October 2023 | VOL. 22, NO. 10 | www.McGowan.pitt.edu
Stories by Cristina D’Imperio
The Diabetic Foot Consortium
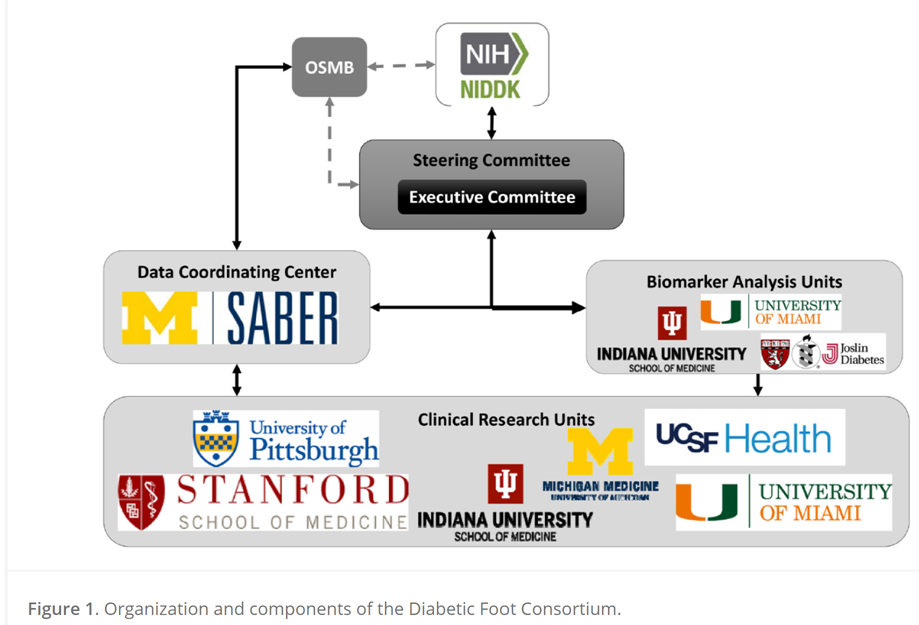
In 2020, six U.S. research institutions launched the first-ever multicenter network to study diabetic foot ulcers (DFUs). Funded by the National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) beginning in 2018, the Diabetic Foot Consortium (DFC) is comprised of the University of Pittsburgh and the McGowan Institute; University of Michigan at Ann Arbor; University of California, San Francisco; Stanford University, Palo Alto California; University of Miami Miller School of Medicine; Indiana University, Indianapolis; and the University of Arizona.
According to the CDC, 37.3 million Americans have diabetes – the equivalent of 11.3% of the U.S. population. Diabetes is ranked as the 8th leading cause of death in the U.S., contributing to more than 100,000 deaths annually.
One of the most frequent complications in diabetes is DFUs. There is a lifetime incidence of foot ulcers between 19% and 34% in diabetics. More than half of DFUs become infected, and approximately 20% of moderate or severe infections lead to amputation. DFUs are the leading cause of lower limb amputations in the U.S.
The DFC aims to lay the foundation for a clinical trial network to test how to improve diabetic wound healing and prevent amputations among the millions of American adults with diabetes.
Chandan K. Sen, PhD, Director of the McGowan Institute, states, “The McGowan Institute will be home to the Pennsylvania site of the DFC, and the Joslin Diabetes Center at Harvard will be our satellite site. Clinical research across 20 UPMC wound care sites supported by innovative basic science research and industry partnerships will position the McGowan Institute as a national leader in this emergent health care discipline.”
Read more about the DFC here and here.
RESOURCES AT THE MCGOWAN INSTITUTE
November Histology Special – Herovici Staining
The Herovici combination of aniline blue or picro methyl blue and picro acid fuchsin results in Type I mature collagen staining red while reticulum and newly formed Type III collagen stains blue.
You’ll receive 25% off your Herovici staining in November when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: Please contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
* 25% off applies to routine sample preparation (processing, embedding, cutting, and staining). It does not apply to specialized services such as immunohistochemistry workups, and IHC staining.
SCIENTIFIC ADVANCES
McGowan Institute and Dr. Vo Part of New Orland Bethel Family Musculoskeletal Research Center
 Nam Viet Vo, PhD (pictured right), McGowan affiliated faculty, is part of the founding leadership team of the new Orland Bethel Family Musculoskeletal Research Center (BMRC).
Nam Viet Vo, PhD (pictured right), McGowan affiliated faculty, is part of the founding leadership team of the new Orland Bethel Family Musculoskeletal Research Center (BMRC).
Dr. Vo is tenured Professor of Orthopaedic Surgery and Deputy Vice Chair of Research in the Department of Orthopaedic Surgery at the University of Pittsburgh and holds joint appointments in the Department of Pathology, Clinical and Translational Science Institute, and University of Pittsburgh/UPMC Aging Institute.
The new Bethel Family Musculoskeletal Research Center is the result of a $25 million gift from the Orland Bethel Family Foundation and a matching $25 million co-investment from the University of Pittsburgh School of Medicine.
 Orland Bethel (pictured left), 87, is the founder of Hillandale Farms, one of the U.S.’s largest egg suppliers. Mr. Bethel suffered from chronic, debilitating spinal pain, and after consulting numerous surgeons, ultimately sought help from Joon Y. Lee, MD, a specialist in spinal surgery and the new founding directing of the BMRC. Mr. Bethel’s surgery and treatment were successful, resulting in greater mobility and function and well as significantly reduced pain.
Orland Bethel (pictured left), 87, is the founder of Hillandale Farms, one of the U.S.’s largest egg suppliers. Mr. Bethel suffered from chronic, debilitating spinal pain, and after consulting numerous surgeons, ultimately sought help from Joon Y. Lee, MD, a specialist in spinal surgery and the new founding directing of the BMRC. Mr. Bethel’s surgery and treatment were successful, resulting in greater mobility and function and well as significantly reduced pain.
Mr. Bethel told the Pittsburgh Post-Gazette that now, nearly a decade after his surgery, he has “no problems” with his back and has even returned to playing golf.
“I know how the University of Pittsburgh and UPMC changed my life and can only imagine how the lives of others — worse off than I was — will be improved thanks to the ongoing research at this new center,” said Mr. Bethel. “My family and I are pleased to offer our support to the spectacular work of Dr. Joon Lee and the other surgeons, physicians, and researchers within the department […] through the creation of the Orland Bethel Family Musculoskeletal Research Center.”
The center is anticipated to open in 2024 and will host a variety of initiatives, including the BMRC Core Laboratories, Bethel Research Fellow, and an annual conference. Some of the goals of the BMRC are to elevate orthopaedic work and innovate applicable treatments, including understanding molecular, genetic and biological mechanisms in spine-related diseases; understanding the influence of gender and age on disease and recovery from musculoskeletal disorders; developing therapies for osteoarthritis; and applying machine learning algorithms for orthopaedic issues.
The McGowan Institute, alongside the Department of Orthopaedic Surgery and Department of Physical Medicine and Rehabilitation in the School of Medicine; the School of Health and Rehabilitation Sciences; the Department of Bioengineering in the Swanson School of Engineering; and the Pittsburgh Center for Pain Research will work together as collaborators in the new BMRC.
“This generous gift is nothing short of transformative. This investment will have both immediate and generational impact. With the help of Mr. Bethel and his family, we can work to immediately change how we approach the big questions, improve cross campus collaboration and allow creativity to solve musculoskeletal problems ‘outside-the-box,’” Dr. Lee said. “The influence of this gift will be felt across the entire system — from young medical school students, through accomplished researchers, on to improvements in patient care.”
Visit the BMRC here.
Dr. Roy Receives R01 Funding to Study Breast Cancer Cells
 Partha Roy, PhD (pictured), McGowan affiliated faculty, has been awarded an R01 by the National Cancer Institute.
Partha Roy, PhD (pictured), McGowan affiliated faculty, has been awarded an R01 by the National Cancer Institute.
Dr. Roy’s project, titled “Transcriptional Regulation of Dormancy and Emergence in Breast Cancer,” began in September and seeks to address the relationship between metastatic outgrowth of extravasated cancer calls in breast cancer patients and patient survival rates.
Breast cancer is the second leading cause of cancer death in women in the U.S. and is largely caused by the metastatic growth of disseminated cells.
While the dissemination of cancer cells occurs during the early stages of tumor growth, the metastatic outgrowth of extravasated cancer cells can take years, primarily because of the dormant behavior of cancer cells.
Dr. Roy’s study hypothesizes that the survival of breast cancer patients should be improved if the dormancy of the cells if prolonged or if the metastatic growth is slowed. His project further hypothesizes that both of these actions are related to the Myocardin-related transcription factor (MRTF). The study seeks to determine the role of MRTF/SRF (serum-response factor) in the regulation of dormancy or growth in breast cancer cells as well as to test whether MRTF-SRF promotes the growth of breast cancer cells.
Upon completion of the project, Dr. Roy and his team hope to identify gene regulation programs and other treatments to slow the growth of breast cancer and increase patient survival rates.
Dr. Lord Awarded $1.3 Million to Study Cell Signaling
 Nathan Lord, PhD (pictured), McGowan affiliated faculty, is the recipient of new funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Nathan Lord, PhD (pictured), McGowan affiliated faculty, is the recipient of new funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
The Lord Lab studies the ways in which developing embryos encode instructions for, or signal to, their cells, frequently overcoming genetic mutations and unexpected environmental perturbations. Through these studies, the lab aims to reveal the pathways and principles underlying healthy, robust development.
Dr. Lord’s project, titled “Decoding the Spatial Grammar of Developmental Signaling,” received more than $1.3 million from the NIH to find new methods to create and test developmental signaling patterns and to devise modeling frameworks that can predict developmental outcomes in various patterns of signaling.
Dr. Lord’s strategy involves a new patterning platform that will enable researchers to create signaling patterns in over 5 million cells in a single experiment. Ultimately, this will help to guide the design of error-correcting, synthetic developmental systems and tissues in vitro.
According to Nature, such tissues in vitro can ultimately be used for “fundamental mechanistic studies on development, regeneration and repair in human tissues, and can also be used in diagnostics, disease modelling, drug discovery, and personalized medicine.”
Investing in the Future of Engineering: McGowan Faculty-Led Student Programs Receive an Additional $3.28 Million in NIH Funding
 The Swanson School of Engineering at the University of Pittsburgh has announced the Biomechanics in Regenerative Medicine (BiRM) and the Cardiovascular Bioengineering Training Program (CBTP) both received National Institutes of Health (NIH) T32 grant renewals for an additional five years.
The Swanson School of Engineering at the University of Pittsburgh has announced the Biomechanics in Regenerative Medicine (BiRM) and the Cardiovascular Bioengineering Training Program (CBTP) both received National Institutes of Health (NIH) T32 grant renewals for an additional five years.
Both programs prepare predoctoral students for careers in health-related research.
BiRM is led by David Vorp, PhD (pictured right), McGowan affiliated faculty and Senior Associate Dean for Research at Swanson. The program is in partnership with Carnegie Mellon University and integrates multiscale biomechanics such as theory, modeling, experimental method design, robotics, AI, and advanced manufacturing with regenerative medicine.
Trainees of BiRM seek to address pressing healthcare needs by translating scientific discoveries into effective therapies. Predoctoral trainees are closely mentored via an interdisciplinary training program that includes classes and research supported by faculty in departments ranging from Bioengineering to Obstetrics, Oral Biology, Physical Medicine & Rehabilitation, and more.
The five-year renewal from the NIH grants an additional $1.1 million to BiRM, which has received more than $5.2 million in funding from the NIH since its inception in 2005. The funding will go towards tuition, stipends, and benefits for four students each year.
 Like BiRM, CBTP also received renewed funding. CBTP is led by Sanjeev Shroff, PhD (pictured left), McGowan core faculty and Interim Dean at Swanson. CBTP provides trainees with core and elective courses, clinical internship and rotation, research activities, and training opportunities in order to prepare students for productive careers in cardiovascular engineering.
Like BiRM, CBTP also received renewed funding. CBTP is led by Sanjeev Shroff, PhD (pictured left), McGowan core faculty and Interim Dean at Swanson. CBTP provides trainees with core and elective courses, clinical internship and rotation, research activities, and training opportunities in order to prepare students for productive careers in cardiovascular engineering.
“Students are exposed first-hand to real-world clinical problems requiring bioengineering input for their solution,” notes Dr. Shroff. “The program is designed to provide students with both breadth and depth in engineering and biological sciences and includes a formal exposure to biostatistics, bioethics, and professional and career development issues. Upon completion, students are well-versed in both basic and clinical aspects of cardiovascular engineering and are well prepared for rewarding careers in a growing field.”
CBTP’s five-year renewal from the NIH is $2.17 million. The program has received more than $7.54 million in NIH funding since 2005.
“NIH-funded training grants are prestigious and highly competitive,” Dr. Vorp states. “Having […] bioengineering-associated training grants here at Pitt is a testament to the quality of our mentors and students and the research they conduct.”
Mentors from the McGowan Institute include
Stephen Badylak, MD, PhD, DVM
Harvey Borovetz, PhD
Lance Davidson, PhD
William Federspiel, PhD
Kang Kim, PhD
Steven Little, PhD
John Pacella, MD, MS
Michael Pinsky, MD
Anne Robertson, PhD
Partha Roy, PhD
Guy Salama, PhD
Richard Schaub, PhD
Sruti Shiva, PhD
Jonathan Vande Geest, PhD
Florideliza Villanueva, MD
James H-C. Wang, PhD
Stephen Winowich
Dr. Almarza Awarded R01 Funding for TMD Research
 Alejandro Jose Almarza, PhD (pictured), McGowan affiliated faculty and Associate Professor in Oral Biology in the School of Dental Medicine at the University of Pittsburgh, has received R01 funding from the National Institute of Dental and Craniofacial Research. The project began in September 2023 and has been funded until August 2024.
Alejandro Jose Almarza, PhD (pictured), McGowan affiliated faculty and Associate Professor in Oral Biology in the School of Dental Medicine at the University of Pittsburgh, has received R01 funding from the National Institute of Dental and Craniofacial Research. The project began in September 2023 and has been funded until August 2024.
Dr. Almarza’s project, “Collaborative for Research to Advance TMD Evidence (CREATE),” proposes to bridge the gap in the understanding of the relationship between temporomandibular disorder (TMD) pain and underlying mechanisms.
A recent study found that approximately 11.5 million adults in the U.S experience pain in or near their temporomandibular joint. While in some instances injury can lead to TMDs, the exact cause is still not clear.
Dr. Almarza’s project seeks to address TMD prevention, the development of effective and personalized TMD therapies, and expanding the current work force of those researching and managing TMD pain by taking a bidirectional translational approach.
The project will include the TMD Collaborative for Improving Patient-Centered Translational Research (TMD Impact). The Collaborative’s goal is to establish a national, interdisciplinary trans-NIH patient-centered research consortium to advance the basic and clinical research, training, and translation to evidence-based treatment.
Dr. Almarza’s study has five key components, which also correspond to the goals of the Collaborative: Clinical Phenotyping, Preclinical Research, Data and Biospecimen Management, Education, and a Stakeholder and Engagement Group.
Weaving a Biocarpet: Dr. Vande Geest Receives NIH Funding to Develop Durable Solution to Peripheral Arterial Disease
 Jonathan P. Vande Geest, PhD (pictured), McGowan affiliated faculty, has received a developmental grant from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI).
Jonathan P. Vande Geest, PhD (pictured), McGowan affiliated faculty, has received a developmental grant from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI).
The project, titled “Biocarpet: The Next Generation Endovascular Device for Peripheral Arterial Disease,” began in September 2023 and has received nearly $400,000 in funding.
Dr. Vande Geest’s project seeks to address peripheral arterial disease (PAD), a condition in which blood flow to the arms and legs is reduced due to narrowed or blocked arteries. Nearly 10 million people age 40 and older in the U.S. suffer from PAD.
Age, smoking, diabetes, ethnicity, lifestyle habits, and other medical conditions like obesity and high blood pressure can increase the risk for developing PAD. Untreated PAD can limit mobility and lead to heart attack, stroke, and limb amputation. Current treatments for severe cases of PAD include balloon angioplasty, stent placement, and vascular bypass.
In the abstract for his project, Dr. Vande Geest argues that many of the surgical treatments for PAD prove problematic over time. For instance, balloon angioplasty can lead to restenosis and device failure; stents can fracture due to complications present in advanced PAD; and surgical bypass has a two-year primary patency rate of 67%.
Dr. Vande Geest and his team have developed what they’ve termed the “Biocarpet” in response to the need for more durable solutions to PAD. The Biocarpet is “a fully biodegradable electrospun sheet that takes the shape of the patient’s vascular anatomy following deployment.”
With funding from the NIH, Dr. Vande Geest’s goal is to finalize the design and prototype of the Biocarpet and make progress toward clinical translation.
Donor-Derived Infusion Demonstrates Potential for Transplantation without Long-Term Immunosuppression: Dr. Humar Publishes Phase 1 Trial Results
 Over the past 70 years in the U.S., immunosuppression regimens used in organ transplants have evolved significantly. However, long-term immunosuppressant use can cause organ transplant recipients to experience side effects such as cancer, diabetes, kidney failure, and greater susceptibility to viral, bacterial, and fungal infections.
Over the past 70 years in the U.S., immunosuppression regimens used in organ transplants have evolved significantly. However, long-term immunosuppressant use can cause organ transplant recipients to experience side effects such as cancer, diabetes, kidney failure, and greater susceptibility to viral, bacterial, and fungal infections.
Abhinav Humar, MD (pictured), McGowan affiliated faculty and Clinical Director of the Thomas E. Starzl Transplantation Institute recently published early-stage clinical trial results indicating the potential for transplantation without the need for long-term immunosuppression.
In a Phase 1 trial, Dr. Humar and a team of researchers enrolled 15 patients who were scheduled to receive a living donor transplant. These patients received an immune cell infusion from their donor one week prior to undergoing transplant surgery.
Researchers then compared the patients who received the immune cell infusion to 40 living donor transplant patients who did not receive the infusion. All patients were given immunosuppressant drugs post-surgery.
One year after surgery, researchers found that patients who received the infusion had fewer immune cells that had the potential to react negatively to their transplanted organ.
Senior author and Distinguished Professor of Surgery and Immunology at the University of Pittsburgh, Angus W. Thomson, PhD, DSc, stated that the donor-derived infusion was “preemptively conditioning the prospective [liver] recipient to see donor cells as safe.”
“These trial results are very encouraging,” continued Dr. Thomson. “Right now, we’re seeing preliminary evidence that this intervention is modifying the recipient’s immune response in such a way that we may be able to safely reduce — or even withdraw — immunosuppression. It would be a significant service to the transplantation community if patients are no longer dependent indefinitely on immunosuppressants.”
The study, titled “Donor-derived regulatory dendritic cell infusion modulates effector CD8+ T cell and NK cell responses after liver transplantation,” is published in Science Translational Medicine and can be read here in its entirety.
Prompting Liver Regeneration in End-Stage Liver Disease: Dr. Monga Discusses New Medication
 Satdarshan (Paul) Singh Monga, MD, Director of McGowan’s Cellular Approaches to Tissue Engineering and Regeneration (CATER) Training Program and Founding Director of the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded Pittsburgh Liver Research Center (PLRC), discusses a new medication to help liver regeneration in The Conversation.
Satdarshan (Paul) Singh Monga, MD, Director of McGowan’s Cellular Approaches to Tissue Engineering and Regeneration (CATER) Training Program and Founding Director of the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded Pittsburgh Liver Research Center (PLRC), discusses a new medication to help liver regeneration in The Conversation.
While the liver has the ability to regenerate, damage from alcohol abuse, obesity, and medications can cause the liver to fail. Dr. Monga notes, “Because very few effective treatments are available for illnesses like nonalcoholic fatty liver disease and alcoholic liver disease, many patients worsen and end up needing a liver transplant.”
In 2022, more than 9,528 liver transplant surgeries were performed in the U.S.; however, as of October 2023, there are 10,050 people currently on the liver transplant waitlist. Patients can wait anywhere from 30 days to five years to receive a liver transplant.
Reenabling the liver’s ability to regenerate has the potential to alleviate the need for transplantation.
Dr. Monga and his team found “that activating a particular protein with a new medication can help accelerate regeneration and repair after severe liver injury or partial surgical removal in mice.”
“One way to address liver transplantation shortages is to improve treatments for liver diseases,” continued Dr. Monga. “My team and I believe that improving the liver’s ability to repair itself could help circumvent the need for transplantation. Further study of drugs that promote liver regeneration may help curb the burden of liver disease worldwide.”
Dr. Zervantonakis Awarded Funding for Research on Cell Migration and Extracellular Matrix Remodeling
 Ioannis Zervantonakis, PhD (pictured), McGowan affiliated faculty, has been awarded funding from the National Institute of General Medical Sciences for a study titled, “Macrophage-Fibroblast Communication in Cell Migration and Extracellular Matrix Remodeling.”
Ioannis Zervantonakis, PhD (pictured), McGowan affiliated faculty, has been awarded funding from the National Institute of General Medical Sciences for a study titled, “Macrophage-Fibroblast Communication in Cell Migration and Extracellular Matrix Remodeling.”
According to the project abstract, “a critical knowledge gap exists in understanding the fundamental mechanisms that control intercellular signaling in complex microenvironments.” Dr. Zervantonakis and his team of researchers will address the knowledge gap by first “integrat[ing] intracellular signaling biosensors with a novel microfluidic technology to control paracrine factors and cell-generated forces precisely.” The results of these studies will enable researchers to determine the underlying principles of cell migration. Researchers will then “engineer multi-layer microfluidic devices with integrated imaging-based multiplexed analysis of fibroblast activation and measurement of mechanical forces.”
The team’s integrative approach in studying cell migration and extracellular matrix remodeling (ECM) will utilize bioengineering tools, intracellular signaling reporters, and computational modeling. Ultimately, cell-cell communication “plays a critical role in organ development and healing responses following injury and disease progression.”
AWARDS AND RECOGNITION
Dr. Cooper Receives Dual Accolades: The National Medal of Technology and Innovation from President Biden and Induction into the National Inventors Hall of Fame
 Last month, Rory Cooper, PhD (pictured left), featured in a film. Over the summer, he received the Distinguished Eagle Scout Award, a prestigious award also granted to Neil Armstrong and Steven Spielberg. Now, he has been inducted into the National Inventors Hall of Fame, and President Biden has presented him with the National Medal of Technology and Innovation.
Last month, Rory Cooper, PhD (pictured left), featured in a film. Over the summer, he received the Distinguished Eagle Scout Award, a prestigious award also granted to Neil Armstrong and Steven Spielberg. Now, he has been inducted into the National Inventors Hall of Fame, and President Biden has presented him with the National Medal of Technology and Innovation.
The National Medal of Technology and Innovation is the U.S.’s highest award for technological achievement. Previous laureates include Steve Jobs, Steve Wozniak, and the eBay corporation. The National Inventors Hall of Fame, since its inception 50 years ago, honors “the greatest inventors who have built the world around us, inspire the innovators of tomorrow, and challenge today’s creative thinkers to design the future.”
Dr. Cooper, a biomedical engineer, has dedicated his career to developing technologies that have not only improved manual and electric wheelchairs but have “advanced the health, mobility, and social inclusion of people with disabilities and older adults.”
His inventions include a manual wheelchair that features a larger outer surface area that more comfortably enables users to push with their palms than conventional wheelchair handrims. Users ultimately experience less hand and wrist pain and fewer wrist and shoulder injuries. Another of Dr. Cooper’s major innovations is a digital joystick for electric wheelchairs. It grants users greater mobility via pressure sensing that can be tailored to individual hand and arm function. The joystick’s software can also compensate for hand tremors that might otherwise lead to crashes or injury.
Dr. Cooper, McGowan affiliated faculty, holds more than 20 U.S. patents and is Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories (HERL), the University of Pittsburgh’s first Assistant Vice Chancellor for Research for STEM-health Sciences Collaborations, a Distinguished Professor, a U.S. Army Veteran, and a Paralympic bronze medalist.
“In thinking about Rory in all of his accomplishments, you’d be hard-pressed to find a more effective advocate for people with disabilities,” said McGowan affiliated faculty and University of Pittsburgh’s School of Health and Rehabilitation Sciences Dean Anthony Delitto. “Nothing speaks more loudly about the effectiveness of his advocacy than the inventions and patents that are celebrated with this award, all of which are designed to reduce or eliminate barriers for people with disabilities.”
Dr. Cooper received the National Medal of Technology and Innovation at the White House on October 24 and was inducted into the National Inventors Hall of Fame in Washington, D.C., on October 26.
Watch the National Medal of Technology and Innovation ceremony on C-Span here.
Read Dr. Cooper’s bio on the National Inventors Hall of Fame website here.
Image credit: Ryan K. Morris and the National Science and Technology Medals Foundation
Jamelle Price Selected to Participate in Appalachian Leadership Program
 Mr. Jamelle Price, External Relations Manager at Pitt McGowan, has been selected to participate in the 2023-2024 class of the Appalachian Leadership Institute.
Mr. Jamelle Price, External Relations Manager at Pitt McGowan, has been selected to participate in the 2023-2024 class of the Appalachian Leadership Institute.
The Appalachian Leadership Institute is a leadership training program developed in partnership with the University of Tennessee, Knoxville; The Howard H. Baker Center for Public Policy; Collective Impact; and Tuskegee University. It is part of the Appalachian Regional Commission (ARC), whose mission is to strengthen economic growth and build community in 423 counties across the Appalachian Region.
Mr. Price is one of 40 fellows selected to be in the program from 13 states across the region.
“The Appalachian Leadership Institute supports leaders already doing amazing work across Eastern Kentucky and the entire Appalachian region,” said ARC 2023 States’ co-chair, Kentucky Governor Andy Beshear. “This program capitalizes on the incredibly talented people who are already invested in these special communities. When we invest in our people, we’re building a brighter future for generations to come.”
Program fellows will participate in a nine-month curriculum, which includes six multi-day seminars across the Appalachian Region. The curriculum will focus, in part, on designing economic development project proposals, integrating community assets into long-term economic development strategies, and preparing competitive applications for public grant opportunities.
“I am very excited to be joining the Appalachian Leadership Institute’s Class of 2023-2024,” said Mr. Price. “This is a tremendous opportunity to build my leadership capacity for the Greater Pittsburgh Region in economic development.”
Mr. Price will be working alongside professionals in communication, tourism, healthcare, education, civil service, and more.
“The McGowan Institute is extremely excited to have Jamelle accepted as a 2023-2024 Leadership Institute Fellow, stated Patrick Cantini, Strategy and Business Development Officer at McGowan. “ We firmly stand behind Jamelle in supporting his participation in this prestigious program and look forward to him strengthening the Institute’s connection to other leaders and organizations in the Appalachia region.”
PUBLICATION OF THE MONTH
Author: Lillian M Tran, Camila Macedo, Alan F Zahorchak, Xinyan Gu, Beth Elinoff, Aatur D Singhi, Brian Isett, Adriana Zeevi, Megan Sykes, Kevin Breen, Avantika Srivastava, Erin M Ables, Douglas Landsittel, Mindi A Styn, Abhinav Humar, Fadi G Lakkis, Diana M Metes, Angus W Thomson
Title: Donor-derived regulatory dendritic cell infusion modulates effector CD8+ T cell and NK cell responses after liver transplantation
Summary: Immune cell–based therapies are promising strategies to facilitate immunosuppression withdrawal after organ transplantation. Regulatory dendritic cells (DCreg) are innate immune cells that down-regulate alloimmune responses in preclinical models. Here, we performed clinical monitoring and comprehensive assessment of peripheral and allograft tissue immune cell populations in DCreg-infused live-donor liver transplant (LDLT) recipients up to 12 months (M) after transplant. Thirteen patients were given a single infusion of donor-derived DCreg 1 week before transplant (STUDY) and were compared with 40 propensity-matched standard-of-care (SOC) patients. Donor-derived DCreg infusion was well tolerated in all STUDY patients. There were no differences in postoperative complications or biopsy-confirmed acute rejection compared with SOC patients up to 12M. DCreg administration was associated with lower frequencies of effector T-bet+Eomes+CD8+ T cells and CD16bright natural killer (NK) cells and an increase in putative tolerogenic CD141+CD163+ DCs compared with SOC at 12M. Antidonor proliferative capacity of interferon-γ+ (IFN-γ+) CD4+ and CD8+ T cells was lower compared with antithird party responses in STUDY participants, but not in SOC patients, at 12M. In addition, lower circulating concentrations of interleukin-12p40 (IL-12p40), IFN-γ, and CXCL10 were detected in STUDY participants compared with SOC patients at 12M. Analysis of 12M allograft biopsies revealed lower frequencies of graft-infiltrating CD8+ T cells, as well as attenuation of cytolytic TH1 effector genes and pathways among intragraft CD8+ T cells and NK cells, in DCreg-infused patients. These reductions may be conducive to reduced dependence on immunosuppressive drug therapy or immunosuppression withdrawal.
Source: Science Translational Medicine. 11 Oct 2023. Vol 15, Issue 717
GRANT OF THE MONTH
PI: Partha Roy
Title: Transcriptional Regulation of Dormancy and Emergence in Breast Cancer
Description: Breast cancer ranks second among cancer deaths in women in the United States. Breast cancer-related mortality primarily results from metastatic growth of disseminated cells. While dissemination of cancer cells occurs early during tumor progression, metastatic outgrowth of extravasated cancer cells often takes many years due to the dormant behavior of cancer cells. In principle, the survival of breast cancer patients should be improved if metastatic burden is reduced by either prolonging the dormancy of disseminated tumor cells or slowing down progressive metastatic growth. Our overarching goal is to identify tumor cell-intrinsic transcriptional programs that are critical for dormancy-to-emergence transition and progressive growth of established metastases in breast cancer. In the proposed study, we hypothesize that dormancy-to- emergence switch and progressive metastatic growth of breast cancer cells require the action of Myocardin- related transcription factor (MRTF), a transcriptional coactivator of serum-response factor (SRF). To test this hypothesis, we propose two specific aims. In Aim 1, we will conduct genetic and pharmacological proof-of- concept studies to establish the role of MRTF/SRF transcriptional axis in regulation of dormancy and metastatic growth of breast cancer cells. Aim 2 will test whether MRTF/SRF activity promotes metastatic growth of breast cancer cells through both tumor-intrinsic and –extrinsic mechanisms. The proposed studies will employ a highly comprehensive experimental strategy integrating inducible functional disruption and pharmacological intervention strategies, intravital imaging of tumor cell activity at metastatic sites, organotypic models of lung and liver tumor microenvironment to study tumor dormancy, mouse model studies, use of patient-derived organoids and clinical samples of BC, and multiplexed quantitative IHC. A successful completion of these studies will establish MRTF as a novel regulator of dormancy-emergence behavior of breast cancer cells with mechanistic details, and provide proof-of-concepts for pharmacological intervention of MRTF as a novel strategy to induce dormancy and curb metastatic growth in breast cancer.
Source: National Cancer Institute
Term: September 1, 2023 – August 31, 2028
Amount: $401,019
