of the Center for Inflammation and Regenerative Modeling at the McGowan Institute.
“These lesions are thought to develop because immobility disrupts adequate oxygenation of tissues where the patient is lying down, followed by sudden resumption of blood flow when the patient is turned in bed to change positions,” Dr. Vodovotz said. “This is accompanied by an inflammatory response that sometimes leads to further tissue damage and breakdown of the skin.”
“Pressure ulcers are an unfortunately common complication after spinal cord injury and cause discomfort and functional limitations,” said co-author Gwendolyn A. Sowa, M.D., Ph.D., associate professor of physical medicine and rehabilitation, Pitt School of Medicine. “Improving the individual diagnosis and treatment of pressure ulcers has the potential to reduce the cost of care and improve quality of life for persons living with spinal cord injury.”
To address the complexity of the biologic pathways that create and respond to pressure sore development, the researchers designed a computational, or “in silico,” model of the process based on serial photographs of developing ulcers from spinal cord-injured patients enrolled in studies at Pitt’s Rehabilitation Engineering Research Center on Spinal Cord Injury. Photos were taken when the ulcer was initially diagnosed, three times per week in the acute stage, and once a week as it resolved.
Then they validated the model, finding that if they started with a single small round area over a virtual bony protuberance and altered factors such as inflammatory mediators and tissue oxygenation, they could recreate a variety of irregularly shaped ulcers that mimic what is seen in reality.
They also conducted two virtual trials of potential interventions, finding that anti-inflammatory interventions could not prevent ulcers unless applied very early in their development.
In the future, perhaps a nurse or caregiver could simply send in a photo of a patient’s reddened skin to a doctor using the model to find out whether it was likely to develop into a pressure sore for quick and aggressive treatment to keep it from getting far worse, Dr. Vodovotz speculated.
“Computational models like this one might one day be able to predict the clinical course of a disease or injury, as well as make it possible to do less expensive testing of experimental drugs and interventions to see whether they are worth pursuing with human trials,” he said. “They hold great potential as a diagnostic and research tool.”
The team included co-senior author Gary An, MD, of the University of Chicago; Cordelia Ziraldo, PhD, Alexey Solovyev, PhD, Ana Allegretti, PhD, Shilpa Krishnan, MS, McGowan Institute for Regenerative Medicine affiliated faculty member David Brienza, PhD, Qi Mi, PhD, all of Pitt; and M. Kristi Henzel, MD, PhD, of the Louis Stokes Cleveland Veterans Affairs Medical Center.
Illustration: UPMC/University of Pittsburgh Schools of the Health Sciences.
RESOURCES AT THE MCGOWAN INSTITUTE
September Special at the Histology Lab
Oil Red O (Lipid Stain) is intended for use in the histological visualization of fat cells and neutral fat. The staining has to be performed on fresh samples, as alcohol fixation removes most lipids.
The McGowan Histology Lab is equipped with state of the art Processing, Embedding, and Staining Automation, including Instruments for paraffin and frozen sections alike.
Contact Lori at the McGowan Core Histology Lab about the Frozen Specials of September.
Email perezl@upmc.edu or call 412-624-5265
Mention this ad for a 30% discount on Oil Red O staining. As always, you will receive the highest quality histology in the lowest amount of time.
Did you know the more samples you submit to the histology lab the less you pay per sample?
Contact Lori to find out how!
 Introducing the flow cytometry rewards program
Introducing the flow cytometry rewards program
We want to REWARD you for all your hard work! After all, you deserve it. Starting September 1, 2015, all our Flow Cytometry Researchers will be enrolled in our new FLOW REWARDS program. Complete 20hrs of work on the Quant or 40hrs on the Aria and receive free bonus hours equal to 10% of the target accumulation hours. No additional forms to fill out. No cards to punch or swipe. And the accumulated time never expires. Just continue with your project’s flow cytometry work! It’s that easy. Talk to Lynda to get complete details.
UPCOMING EVENTS
Fourth Annual Regenerative Rehabilitation Symposium
The annual Regenerative Rehabilitation Symposia series is a unique opportunity for students, researchers, and clinicians working in the interrelated fields of regenerative medicine and rehabilitation to meet, exchange ideas, and generate new collaborations and clinical research questions. Jointly organized by the University of Pittsburgh Rehabilitation Institute, the School of Health and Rehabilitation Sciences at the University of Pittsburgh, the McGowan Institute for Regenerative Medicine and the Rehabilitation Research and Development Center of Excellence at the Veterans Affairs Palo Alto Health Care System, the Fourth Annual Symposium on Regenerative Rehabilitation will be held on September 24-26, 2015 in Rochester, MN, hosted by the Mayo Clinic.
For more information on this event, please contact Katy Wharton at: rehabmtg@pitt.edu or whartonkm@upmc.edu or 412-624-5293.
Registration Now Open: 2015 Symposium–Regenerative Surgery: The Cutting Edge
 This year’s symposium, “Regenerative Surgery: The Cutting Edge,” is hosted by LifeNet Health and will be held at The Institute of Regenerative Medicine, Norfolk, Virginia, September 25-26, 2015. McGowan Institute for Regenerative Medicine deputy director Vijay Gorantla, MD, PhD, Associate Professor of Surgery in the Department of Plastic Surgery and the Administrative Medical Director of the Pittsburgh Reconstructive Transplant Program at University of Pittsburgh Medical Center, is the symposium chairman. The goal of this meeting is to provide a state-of-the-art update on groundbreaking research and clinical progress in the fields of regenerative medicine, tissue engineering, and reconstructive transplantation. The symposium will bring together experts to provide thought provoking insights into transformative scientific advances and future frontiers.
This year’s symposium, “Regenerative Surgery: The Cutting Edge,” is hosted by LifeNet Health and will be held at The Institute of Regenerative Medicine, Norfolk, Virginia, September 25-26, 2015. McGowan Institute for Regenerative Medicine deputy director Vijay Gorantla, MD, PhD, Associate Professor of Surgery in the Department of Plastic Surgery and the Administrative Medical Director of the Pittsburgh Reconstructive Transplant Program at University of Pittsburgh Medical Center, is the symposium chairman. The goal of this meeting is to provide a state-of-the-art update on groundbreaking research and clinical progress in the fields of regenerative medicine, tissue engineering, and reconstructive transplantation. The symposium will bring together experts to provide thought provoking insights into transformative scientific advances and future frontiers.
The objectives of the 2015 symposium are to:
- Enhance understanding of cutting edge advances in tissue engineering and regenerative medicine at the interface of reconstructive transplantation
- Improve scientific insights into clinical outcomes after innovative vascularized composite allotransplantation procedures and glean from pioneering surgeon and patient perspectives
- Discover revolutionary progress in smart nanotherapeutics for drug delivery and transplant immunomodulation, whole organ engineering, and organ preservation
- Advance knowledge of state-of-the-art aspects of regenerative surgery including cell and molecular imaging and functional composite micro-tissues
- Recognize unmet needs and exponential progress in restorative surgery in the war- wounded service member
In addition to prestigious lecturers from the University of Arizona, University of Illinois at Chicago, University of Gothenburg, Center for Organ Recovery and Education (CORE), Harvard University, Yale University, Tufts University, and the U.S. Army Institute of Surgical Research, the following McGowan Institute for Regenerative Medicine affiliated faculty members will also present:
- Paulo Fontes, MD: Organ Preservation: The New Frontier in Organ Transplantation
- Michel Modo, PhD: Advanced Imaging in Regenerative Surgery
- Rocky Tuan, PhD: Functional Microtissues – The Future of Clinical Trials
Established in 2013, LifeNet Health hosts its annual scientific symposium for the purpose of inspiring an interactive dialogue among clinicians, administrators, and allograft scientists. The symposium’s annual themes specifically focus on key topics within the field of regenerative medicine for the purpose of inspiring and recognizing the deep appreciation for the scientific method of allograft sciences, thinking and dreaming of the possibilities for allografts and improved patient care overall. Past lecturers, participants, and sponsors represented biotech companies, educational institutions, research facilities, and other health care establishments.
Illustration: LifeNet Health.
Save the Date: 9th Symposium on Biologic Scaffolds for Regenerative Medicine
 The 9th Symposium on Biologic Scaffolds for Regenerative Medicine will be held at the Silverado Resort in Napa, California on April 28th – 30th, 2016.
The 9th Symposium on Biologic Scaffolds for Regenerative Medicine will be held at the Silverado Resort in Napa, California on April 28th – 30th, 2016.
This symposium represents an opportunity to advance the use of biologic scaffolds for regenerative medicine and all general surgery applications. Topics will include the basic science of scaffold remodeling from the molecular level through the macroscopic and clinical level. Approximately 50% of the presentations will involve the clinical perspective in the form of formal studies, anecdotal reports, and surgeon reviews. Speakers will provide an objective opinion of the pros and cons of the use of biologic scaffold materials. It is not the intent of this symposium to discuss only the beneficial aspects of biologic scaffolds or particular products, but also to identify problems, develop strategies for solving these problems, and hopefully initiate collaborations among basic scientists, clinicians, and industry in attendance at the meeting.
This symposium is designed to advance the use of biologic scaffolds for regenerative medicine and all general surgery applications via a series of objective presentations describing the potential benefits and risks associated with the use of such materials, factors that affect performance, and the clinical applications that may benefit most from their use. Topics range from the most basic science of scaffold remodeling at the molecular level through the preclinical and clinical level. It is not the intent of this symposium to discuss only the beneficial aspects of biologic scaffolds, but just as importantly to identify the problem areas, develop strategies for solving these problems, and hopefully initiate collaborations among basic scientists and clinicians in attendance at the meeting. Our feedback from previous symposia (this is a bi-annual event) consistently identifies the equal mix of clinicians and basic scientists as the most beneficial and rewarding aspect of the meeting;
Although all topic areas are considered and will be represented, the 2016 symposium will definitely include the following:
- An in depth account of the most recent findings regarding the mechanisms by which biologic scaffolds facilitate constructive remodeling of tissues; including body wall (skeletal muscle), cardiovascular, reconstructive surgical applications such as breast and pelvic floor, and whole organs such as liver, lung and heart.
- Identification and discussion of manufacturing issues such as tissue source, decellularization methods, and sterilization decisions that affect the quality and performance of biologic scaffolds for surgical applications.
- A review of clinical experiences, especially general surgery, orthopedic and trauma related challenges, neurologic applications, gastrointestinal applications, and cardiovascular applications.
- Identification and discussion of the effect of the host innate immune response upon scaffold remodeling and clinical outcome.
List of invited (and accepted) speakers to date: Robert M. Nerem, PhD (Georgia Institute of Technology), Arnold I. Caplan, PhD (Case Western Reserve University), Jeffrey M. Davidson, Ph.D. (Vanderbilt University), Cyrus Ghajar, PhD (Fred Hutchinson Cancer Research Center), Jeffrey A. Hubbell, PhD (Swiss Federal Institute of Technology, EPFL), Kristen Jones, MD (University of Minnesota), C. James Kirkpatrick MD PhD DSc FRCPath (Johannes Gutenberg University), Robert G. Martindale, MD, PhD (Oregon Health & Science University), Charles D. Mills, PhD (BioMedical Consultants), Laura E Niklason PhD, MD (Yale University), Frederick J. Schoen, MD, PhD (Harvard University), Allan S. Stewart, MD (Mount Sinai Hospital NYC), and Nadia Rosenthal, PhD (Monash University, Australia).
SCIENTIFIC ADVANCES
Pitt Researchers Will Study Sepsis with $1.5M NIH Grant
 Two University of Pittsburgh researchers have been awarded a 4-year, $1.5 million grant from the National Institutes of Health to study the body’s response to the lethal inflammatory disease known as sepsis.
Two University of Pittsburgh researchers have been awarded a 4-year, $1.5 million grant from the National Institutes of Health to study the body’s response to the lethal inflammatory disease known as sepsis.
Led by Timothy Billiar, MD, Pitt’s George Vance Foster endowed professor and chairman of the Department of Surgery and a McGowan Institute for Regenerative Medicine faculty member, and Daolin Tang, MD, PhD, assistant professor of surgery at Pitt and the University of Pittsburgh Cancer Institute (UPCI), researchers plan to explore the relationship between immunometabolism, meaning cellular energy regulation and immune function, and the development of sepsis.
“Despite recent advances in antibiotic therapy and intensive care, severe sepsis and septic shock remain the leading causes of death in intensive care units worldwide, including the United States,” Dr. Billiar said. “Our proposal will provide new insights into our understanding of the pathophysiology of sepsis and may lead to new ways to treat it.”
In the new study, researchers will build on their previous work that showed a protein called PKM2 could contribute to production of a cell-signaling molecule involved in triggering widespread inflammation, as well as test whether inhibiting PKM2 can forestall sepsis development in an animal model.
Pitt’s Center for Medical Innovation Awards Round-1 2015 Pilot Funding
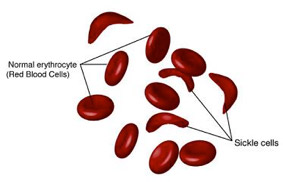 McGowan Institute for Regenerative Medicine affiliated faculty members Marina Kameneva, PhD, Research Professor of Surgery at the University of Pittsburgh School of Medicine, Professor of Bioengineering, and Director of the Artificial Blood Program at the McGowan Institute, and Jonathan Waters, MD, Professor in the Department of Anesthesiology in the University of Pittsburgh School of Medicine, Professor in the Department of Bioengineering, Chief of Anesthesia Services at Magee Womens Hospital of UPMC, and the Medical Director in the Blood Management Division of Procirca, Inc., are members of the team of researchers who were recently awarded a Center for Medical Innovation (CMI) grant for their project, “Reducing alloimmunization and sickle crisis in sickle cell disease patients using a novel method of replacing HbS with donor Hb in autologous RBCs.” The focus of this effort is to develop a novel method for treating sickle cell anemia. Mark Gartner, PhD, in the Department of Bioengineering at Pitt, is also a member of the team.
McGowan Institute for Regenerative Medicine affiliated faculty members Marina Kameneva, PhD, Research Professor of Surgery at the University of Pittsburgh School of Medicine, Professor of Bioengineering, and Director of the Artificial Blood Program at the McGowan Institute, and Jonathan Waters, MD, Professor in the Department of Anesthesiology in the University of Pittsburgh School of Medicine, Professor in the Department of Bioengineering, Chief of Anesthesia Services at Magee Womens Hospital of UPMC, and the Medical Director in the Blood Management Division of Procirca, Inc., are members of the team of researchers who were recently awarded a Center for Medical Innovation (CMI) grant for their project, “Reducing alloimmunization and sickle crisis in sickle cell disease patients using a novel method of replacing HbS with donor Hb in autologous RBCs.” The focus of this effort is to develop a novel method for treating sickle cell anemia. Mark Gartner, PhD, in the Department of Bioengineering at Pitt, is also a member of the team.
Illustration: National Institutes of Health.
New Publications Show Promise of Improved Artificial Lung Devices
 ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, announced recently the publication of new data by University of Pittsburgh researchers on two groundbreaking technologies it has previously licensed from the University. The new technologies, which help enhance the performance of artificial lung devices, were developed by McGowan Institute for Regenerative Medicine faculty member William Federspiel, PhD, Professor of Bioengineering at the University of Pittsburgh, and his team in the Medical Devices Laboratory of the McGowan Institute. Dr. Federspiel is also a co-founder of ALung Technologies.
ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, announced recently the publication of new data by University of Pittsburgh researchers on two groundbreaking technologies it has previously licensed from the University. The new technologies, which help enhance the performance of artificial lung devices, were developed by McGowan Institute for Regenerative Medicine faculty member William Federspiel, PhD, Professor of Bioengineering at the University of Pittsburgh, and his team in the Medical Devices Laboratory of the McGowan Institute. Dr. Federspiel is also a co-founder of ALung Technologies.
Artificial lung devices are used to support patients with lung failure. They work by passing a patient’s blood over an artificial membrane which removes carbon dioxide and delivers oxygen to the blood, independently of the native lung. While today’s artificial lung technology is very good, a clinical need still exists for more efficient, minimally invasive devices. The new techniques developed by Dr. Federspiel use a combination of two biochemical approaches that work synergistically to more than double the rate of carbon dioxide removal across the artificial lung membrane. The work of Dr. Federspiel’s team was recently published in the journals Acta Biomaterialia and the Journal of Material Science: Materials in Medicine, designated in the latter as an “Editor’s Choice” paper.
ALung’s license agreement with the University Pittsburgh for this new technology includes two pending patent applications. “These novel technologies fit nicely within our broader intellectual property portfolio of methods for enhancing gas exchange,” said Peter DeComo, ALung Chairman and CEO. “The continued refinement of these techniques, as highlighted in the new publications, helps pave the way for the future development of more effective artificial lung devices for the millions of patients with acute and chronic lung failure. We offer Dr. Federspiel and his team our congratulations on their most recent publications.
ALung’s Hemolung Respiratory Assist System, a minimally invasive extracorporeal CO2 removal system for treating patients with acute respiratory failure, also incorporates technology licensed from the University of Pittsburgh.
New High Definition Fiber Tracking
 A powerful new imaging technique called High Definition Fiber Tracking (HDFT) will allow doctors to clearly see for the first time neural connections broken by traumatic brain injury (TBI) and other disorders. As reported in Discover Magazine by Bijal P. Trivedi, MS, MA, head bumps, jolts, or exposure to a blast can snap fragile nerves in the brain that carry signals from one part of the body to another. But there is no diagnostic technique that can visualize which nerve fibers, or neurons, are broken. When a nerve snaps, communication between different brain regions is disrupted, just like a damaged circuit in a computer. Not being able to locate the damage is an enormous setback to recovery and rehabilitation for the approximately 1.7 million people who suffer TBI each year in the U.S. That number doesn’t include the more than 300,000 soldiers with brain damage inflicted during military combat between 2000 and 2014.
A powerful new imaging technique called High Definition Fiber Tracking (HDFT) will allow doctors to clearly see for the first time neural connections broken by traumatic brain injury (TBI) and other disorders. As reported in Discover Magazine by Bijal P. Trivedi, MS, MA, head bumps, jolts, or exposure to a blast can snap fragile nerves in the brain that carry signals from one part of the body to another. But there is no diagnostic technique that can visualize which nerve fibers, or neurons, are broken. When a nerve snaps, communication between different brain regions is disrupted, just like a damaged circuit in a computer. Not being able to locate the damage is an enormous setback to recovery and rehabilitation for the approximately 1.7 million people who suffer TBI each year in the U.S. That number doesn’t include the more than 300,000 soldiers with brain damage inflicted during military combat between 2000 and 2014.
Often such damage is invisible on CT scans, which use X-rays to visualize blockages, bleeds, tumors, and skull fractures. MRI uses radio waves to create more detailed images, revealing bleeds, tumors, and crude structural damage, but it cannot detect broken nerves. Even functional MRI (fMRI), which measures brain activity by tracking blood flow, can’t detect the loss of neurons.
McGowan Institute for Regenerative Medicine affiliated faculty member David Okonkwo, MD, PhD, Professor and Executive Vice Chair of Neurological Surgery, and Professor of Sports Medicine and Nutrition at the University of Pittsburgh, Director of Neurotrauma and of the Scoliosis and Spinal Deformity Program at UPMC, and the Clinical Director of the Brain Trauma Research Center, knew that brain injuries were easily overlooked. Even today, when a head injury or coma patient is brought to the ER, the person gets a CT scan to determine if there is a blood clot in the brain that requires surgery. The problem, says Dr. Okonkwo, is that in 9 out of 10 cases, those patients have a normal CT scan and are told they’re fine. “But in many cases, they are not normal,” he adds. “And they will be the first ones to share with you 3 months, 6 months later, the ways in which their life has changed.” He says that in most hospitals, trying to diagnose a TBI is pretty much like trying to find a bone fracture before X-ray machines were invented.
But that changed for Dr. Okonkwo in fall 2009 when Walter Schneider, PhD, a psychologist at Pitt, visited from across campus. Dr. Schneider is fascinated by technology, and he’d come to talk about a new way to image the major tracts of the brain. Tracts are bundled cables of axons that link one region of the brain to another — like superhighways — and conduct information. An axon is the long, skinny “tail” of a nerve cell, or neuron, which transmits electrical signals from one neuron to another elsewhere in the brain. Within a specific tract, all the nerve cells begin in the same location and end in a common location. Each tract has a predominant function: The corticospinal tract controls movement; the cingulate tract, memory; and the arcuate handles language. When an axon is injured, communication between particular neurons is lost; when an entire tract is severed, two brain regions can no longer talk to each other.
With financing from the Defense Advanced Research Projects Agency, Dr. Schneider launched the 2009 Pittsburgh Brain Competition to lure the best minds to work on brain connectivity mapping. Dr. Okonkwo began collaborating with Dr. Schneider to test the HDFT technology in a research trial by recruiting patients with brain injuries. Other team members then worked with Dr. Okonkwo and Juan Fernandez-Miranda, MD, a Pittsburgh neurosurgeon and neuroanatomist and the Director of the Fiber Tractography (HDFT) Lab, on an iPad app to create a tool that was clinically relevant and useful to neurosurgeons as they performed brain surgery or searched for damage in an injured patient. Seeing a detailed scan of the brain is clinically important, both in a diagnostic sense as well as a therapeutic one, says Dr. Okonkwo.
Part of the process of pushing this new technology is building the technical infrastructure that will allow Drs. Okonkwo and Schneider to better acquire MRI data, analyze and interpret it, and present brain images to clinicians and patients in a way that’s intuitive. The scan now takes 22 minutes, the analysis just 4 hours. Currently the only way to get a high-definition scan of brain fibers is to participate in a research trial. That will remain true for the next 3 to 5 years until the FDA approves the technology. But already, Drs. Okonkwo and Schneider are glimpsing the fruits of their efforts: They’re helping patients understand the consequences of their brain injuries.
AWARDS AND RECOGNITION
Dr. Steven Little Elected Fellow of the Biomedical Engineering Society
 McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, Associate Professor, CNG Faculty Fellow and Chair of the Department of Chemical and Petroleum Engineering at the University of Pittsburgh’s Swanson School of Engineering, has been elected a Class of 2015 Fellow of the Biomedical Engineering Society (BMES).
McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, Associate Professor, CNG Faculty Fellow and Chair of the Department of Chemical and Petroleum Engineering at the University of Pittsburgh’s Swanson School of Engineering, has been elected a Class of 2015 Fellow of the Biomedical Engineering Society (BMES).
Founded in 1968, BMES is an interdisciplinary professional society for biomedical engineering and bioengineering. Fellow status is awarded to Society members who demonstrate exceptional achievements and experience in the field of biomedical engineering, and a record of membership and participation in the Society. Dr. Little holds eight US patents and provisional applications for patents including
- new methods to fabricate controlled release vehicles in a high throughput fashion
- dissolvable synthetic-vasculature
- novel complex delivery vehicles
- a description of the first degradable, artificial cell
He has authored/co-authored 70 articles in highly prestigious archival journals in his fields of specialization (controlled release, biomimetic materials, tissue engineering/regenerative medicine, and drug delivery).
“Dr. Little’s election as BMES Fellow recognizes his seminal contributions to bioengineering education and research during his academic career,” noted McGowan Institute for Regenerative Medicine faculty member Harvey Borovetz, PhD, Distinguished Professor and Former Chair of Bioengineering and the Robert L. Hardesty Professor of Surgery at Pitt, and BMES Fellow who nominated Dr. Little. “In addition to his remarkable achievements in his research, Dr. Little is a prolific classroom instructor whose courses are among the most highly rated in the Swanson School of Engineering. He is the mentor for numerous MS and PhD candidates; his lab is a magnet for undergraduate students, with more than 40 undergraduate interns being mentored by Dr. Little to date. We are very proud to recognize Dr. Steven Little as a Class of 2015 Fellow of the Biomedical Engineering Society.”
Dr. Little joins the ranks of several McGowan Institute for Regenerative Medicine affiliated faculty members and BMES Fellows:
- Clifford Brubaker, PhD, Distinguished Service Professor and Dean Emeritus of the School of Health and Rehabilitation Sciences at the University of Pittsburgh
- Rory Cooper, PhD, FISA/PVA Endowed Chair and Distinguished Professor of the Department of Rehabilitation Science and Technology, School of Health and Rehabilitation Sciences, University of Pittsburgh
- William Federspiel, PhD, the William Kepler Whiteford Professor of Bioengineering at the University of Pittsburgh
- Philip LeDuc, PhD, William J. Brown Professor of Mechanical Engineering with appointments in Biomedical Engineering, Biological Sciences, and Computational Biology at Carnegie Mellon University
- Sanjeev Shroff, PhD, Distinguished Professor and the Gerald E. McGinnis Chair in Bioengineering and Professor of Medicine at the University of Pittsburgh
- David Vorp, PhD, Associate Dean for Research in the Swanson School of Engineering and the William Kepler Whiteford Professor of Bioengineering at the University of Pittsburgh
- William Wagner, PhD, Director of the McGowan Institute for Regenerative Medicine and Professor of Surgery, Bioengineering and Chemical Engineering, University of Pittsburgh
- Savio L-Y. Woo, PhD, DSc, DEng, Distinguished University Professor of Bioengineering and the Founder and Director of the Musculoskeletal Research Center, University of Pittsburgh
Illustration: University of Pittsburgh.
Dr. Anna Balazs: NSF Interview
 Annually, the National Science Foundation’s (NSF) Directorate for Mathematical and Physical Sciences invites media and members of the public to a series of lectures that helps promote a national discussion of issues that scientists expect to shape their research in the coming years. McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, was a Distinguished Lecturer for the NSF on June 22, 2015.
Annually, the National Science Foundation’s (NSF) Directorate for Mathematical and Physical Sciences invites media and members of the public to a series of lectures that helps promote a national discussion of issues that scientists expect to shape their research in the coming years. McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, was a Distinguished Lecturer for the NSF on June 22, 2015.
Dr. Balazs’ presentation was entitled, “From Pendulums to Heartbeats: Inspirations for Designing Active, Responsive Materials,” and was held at the NSF in Arlington, Virginia. Dr. Balazs joined renowned speakers from the University of Colorado/NIST, University of California—Los Angeles, New York University, Cornell University, Harvard University, and the University of Wisconsin in the 2014-2015 series of lectures.
Following her presentation, Public Affairs Specialist Ms. Ivy Kupec from the NSF interviewed Dr. Balazs. Their conversation spanned Dr. Balazs’ journey from Hungary, inspiration from her parents, and the future of biosensing. Read the interview here.
Illustration: University of Pittsburgh.
Dr. MaCalus Hogan Named to List of Who’s Who In Black Pittsburgh
 McGowan Institute for Regenerative Medicine affiliated faculty member MaCalus V. Hogan, MD, assistant professor and the associate residency program director in the Department of Orthopaedic Surgery at the University of Pittsburgh Medical Center and School of Medicine, was recently named to the list of Who’s Who In Black Pittsburgh. The official unveiling reception for the Inaugural Edition of Who’s Who In Black Pittsburgh was held July 16, 2015, at the Herberman Conference Center at UPMC Cancer Pavilion, Pittsburgh. Since beginning in 1989 with a single publication, Who’s Who Publishing now produces annual directories of African American achievers in 25 cities, with Pittsburgh being the latest edition.
McGowan Institute for Regenerative Medicine affiliated faculty member MaCalus V. Hogan, MD, assistant professor and the associate residency program director in the Department of Orthopaedic Surgery at the University of Pittsburgh Medical Center and School of Medicine, was recently named to the list of Who’s Who In Black Pittsburgh. The official unveiling reception for the Inaugural Edition of Who’s Who In Black Pittsburgh was held July 16, 2015, at the Herberman Conference Center at UPMC Cancer Pavilion, Pittsburgh. Since beginning in 1989 with a single publication, Who’s Who Publishing now produces annual directories of African American achievers in 25 cities, with Pittsburgh being the latest edition.
In additional to patient care Dr. Hogan oversees the surgical training of 40 resident and fellow physicians. Dr. Hogan has extensive research interest in musculoskeletal regenerative medicine with a focus on tendon, ligament, and cartilage bioengineering. He earned a Bachelor of Science degree from Xavier University of Louisiana and received a Doctor of Medicine from Howard University. He completed his orthopaedic surgery residency training at The University of Virginia. He completed his foot and ankle fellowship at the Hospital for Special Surgery.
Dr. Hogan was named a Top 40, Under 40 physician by the National Medical Association, and was recently awarded the Top 40, Under 40 Alumni award by Xavier University of Louisiana. For his research, Dr. Hogan and his team have received a number of grants and awards, including the 2013 American Orthopaedic Foot and Ankle Society J. Leonard Goldner Award for their work on Achilles tendon regeneration.
Congratulations, Dr. Hogan!
Badylak Lab Student to Receive Acta Student Award
 McGowan Institute for Regenerative Medicine congratulates Denver Faulk, a graduate student within the Bioengineering Department at the University of Pittsburgh and within the laboratory of McGowan Institute deputy director Stephen Badylak, DVM, PhD, MD, on his selection to receive an Acta Student Award for his primary contribution to the manuscript, “The effect of detergents on the basement membrane complex of a biologic scaffold material.”
McGowan Institute for Regenerative Medicine congratulates Denver Faulk, a graduate student within the Bioengineering Department at the University of Pittsburgh and within the laboratory of McGowan Institute deputy director Stephen Badylak, DVM, PhD, MD, on his selection to receive an Acta Student Award for his primary contribution to the manuscript, “The effect of detergents on the basement membrane complex of a biologic scaffold material.”
As noted by the judges, not only did Mr. Faulk’s paper demonstrate exceptional value to the materials community, but his personal credentials and recommendations were also exemplary. His research is focused on investigating whole liver extracellular matrix scaffolds for engineering an implantable liver graft for patients with end-stage liver failure.
Mr. Faulk will accept his award at the Materials Science & Technology (MS&T) 2015 Meeting in Columbus, Ohio, on October 5, 2015, during the American Society for Metals (ASM) Leadership Luncheon. He will receive a prize of $2,000 and reimbursement of his travel expenses.
Congratulations, Mr. Faulk!
Badylak Lab Student to Be Awarded TERMIS-AM Recognition
 McGowan Institute for Regenerative Medicine is pleased to announce that Timothy Keane, National Science Foundation Graduate Research Fellow in the Stephen Badylak Laboratory of the McGowan Institute and a student within the Department of Bioengineering, University of Pittsburgh, will be awarded the 2015 Mary Ann Liebert, Inc. Outstanding Student Award during the upcoming 2015 TERMIS World Congress. The 2015 TERMIS World Congress will be held from September 8-11 at the Marriott Copley Place in Boston, Massachusetts. The TERMIS-AM Mary Ann Liebert, Inc. Outstanding Student award presentation will be conducted on Thursday, September 10th during the Gala Event that will be held at the Museum of Science. The awards presentation (presentation of plaques) will begin at 8:00 PM.
McGowan Institute for Regenerative Medicine is pleased to announce that Timothy Keane, National Science Foundation Graduate Research Fellow in the Stephen Badylak Laboratory of the McGowan Institute and a student within the Department of Bioengineering, University of Pittsburgh, will be awarded the 2015 Mary Ann Liebert, Inc. Outstanding Student Award during the upcoming 2015 TERMIS World Congress. The 2015 TERMIS World Congress will be held from September 8-11 at the Marriott Copley Place in Boston, Massachusetts. The TERMIS-AM Mary Ann Liebert, Inc. Outstanding Student award presentation will be conducted on Thursday, September 10th during the Gala Event that will be held at the Museum of Science. The awards presentation (presentation of plaques) will begin at 8:00 PM.
At the award presentation ceremony, Mr. Keane will be presented with a plaque recognizing his outstanding research accomplishments within the tissue engineering and regenerative medicine field. He will also receive a complimentary registration to the 2015 TERMIS World Congress as well as an honorarium of $500 and reimbursement up to $1,000 to be used towards his travel expenses (airfare, hotel) to attend the conference.
Mr. Keane’s award-winning manuscript will be published in the journal, Tissue Engineering, Part A. His research projects involve investigating the role of decellularized extracellular matrix (ECM) in limb and muscle regeneration, specifically the tissue remodeling response to ECM that has been subjected to various degrees of decellularization.
Congratulations, Mr. Keane!
Wiegand Summer Internship
 The Wiegand Summer Internship, which is made possible through an endowment from Mr. and Mrs. Bruce Wiegand, who are friends and supporters of the Institute, is designed to provide an opportunity for a high school senior to experience the opportunities and excitement of a career in science and engineering with a focus on regenerative medicine.
The Wiegand Summer Internship, which is made possible through an endowment from Mr. and Mrs. Bruce Wiegand, who are friends and supporters of the Institute, is designed to provide an opportunity for a high school senior to experience the opportunities and excitement of a career in science and engineering with a focus on regenerative medicine.
The 2015 Wiegand Intern was Makenna Laffey who is a graduate of Pine-Richland High School. Ms. Laffey served her internship in the lab of McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD, associate professor in the Departments of Plastic Surgery in the School of Medicine and Bioengineering in the School of Engineering at the University of Pittsburgh, director of the Plastic Surgery Research Laboratory in the Department of Plastic Surgery in Pitt’s School of Medicine, and co-director of the Adipose Stem Cell Center of the McGowan Institute. Ms. Laffey’s responsibilities included assisting in the preparation of histology specimens, fabrication and evaluation of controlled release polymers for controlled release drug delivery, and data analysis.
At the conclusion of her 4-week internship, Ms. Laffey gave a presentation highlighting her experiences and observations. Attending were her collaborators and mentors from the Marra Lab and members of her family. She will be attending Georgia Tech in the fall, majoring in Biomedical Engineering.
Illustration: (left to right) Ryan Schroth, Kassandra O’Brien, Mayara Silva, Makenna Laffey, Kacey Marra, PhD, and Chris Mahoney.
Polish Mentee of Dr. Stephen Badylak Receives MIT Recognition
 Katarzyna Nawrotek, PhD Eng, of the Polytechnic University of Lodz (Poland) received recognition by Massachusetts Institute of Technology Technology Review as an Innovator Under 35. Her mentor is McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor in the Department of Surgery and director of the Center for Pre-Clinical Tissue Engineering within the Institute. Dr. Nawrotek is a participant in the Foundation for Polish Science’s SKILLS FNP Mentoring Program.
Katarzyna Nawrotek, PhD Eng, of the Polytechnic University of Lodz (Poland) received recognition by Massachusetts Institute of Technology Technology Review as an Innovator Under 35. Her mentor is McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor in the Department of Surgery and director of the Center for Pre-Clinical Tissue Engineering within the Institute. Dr. Nawrotek is a participant in the Foundation for Polish Science’s SKILLS FNP Mentoring Program.
Between the United States and Europe, more than 600,000 people suffer nerve damage which includes either cut or crushed nerves each year. These injuries create obstacles in the path through which the nervous impulses from the brain should slow, which can lead to loss of feeling or even movement in the affected area. The young, Polish innovator Dr. Nawrotek wants to minimize these effects, and to this end she has created a new technology that produces personalized, biocompatible channels that help to regenerate the damaged nervous tissue, and which dissolve once their function has been served.
The technology used by this researcher produces channels made of chitosan, a polymer obtained from chitin which is found naturally in crustacean shells, a mere 10 minutes prior to the operation. The polymer-based implants used currently are prepared much further in advance and stored until needed. In this way, Dr. Nawrotek´s technology allows the design of the implants to be personalized for the needs of the specific patient, already waiting on the operating table.
“Chitosan´s properties are similar to those of the extracellular matrix,” Dr. Nawrotek explains. This makes the chitosan an ideal substance for the channels that act as protective scaffolding for the damaged nerve, since it is not rejected by the human body and is bioabsorbable. “The degradation rate of the chitosan can be controlled through certain chemical modifications,” Dr. Nawrotek continues, “in such a way that, depending on the amount of time required for the nerve to regenerate, we can define the amount of time the implant must remain stable before it begins to disintegrate.”
The aim of the SKILLS FNP Mentoring Program is to enable its participants (young scientists working in Poland) to make contacts and gain mentors among experienced scholars – in Poland and abroad – with recognized academic achievements to their name. The program supports one-to-one mentoring.
Illustration: Massachusetts Institute of Technology Technology Review.
Regenerative Medicine Podcast Update
 The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#150 –– Mr. Patrick Cantini is the Strategy and Business Development Officer at the McGowan Institute. Mr. Cantini discusses the upcoming Fourth Annual Symposium on Regenerative Rehabilitation.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
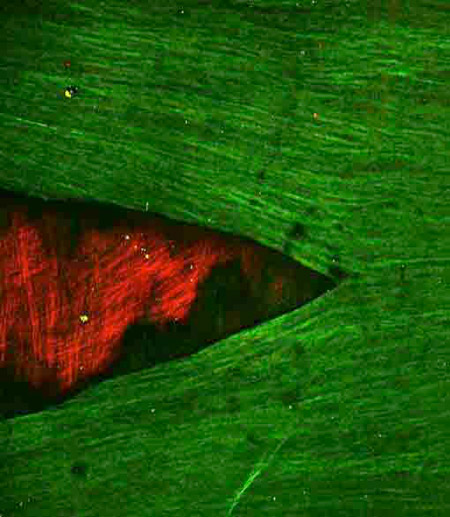
Crack in the internal elastic lamina of human cerebral vessels formed as a result of loading in uniaxial tension in the circumferential direction (vertical in image).
Multi-photon microscopy images from en face preparations of the human basilar artery displaying autofluorescent elastin, by utilizing two-photon emission (2PE) spectroscopy. The IEL (green) can be seen to be retracted and underlying medial collagen fibers (red) are visible using the signal from second harmonic generation.
From: A.M. Robertson, M.R. Hill, D. Li, Structurally motivated damage models for arterial walls – theory and application, in: D. Ambrosi, A. Quarteroni, G. Rozza (Eds.), in: Modelling of Physiological Flows, Modeling, Simulation and Applications, vol. 5, Springer-Verlag, 2011.
