June 2021 | VOL. 20, NO. 6| www.McGowan.pitt.edu
Injection of Light-Sensitive Proteins Restores Blind Man’s Vision

After 40 years of blindness, a 58-year-old man can once again see images and moving objects, thanks to an injection of light-sensitive proteins into his retina.
The study, published in Nature Medicine, is the first successful clinical application in optogenetics, in which flashes of light are used to control gene expression and neuron firing. The technique is widely used in laboratories to probe neural circuitry and is being investigated as a potential treatment for pain, blindness, and brain disorders.
The clinical trial, run by the company GenSight Biologics based in Paris, enrolls people with retinitis pigmentosa (RP): a degenerative disease that kills off the eye’s photoreceptor cells, which are the first step in the visual pathway. In a healthy retina, photoreceptors detect light and send electrical signals to retinal ganglion cells (RGCs), which then transmit the signal to the brain. GenSight’s optogenetic therapy skips the damaged photoreceptor cells entirely by using a virus to deliver light-sensitive bacterial proteins into the RGCs, allowing them to detect images directly.
The researchers injected the virus into the eye of a man with RP, then waited four months for protein production by the RGCs to stabilize before testing his vision. McGowan Institute for Regenerative Medicine affiliated faculty member José-Alain Sahel, MD, Chair and Distinguished Professor of the Department of Ophthalmology at the University of Pittsburgh School of Medicine, Director of the UPMC Eye Center, the Eye and Ear Foundation Endowed Chair of Ophthalmology, and leader of the study, says that one of the challenges was regulating the amount and type of light entering the eye, because a healthy retina uses a variety of cells and light-sensitive proteins to see a wide range of light. “No protein can replicate what the system can do,” he says. So, the researchers engineered a set of goggles that captured the visual information around the man and optimized it for detection by the bacterial proteins.
Using a camera, the goggles analyze changes in contrast and brightness and convert them in real time into what Dr. Sahel describes as a ‘starry sky’ of amber-colored dots. When the light from these dots enters a person’s eye, it activates the proteins and causes the RGCs to send a signal to the brain, which then resolves these patterns into an image.
The trial participant had to train with the goggles for several months before his brain adjusted to interpret the dots correctly. “He was like an experimentalist, a scientist trying to understand what he was seeing and make sense of it,” Dr. Sahel says. Eventually, he was able to make out high-contrast images, including objects on a table and the white stripes in a crosswalk. When the researchers recorded his brain activity, they found that his visual cortex reacted to the image in the same way as it would have if he had normal sight.
The man still can’t see without the goggles, but Dr. Sahel says that he wears them for several hours per day and that his vision has continued to improve in the two years since his injection. Six other people were injected with the same light-sensitive proteins last year, but the COVID-19 pandemic delayed their training with the goggles. Dr. Sahel expects to have their results within about a year.
Safe and permanent
“It’s a big step for the field,” says John Flannery, PhD, a neurobiologist at the University of California, Berkeley. “The most important thing is that it seems to be safe and permanent, which is really encouraging.” Because the retina contains around 100 times more photoreceptors than do RGCs, the resolution of images detected by RGCs will never be as good as natural vision. But Dr. Flannery says it is exciting that the brain can interpret images accurately.
Others say that more research is needed. “It’s interesting, but it’s an N of 1,” says Sheila Nirenberg, PhD, a neuroscientist at Weill Cornell Medical College in New York City. She says she looks forward to seeing whether the other people in the trial, including some who were injected with higher doses of the protein, have similar results.
GenSight is one of several companies developing optogenetics as a treatment for RP and other disorders of the retina. In March, Dr. Nirenberg’s company Bionic Sight announced that four of the five people with RP it had treated with a similar optogenetic therapy and a virtual-reality headset had recovered some level of vision, although the full trial results have not yet been published. And Swiss pharma giant Novartis is developing a therapy based on a different protein that is so light sensitive that goggles might not be needed. That therapy has not yet entered clinical trials.
Karl Deisseroth, MD, PhD, a neuroscientist at Stanford University in California who co-developed optogenetics as a lab technique, says the study is important because it is the first time that the technique’s effects have been shown in people. “It will be interesting to try this with more light-sensitive opsins” that might not require goggles, he says. But he expects optogenetics to be most useful as a research tool that leads to therapies, rather than a therapy itself. “What we hope to see even more of is optogenetics-guided human and clinical studies,” he says.
Illustration: Experimental setup for studying behavioral responses and brain activity. A visual detection task from the study. The man is asked to determine the presence or absence of a black cup on the white desk. Credit: J.-A. Sahel et al./Nature Medicine.
RESOURCES AT THE MCGOWAN INSTITUTE
July Histology Special – TUNEL Assay
Apoptosis, or “programmed cell death”, is a form of cell death designed to eliminate compromised or senescent cells. Apoptosis is controlled by multiple signaling and effector pathways that in turn, are activated in response to external growth, survival, or death factors.
A method for examining apoptosis via DNA fragmentation is the TUNEL assay. This technique can detect early-stage apoptosis in systems where chromatin condensation has begun and strand breaks are fewer, even before the nucleus undergoes recognizable morphologic changes.
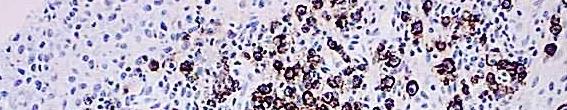
The McGowan Histology Core offers TUNEL staining on paraffin embedded or frozen tissue.
You will receive 30% off TUNAL Staining for the entire month of July when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
Sense of Touch Improves Control of Robotic Arm

Most able-bodied people take their ability to perform simple daily tasks for granted—when they reach for a warm mug of coffee, they can feel its weight and temperature and adjust their grip accordingly so that no liquid is spilled. People with full sensory and motor control of their arms and hands can feel that they’ve made contact with an object the instant they touch or grasp it, allowing them to start moving or lifting it with confidence.
But those tasks become much more difficult when a person operates a prosthetic arm, let alone a mind-controlled one.
In a paper published in Science, a team of bioengineers from the University of Pittsburgh Rehab Neural Engineering Labs describe how adding brain stimulation that evokes tactile sensations makes it easier for the operator to manipulate a brain-controlled robotic arm. In the experiment, supplementing vision with artificial tactile perception cut the time spent grasping and transferring objects in half, from a median time of 20.9 to 10.2 seconds. McGowan Institute for Regenerative Medicine affiliated faculty member Michael Boninger, MD, Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh, School of Medicine with joint appointments in the Departments of Bioengineering, Rehabilitation Science and Technology, and the Clinical and Translational Science Institute, is a co-author on this study.
“In a sense, this is what we hoped would happen—but perhaps not to the degree that we observed,” said co-senior author Jennifer Collinger, PhD, associate professor in the Pitt Department of Physical Medicine and Rehabilitation. “Sensory feedback from limbs and hands is hugely important for doing normal things in our daily lives, and when that feedback is lacking, people’s performance is impaired.”
Study participant Nathan Copeland, whose progress was described in the paper, is the first person in the world who was implanted with tiny electrode arrays not just in his brain’s motor cortex but in his somatosensory cortex as well—a region of the brain that processes sensory information from the body. Arrays allow him to not only control the robotic arm with his mind, but also to receive tactile sensory feedback, which is similar to how neural circuits operate when a person’s spinal cord is intact.
“I was already extremely familiar with both the sensations generated by stimulation and performing the task without stimulation. Even though the sensation isn’t ‘natural’—it feels like pressure and gentle tingle—that never bothered me,” said Mr. Copeland. “There wasn’t really any point where I felt like stimulation was something I had to get used to. Doing the task while receiving the stimulation just went together like PB&J.”
After a car crash that left him with limited use of his arms, Mr. Copeland enrolled in a clinical trial testing the sensorimotor microelectrode brain-computer interface (BCI) and was implanted with four microelectrode arrays developed by Blackrock Microsystems (also commonly referred to as Utah arrays).
This paper is a step forward from an earlier study that described for the first time how stimulating sensory regions of the brain using tiny electrical pulses can evoke sensation in distinct regions of a person’s hand, even though they lost feeling in their limbs due to spinal cord injury. In this new study, the researchers combined reading the information from the brain to control the movement of the robotic arm with writing information back in to provide sensory feedback.
In a series of tests, where the BCI operator was asked to pick up and transfer various objects from a table to a raised platform, providing tactile feedback through electrical stimulation allowed the participant to complete tasks twice as fast compared to tests without stimulation.
In the new paper, the researchers wanted to test the effect of sensory feedback in conditions that would resemble the real world as closely as possible.
We didn’t want to constrain the task by removing the visual component of perception,” said co-senior author Robert Gaunt, PhD, associate professor in the Pitt Department of Physical Medicine and Rehabilitation. “When even limited and imperfect sensation is restored, the person’s performance improved in a pretty significant way. We still have a long way to go in terms of making the sensations more realistic and bringing this technology to people’s homes, but the closer we can get to recreating the normal inputs to the brain, the better off we will be.”
Dr. Kacey Marra Receives $2M DOD Grant for Nerve Guide Work

McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD, Professor, Departments of Plastic Surgery and Bioengineering, Vice Chair of Research, Department of Plastic Surgery, recently was awarded a 2-year, $2,085,067 Department of Defense (DOD) Congressionally Directed Medical Research Programs, Peer Reviewed Medical Research Program (PRMRP), Technology/Therapeutic Development Award (TTDA) grant to obtain regulatory approval for her novel nerve guide. This will provide funds to finish the necessary testing of the nerve guide to get approval for the first human clinical trial. The co-investigator is Anthony Windebank, MD, Professor of Neurology, Mayo Clinic, Rochester, Minnesota, where the team will optimize the manufacturing process of the nerve guides under GMP conditions.
The project title is “Development of an Implantable Medical Device for Human Extremity Nerve Injuries,” and will run from July 1, 2021-June 30, 2023.
The PRMRP TTDA is a product-driven award mechanism intended to provide support for the translation of promising preclinical findings into products for clinical applications, including prevention, detection, diagnosis, treatment, or quality of life, in at least one of the Congressionally directed PRMRP Topic Areas. Products in development should be responsive to the healthcare needs of military Service Members, Veterans, and/or beneficiaries.
Congratulations, Dr. Marra!
Landmark Data Presented for ZFUZE Surgical Polymer

DiFusion Technologies, Inc. recently announced landmark data for the company’s ZFUZE Surgical Polymer and how it compares to traditional biomaterials like Titanium and Poly-Ether-Ether-Ketone (PEEK). The data and the importance of the immune system response in new technologies such as ZFUZE were debated by Stephen Badylak, DVM, MD, PhD, Deputy Director of the McGowan Institute for Regenerative Medicine, Professor in the Department of Surgery at the University of Pittsburgh, and Director of the Center for Pre-Clinical Tissue Engineering within the Institute, at the International Society for Advancement of Spine Surgery (ISASS) in late May 2021 in Miami. Dr. Badylak is a member of the Scientific Advisory Board of DiFusion Technologies, Inc.
The immune system plays a vital role in tissue healing, but only recently have experts come to understand the importance of the immune system in normal tissue/organ development, remodeling following injury, and regeneration and how important it is to consider the immune response in the design and development of implantable medical materials and devices. Medical practitioners are now considering the “host response” for medical implants as the human immune system sees Titanium and PEEK as “foreign” which triggers a chronic long-term inflammatory response.
Dr. Badylak is among the foremost experts the immune response to biomaterials. He discussed data related to ZFUZE at the ISASS meeting. “Imagine having two materials and making identical devices in terms of the mechanical properties, such as pore size, but one material (PEEK) promotes a prolonged inflammatory response and the other material (ZFUZE) promotes a pro-healing remodeling response,” said Dr. Badylak. “We have tested over 300 different biomaterials with respect to the type of macrophage response that is elicited and have never seen immunomodulation like we have for ZFUZE in anything other than a naturally occurring molecule.” Watch a video of Dr. Badylak explaining the technology here.
“Dr. Badylak’s presentation at ISASS was a wake-up call to the industry. For many years orthopedic OEM’s have borrowed materials like titanium and PEEK from the aerospace industry, based on strength or surface topography. He showed us an entirely new way of looking at the host response to medical implants,” said Jeffrey Fernyhough, MD, Founder and CEO, Florida Back Institute. “Companies should be looking to material biocompatibility first and foremost when selecting future biomaterials.”
Dr. Badylak’s Osteoimmunology Study was followed by a Clinical Patient review of ZFUZE surgeries performed by Paul Kraemer, MD, of the Indiana Spine Group. “We have reviewed more than 25 surgeries via CT scan and have documented early boney ingrowth and ongrowth in every patient to date. The big takeaway from these cases is how ZFUZE performs in immuno-compromised patients such as diabetics, osteopenic patients, and even smokers,” Paul Kraemer, MD.
“Host rejection of medical implants is a major concern across virtually every sector of medicine. The results we have gleaned via Dr. Badylak’s work not only have major implications in orthopedics but in regenerative medicine as a whole,” said Derrick Johns, Founder and CEO of DiFusion Technologies. “Pairing Dr. Badylak’s osteoimmunology data with Dr. Kraemer’s clinical results at ISASS is a difficult challenge for traditional implant makers to overcome and puts DiFusion at the forefront of biomaterial OEM’s.”
Illustration: DiFusion Technologies, Inc.
Engineering Smarter Stents

An estimated two million people will need a coronary artery stent every year. A small mesh tube inserted into a narrow or blocked coronary artery, a stent can help ensure blood can continue to flow through the artery unimpeded. Today, many also contain a coating that releases a steady dose of medication to improve healing and keep the blockage from coming back.
Stents, however, are not without their risks: Restenosis—the re-narrowing of an artery—is one risk of coronary artery stents and occurs in three to 10 percent of cases in the first six to nine months. There is also a risk of blood clot formation, or thrombosis, that can also occur as the stent’s medicinal coating dissolves, exposing a metal surface.
McGowan Institute for Regenerative Medicine affiliated faculty member Youngjae Chun, PhD, Associate Professor of Industrial Engineering with a secondary appointment in the Department of Bioengineering at the University of Pittsburgh Swanson School of Engineering, is part of a consortium from industry, academia, and research that will seek to revolutionize the design of heart stents. The new stents will feature ultra-low-profile struts and a uniquely textured “smart” surface that will help improve healing and lessen the risks of restenosis and thrombosis.
The consortium, which includes members from industry, academia, and research, recently received $2 million in funding from the South Korean Ministry of Trade, Industry and Energy’s Outstanding Company Research Center Promotion Project (ATC+).
“The uniqueness of our stent is in both the thin struts that may reduce the risk of restenosis and the smart surface, which can help improve healing and prevent clots,” said Dr. Chun, who is a co-principal investigator. “When you introduce specialized micro and nanopatterns on the material—like grouped patterns, dimples, cavities, or diamond patterns—you can improve biocompatibility.”
Most widely used drug-eluting stents (DES) have a polymer coating mixed with a drug that is released over several months to help prevent restenosis. After that period, however, the metal stent is exposed. The unique, patterned surface on the proposed DES design would encourage endothelial cells—cells that form a barrier between vessels and tissue to control the flow of fluids in the body—to grow on the surface of the stent, helping to speed healing and reduce the risk of blood clot formation.
Dr. Chun’s lab will provide the computational modeling of the stent, as well as the creation of the smart surface. They will work with lead investigator and DES manufacturing company Osstem Cardiotech, as well as Daegu Gyeongbuk Medical Innovation Foundation, located in South Korea.
The project, “Development of a Coronary Artery Drug Eluting Stent That Contains Smart 60um Ultra-Thin Struts and Surface Structures for Rapid Vascular Healing Process,” began in April 2021 and will last four years.
Novel Immunotherapy Boosts Long-Term Stroke Recovery in Mice
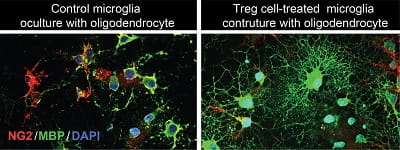
McGowan Institute for Regenerative Medicine faculty member Donna Stolz, PhD, Associate Director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and an Associate Professor in the Departments of Cell Biology and Pathology at the University of Pittsburgh, is a co-author on a study where it was found by University of Pittsburgh School of Medicine neurologists that specialized immune cells that accumulate in the brain in the days and weeks after a stroke promote neural functions in mice, pointing to a potential immunotherapy that may boost recovery after the acute injury is over.
The study, published in the journal Immunity, demonstrated that a population of specialized immune cells, called regulatory T (Treg) cells, serve as tissue repair engineers to promote functional recovery after stroke. Boosting Treg cells using an antibody complex treatment, originally designed as a therapy after transplantation and for diabetes, improved behavioral and cognitive functions for weeks after a stroke in mice compared to those that did not receive the antibody complex.
“The beauty of this treatment is in its wide therapeutic window,” said senior author Xiaoming Hu, MD, PhD, Associate Professor in the Department of Neurology at Pitt’s School of Medicine. “With most strokes, you have four and a half hours or less when you can give medication called tPA to reopen a blocked blood vessel and expect to rescue neurons. We’re excited to identify a mechanism that may promote brain recovery by targeting non-neuronal cells well after this window closes.”
Previous research in stroke has been focused on developing new drugs to reduce neuronal death. And whereas these acute stroke treatments quickly lose effectiveness after neurons die, Treg cells remain active for weeks after the injury.
True to their name, Treg cells are immune cells that regulate the immune response, including curtailing excessive inflammation that could harm more than help. Hu and her colleagues observed that the levels of Treg cells infiltrating the brain began to increase about a week after a stroke and continued increasing up to five weeks later. So, they did multiple tests in mice after they’d had strokes, paying particular attention to the brain’s white matter—which is the brain tissue through which neurons pass messages, turning thoughts into actions, like lifting food to your mouth or saying the name of an object you’re looking at.
Mice that were genetically unable to produce Treg cells fared worse than mice with a robust Treg cell response. Interestingly, it was only in the latter phases of stroke recovery that the Treg cell-depleted mice suffered impairments in white matter integrity and behavioral performance compared to mice with a normal Treg cell response.
Additionally, when normal mice were given an antibody complex called “IL-2:IL-2Ab” to boost their Treg cell levels after a stroke, their white matter integrity improved even more and neurological functions were rescued over the long term. The mice with more Treg cells had an easier time moving and had better memories, allowing them to navigate mazes faster after a stroke than their non-treated counterparts.
“This strongly suggests that, rather than working to preserve white matter structure and function immediately after a stroke, Treg cells influence long-term white matter repair and regeneration,” said Dr. Hu, also a member of the Pittsburgh Institute of Brain Disorders and Recovery and a U.S. Department of Veterans Affairs (VA) investigator. “Our findings pave the way for a therapeutic approach to stroke and other neurological disorders that involve excessive brain inflammation and damage to the white matter. Treg cells appear to hold neurorestorative potential for stroke recovery.”
Dr. Hu stressed that there are still many hurdles to cross before Treg cells could be used in humans for stroke recovery. Namely, research is needed to determine the best way to boost the number of Treg cells in stroke victims. This could be done by improving IL-2:IL-2Ab so that it better stimulates production of Treg cells with fewer side effects, or a personalized therapy could be developed where some cells are taken from an established Treg cell bank and used to grow custom Treg cells in the lab, which could then be infused back into the patient.
Illustration: Treg cells boost neuroregeneration: These images show how regulatory T cells (Treg cells) boost the ability of microglia cells to promote the regeneration of the brain’s white matter (right), compared to a sample not treated with Treg cells (left). Xiaoming Hu/University of Pittsburgh.
Pre-Pandemic Hospital Surge Capacity ‘Time Capsule’

A University of Pittsburgh School of Medicine-led survey of dozens of surge capacity managers at hospitals nationwide captures the U.S. health care system’s pandemic preparedness status in the months before the first COVID-19 cases were identified in China. McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, Executive Vice President, and Chief Healthcare Innovation Officer, UPMC; Associate Vice Chancellor for Healthcare Innovation, University of Pittsburgh Schools of the Health Sciences; Distinguished Professor and Mitchell P. Fink Endowed Chair, Department of Critical Care Medicine, University of Pittsburgh and UPMC; Senior editor, JAMA, is a co-author of the study published in the journal JAMA Network Open.
The investigation details the strain experienced by U.S. hospitals during the 2017-18 influenza season, which was marked by severe illness and the highest infectious disease-related hospitalization rates in at least a decade. At the time, pandemic planning within hospitals was not reported as being a high priority.
“The timing for our survey couldn’t have been better—ultimately it serves as a pre-COVID-19 time capsule of our preparedness to accommodate surges in patients needing hospitalization for acute illness,” said senior author David Wallace, MD, MPH, associate professor in Pitt’s departments of Critical Care Medicine and Emergency Medicine. “It was surprising to hear very detailed stories of the strain hospitals were under during the 2017-18 flu season, and yet have no pandemic planning come out of it.”
The 2017-18 flu season was associated with more than 27.7 million medical visits, nearly a million hospitalizations and almost 80,000 deaths, according to the U.S. Centers for Disease Control and Prevention. That is more than double the deaths in a typical flu season and the highest hospitalization rate since seasonal influenza surveillance was instituted in 2005.
Dr. Wallace and his team—which included specialists in health policy, medical anthropology, and infectious diseases—interviewed surge capacity managers at a random sampling of 53 hospitals across the U.S. starting in April 2018, at the tail end of the flu season. Using a structured survey, they recorded detailed interviews about everything from ICU bed capacity and staffing ratios to the perceived effect of strain on quality of patient care and staff well-being.
All of those surveyed reported experiencing hospital strain during the 2017-18 flu season. Strain was generally described as the result of high patient occupancy causing demand to outstrip the supply of resources—in fact or in perception.
The “4 S’s”—staff, stuff, space, and systems—were reported as the widespread challenges that surge capacity managers consistently faced in continuing health care operations during the flu season. Staff was a particular concern, due to fatigue or staff being out sick with flu or caring for ill family.
“This demonstrates that the perceptions of strain on staffing, patient care and capacity that we have seen during the COVID-19 pandemic were already present with prior epidemics,” said lead author Gavin Harris, MD, an assistant professor in the Emory University School of Medicine, who did this research while at Pitt. “Less than two years before COVID-19 took off in the U.S., we were experiencing a preview of the strain that a fast-spreading, severe respiratory infection places on our health system.”
In fall 2013—four years before this challenging flu season—the U.S. Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response produced the Interim Healthcare Coalition Checklist for Pandemic Planning report, which identified eight categories hospitals should address when planning for crises, specifically surges in acute care needs. None of the survey participants commented on all eight categories, nor did any specifically report using the checklist.
“Hospitals have a tendency to deal with what’s right in front of them, the present,” Dr. Wallace said. “In doing that, we must also learn when certain levers—like a pandemic preparedness checklist—must be pulled. That is done through reflecting after a crisis subsides and looking for opportunities to improve before the next crisis hits. If the past year has taught us anything, it’s that infectious diseases aren’t going away, and we’ll always get a chance to put lessons learned to work.”
Additional authors on this research are Kimberly Rak, PhD, MPH, Jeremy Kahn, MD, MSc, Olivia Mancing, and Julia Driessen, PhD, all of Pitt.
Shifting Gears Toward Chemical Machines
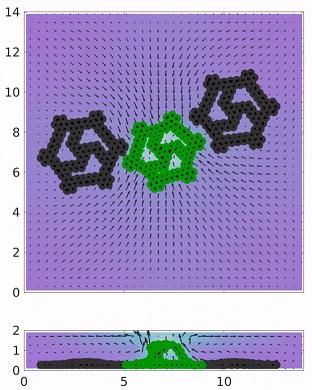
The gear is one of the oldest mechanical tools in human history and led to machines ranging from early irrigation systems and clocks, to modern engines and robotics. For the first time, researchers at the University of Pittsburgh Swanson School of Engineering have utilized a catalytic reaction that causes a two-dimensional, chemically coated sheet to spontaneously “morph” into a three-dimensional gear that performs sustained work.
The findings indicate the potential to develop chemically driven machines that do not rely on external power, but simply require the addition of reactants to the surrounding solution. Published in the Cell Press journal Matter, the research was developed by Anna Balazs, PhD, Distinguished Professor of Chemical and Petroleum Engineering and the John A. Swanson Chair of Engineering. Lead author is Abhrajit Laskar, PhD, and co-author is Oleg Shklyaev, PhD, both post-doctoral associates.
“Gears help give machines mechanical life; however, they require some sort of external power, such as steam or electricity, to perform a task. This limits the potential of future machines operating in resource-poor or remote environments,” Dr. Balazs explains. “Abhrajit’s computational modeling has shown that chemo-mechanical transduction (conversion of chemical energy into motion) at active sheets presents a novel way to replicate the behavior of gears in environments without access to traditional power sources.”
In the simulations, catalysts are placed at various points on a two-dimensional sheet resembling a wheel with spokes, with heavier nodes on the sheet’s circumference. The flexible sheet, approximately a millimeter in length, is then placed in a fluid-filled microchamber. A reactant is added to the chamber that activates the catalysts on the flat “wheel”, thereby causing the fluid to spontaneously flow. The inward fluid flow drives the lighter sections of the sheet to pop up, forming an active rotor that catches the flow and rotates.
“What is really distinctive about this research is the coupling of deformation and propulsion to modify the object’s shape to create movement,” Dr. Laskar says. “Deformation of the object is key; we see in nature that organisms use chemical energy to change their shape and move. For our chemical sheet to move, it also has to spontaneously morph into a new shape, which allows it to catch the fluid flow and perform its function.”
Additionally, Drs. Laskar and Shklyaev found that not all the gear parts needed to be chemically active for motion to occur; in fact, asymmetry is crucial to create movement. By determining the design rules for the placement, Drs. Laskar and Shklyaev could direct the rotation to be clockwise or counterclockwise. This added “program” enabled the control of independent rotors to move sequentially or in a cascade effect, with active and passive gear systems. This more complex action is controlled by the internal structure of the spokes, and the placement within the fluid domain.
“Because a gear is a central component to any machine, you need to start with the basics, and what Abhrajit has created is like an internal combustion engine at the millimeter scale,” Dr. Shklyaev says. “While this won’t power your car, it does present the potential to build the basic mechanisms for driving small-scale chemical machines and soft robots.”
In the future, Dr. Balazs will investigate how the relative spatial organization of multiple gears can lead to greater functionality and potentially designing a system that appears to act as if it were making decisions.
“The more remote a machine is from human control, the more you need the machine itself to provide control in order to complete a given task,” Dr. Balazs said. “The chemo-mechanical nature of our devices allows that to happen without any external power source.”
These self-morphing gears are the latest evolution of chemo-mechanical processes developed by Drs. Balazs, Laskar, and Shklyaev. Other advances include creating crab-like sheets that mimic feed, flight, and fight responses; and sheets resembling a “flying carpet” that wrap, flap, and creep.
Illustration: Transmission of rotational motion from an active gear to two passive gears. In a fluidic chamber, an active gear can rotate multiple passive gears, which are placed to break the symmetry of the flow field. Balazs Lab/University of Pittsburgh Swanson School of Engineering.
AWARDS AND RECOGNITION
Dr. Peter Rubin Named in Newsweek’s America’s Best Plastic Surgeons 2021

Newsweek magazine, this year, for the first time, partnered with Statista Inc., the global market research and customer data firm, to find America’s Best Plastic Surgeons. The data was
ranked into four categories: Breast Augmentation, Facelift, Liposuction, and Rhinoplasty. To determine the winners, Newsweek conducted a national survey among plastic surgeons, asking them to recommend the best plastic surgeons in their state as well as across the U.S. Additionally, participants were asked to rank their peers according to several quality dimensions. The rankings feature the top 200 plastic surgeons for Breast Augmentation and the top 150 for Facelift, Liposuction, and Rhinoplasty. In total, 387 individual plastic surgeons were ranked, with some being recognized for more than one procedure.
McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, was recognized in three of the four categories. His rankings include: #72 (of 200) Breast Augmentation, #115 (of 150) Facelift, and #55 (of 150) Liposuction. Congratulations, Dr. Rubin!
Dr. Rubin is Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh. He earned his undergraduate degree in biology from Grinnell College and his MD degree from Tufts University School of Medicine. He completed a residency training program in general surgery at Boston University/Boston City Hospital. He took time away from the clinic to pursue a two-year fellowship in surgical basic science at Massachusetts General Hospital/Harvard Medical School. He completed a three-year residency in plastic surgery at Harvard Medical School and joined the plastic surgery faculty at the University of Pittsburgh.
Dr. Rubin is well-recognized for his surgical skills and innovative solutions to complex aesthetic and reconstructive problems. He is Founder and Director of the Life After Wright Loss Surgical Body Contouring Program at the University of Pittsburgh Medical Center. In addition to his active clinical program, Dr. Rubin directs a basic science research program in the biology of adipose derived stem cells and serves as Co-Director of the Adipose Stem Cell Center at the University of Pittsburgh. He is the principal investigator in an NIH funded line of research aimed at developing cell-based methods for clinical soft tissue reconstruction after cancer therapy. He directs a related line of research aimed at soft tissue reconstruction for injured military personnel as an investigator for the Department of Defense Armed Forces Institute for Regenerative Medicine (AFIRM). To facilitate the rapid translation of new technology, he founded the Center for Innovation in Restorative Medicine (CIRM) at the University of Pittsburgh.
This “clinical accelerator” has a staff of experts in regulatory affairs, clinical trials implementation, and preclinical testing and has successfully taken new therapies through the IND and IDE process. He has moved his work from the laboratory to the clinic, and he is conducting clinical trials funded by the Department of Defense that are assessing new minimally invasive therapies to improve the lives of our wounded warriors.
His many scientific leadership positions include current Director, American Board of Plastic Surgery; Co-Chair of the American Society of Plastic Surgeons (ASPS) Task Force on Regenerative Medicine; Regulatory Chair for ASPS; Vice President of Finance for ASPS, past president of the International Society of Adipose Therapeutics and Science (IFATS), Board Chair of IFATS, and past Chairman of the Plastic Surgery Research Council. Dr. Rubin is the recipient of a Presidential Early Career Award for Scientists and Engineers (PECASE). The Presidential Award is the highest honor bestowed by the United States government on outstanding scientists and engineers early in their research careers. It is intended to recognize some of the finest scientists who show exceptional potential for leadership at the frontiers of scientific knowledge during the twenty-first century. He has served as editor for four textbooks, published over 180 peer reviewed articles, and presented over 500 invited lectures.
54 Current McGowan Institute Affiliated Faculty Members Ranked in Top 2% of World Scientists
 According to a report by Stanford University, 54 current McGowan Institute for Regenerative Medicine affiliated faculty members are in the top 2% of world scientists. The report covered scientists globally from a wide range of fields. The ranking is based on citations from Scopus, assessing scientists for career-long citation impact up until the end of 2019, and for citation impact during the single calendar year 2019. More information on the ranking method is found here.
According to a report by Stanford University, 54 current McGowan Institute for Regenerative Medicine affiliated faculty members are in the top 2% of world scientists. The report covered scientists globally from a wide range of fields. The ranking is based on citations from Scopus, assessing scientists for career-long citation impact up until the end of 2019, and for citation impact during the single calendar year 2019. More information on the ranking method is found here.
Of particular note, four of the McGowan Institute affiliated faculty members listed were ranked in the 1-10 range for their respective fields. They include:
Krzysztof Matyjaszewski, PhD: #1 Polymers
Derek Angus, MD, MPH: #6 Emergency & Critical Care Medicine
Stephen Badylak, DVM, PhD, MD: #7 Biomedical Engineering
John Kellum, MD: #9 Emergency & Critical Care Medicine
The remaining McGowan Institute affiliated faculty members who made the list and their associated subject fields are:
Ivet Bahar, PhD: Bioinformatics
Anna Balazs, PhD: Chemical Physics
Elia Beniash, PhD: Dentistry
Christopher Bettinger, PhD: Nanoscience & Nanotechnology
Timothy Billiar, MD: Surgery
Michael Boninger, MD: Rehabilitation
Charleen Chu, MD, PhD: Biochemistry & Molecular Biology
Gilles Clermont, MD: Emergency & Critical Care Medicine
Rory Cooper, PhD: Rehabilitation
Xinyan Tracy Cui, PhD: Biomedical Engineering
Anthony Delitto, PhD, PT: Rehabilitation
Anthony Demetris, MD: Surgery
Mary Amanda Dew, PhD: Psychiatry
Adam Feinberg, PhD: Biomedical Engineering
Freddie Fu, MD: Orthopedics
Jörg Gerlach, MD, PhD: Biomedical Engineering
Thomas Gilbert, PhD: Biomedical Engineering
George Gittes, MD: Pediatrics
Joseph Glorioso III, PhD: Biotechnology
Abhinav Humar, MD: Surgery
Valerian Kagan, PhD: Biochemistry & Molecular Biology
Robert Kormos, MD: Surgery
Prashant Kumta, PhD: Energy
Eric Lagasse, PharmD, PhD: Immunology
Michael Lotze, MD: Immunology
James Luketich, MD: Respiratory System
Kacey Marra, PhD: Biomedical Engineering
Dennis McNamara, MD: Cardiovascular System & Hematology
George Michalopoulos, MD, PhD: Gastroenterology & Hepatology
Satdarshan Paul Singh Monga, MD: Gastroenterology & Hepatology
Bradley Nindl, PhD: Sport Sciences
Michael Pinsky, MD: Emergency & Critical Care Medicine
Thomas Rando, MD, PhD: Developmental Biology
Peter Rubin, MD: Surgery
José Alain Sahel, MD: Ophthalmology & Optometry
Sruti Shiva, PhD: Biochemistry & Molecular Biology
Ian Sigal, PhD: Ophthalmology & Optometry
Carl Snyderman, MD: Otorhinolaryngology
Rocky Tuan, PhD: Biomedical Engineering
Jessie VanSwearingen, PhD: Rehabilitation
Sachin Velankar, PhD : Polymers
Raman Venkataramanan, PhD: Pharmacology & Pharmacy
Yoram Vodovotz, PhD: Emergency & Critical Care Medicine
David Vorp, PhD: Biomedical Engineering
William Wagner, PhD : Biomedical Engineering
Jonathan Waters, MD: Anesthesiology
Simon Watkins, PhD: Immunology
Alan Wells, MD, DMS: Biochemistry & Molecular Biology
David Whitcomb, MD, PhD: General & Internal Medicine
Frank Witte, MD, PhD: Biomedical Engineering
See the full list of the Top 2% of World Scientists here.
Congratulations, all!
2020-2021 Engineering Graduate Student Organization Award Recipients
The University of Pittsburgh’s Swanson School of Engineering’s (SSOE’s) Engineering Graduate Student Organization (EGSO) recently announced the winners of the Outstanding Teaching Assistant award and Outstanding Research Assistant award in each of the SSOE departments. The winners from the following McGowan Institute for Regenerative Medicine affiliated faculty member labs include:
| Emily Ackerman, in the lab of Jason Shoemaker, PhD, as the 2020-2021 Outstanding Research Assistant in Chemical Engineering! |  |
| Ronald Fortunato, in the lab of Spandan Maiti, PhD, as the 2020-2021 Outstanding Research Assistant in Mechanical Engineering and Materials Science! |  |
| Monica Shapiro, in the lab of Robert Parker, PhD, as the 2020-2021 Outstanding Teaching Assistant in Chemical Engineering! |  |
| Kevin Stieger, in the lab of Takashi Kozai, PhD, as the 2020-2021 Outstanding Research Assistant in Bioengineering! |  |
Winners were invited to the virtual EGSO Awards ceremony to receive their awards scheduled for Wednesday, April 14, at 6:00 PM.
Congratulations, all!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#222 –– Dr. Juliana Blum discusses her work at Humalyte, where she is working on the development and manufacture of bioengineered human tissues.
*Humacyte’s products are not yet approved by the U.S. Food and Drug Administration and are currently in clinical evaluation.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: José-Alain Sahel, Elise Boulanger-Scemama, Chloé Pagot, Angelo Arleo, Francesco Galluppi, Joseph N. Martel, Simona Degli Esposti, Alexandre Delaux, Jean-Baptiste de Saint Aubert, Caroline de Montleau, Emmanuel Gutman, Isabelle Audo, Jens Duebel, Serge Picaud, Deniz Dalkara, Laure Blouin, Magali Taiel & Botond Roska
Title: Partial recovery of visual function in a blind patient after optogenetic therapy
Summary: Optogenetics may enable mutation-independent, circuit-specific restoration of neuronal function in neurological diseases. Retinitis pigmentosa is a neurodegenerative eye disease where loss of photoreceptors can lead to complete blindness. In a blind patient, we combined intraocular injection of an adeno-associated viral vector encoding ChrimsonR with light stimulation via engineered goggles. The goggles detect local changes in light intensity and project corresponding light pulses onto the retina in real time to activate optogenetically transduced retinal ganglion cells. The patient perceived, located, counted and touched different objects using the vector-treated eye alone while wearing the goggles. During visual perception, multichannel electroencephalographic recordings revealed object-related activity above the visual cortex. The patient could not visually detect any objects before injection with or without the goggles or after injection without the goggles. This is the first reported case of partial functional recovery in a neurodegenerative disease after optogenetic therapy.
Source: Nature Medicine. 2021 May 24. doi: 10.1038/s41591-021-01351-4. Online ahead of print.
GRANT OF THE MONTH
PI: William Wagner
Co-PI: John Alcorn, Stephen Badylak, Louis Falo, William Federspiel, Neeraj Gandhi, Eric Lagasse, Steven Little, Alan Wells, and Cecilia Yates
Title: Therapies for COVID-related Disease and Technology Development
Description: Since December 2020, the FDA (Federal Drug Administration) emergency use authorization of two vaccines directed against SARS-CoV-2 (COVID 19 or Coronavirus) has changed the trajectory and future of personal and public health management of COVID-19. While these vaccines show success rates as high as 95% in preventing severe COVID-19 adverse events, these vaccine studies did not investigate the efficacy of these vaccines in the context of a patient receiving immunomodulatory therapies for either COVID-19 or a separate comorbidity. The Badylak laboratory at the University of Pittsburgh completed a pilot study investigating the efficacy of recently characterized, immunomodulatory matrix-bound nanovesicles (MBV) in treatment of viral-induced pulmonary infection and inflammation. In this study, preliminary results demonstrate a significant reduction in overall lung inflammation and promotion of a systemic immune response that is both anti-viral and anti-inflammatory in properties. While promising, the observed immunomodulatory effect of MBV has an unknown effect on the well-described and predictable vaccine-driven immune response. Therefore, with more patients becoming vaccinated against COVID-19 every day, it is of immense importance to pursue investigation into the effect of MBV on a normal vaccine response. The translational viability of MBV therapy for COVID-19 will only be successful if the patient’s ability to mount a physiologic vaccine response is preserved throughout and following treatment.
In this project, we will use funds to investigate two central questions: 1.) Can immunomodulatory MBV be administered by intravenous (IV, systemic) injection to treat COVID-19 mediated lung infection, and 2.) does administration of MBV have any adverse effects on the host ability to mount an effective (virus neutralizing) immune response? Using murine models of COVID-19 and H1N1, we will investigate the therapeutic potential of MBV in these two examples of viral-mediated lung inflammation. Since the COVID-19 vaccines are not available at the present moment for research use, we will use the influenza vaccine as a model response. Since vaccine responses are well-conserved across the type of vaccine, we will be able to extrapolate results from this study and apply them to our understanding of the COVID-19 vaccine.
To address question 1, we will use the transgenic ACE2 (angiotensin-converting enzyme expressing mice) (wild-type mice cannot be infected with COVID-19 since they lack the ACE2 receptor) to study the efficacy of MBV in mediating COVID-19 lung inflammation. To address question 2, we will use wildtype mice. To study the effect of MBV on the normal vaccine response, animals will be treated with MBV before and during vaccination with the H1N1 (influenza A virus subtype) vaccine. After the vaccine period, animals will then be infected with live H1N1 and survival and lung inflammation will be used as metrics to determine safety and compatibility of MBV with vaccines.
Source: Pennsylvania Department of Human Services (DHS)
Term: 2 Years
Amount: $4 million
