August 2022 | VOL. 21, NO. 8| www.McGowan.pitt.edu
Addressing Chronic Rejection in Heart Transplants

McGowan Institute for Regenerative Medicine affiliated faculty member Hēth Turnquist, PhD (pictured), Associate Professor in the University of Pittsburgh School of Medicine, Department of Surgery, T.E. Starzl Transplantation Institute, with a secondary appointment in the Department of Immunology, is the principal investigator of the newly funded National Institutes of Health/National Heart, Lung, and Blood Institute 5-year grant entitled, “Immunoregulatory Mechanisms of IL-33 in Heart Transplantation.”
The project will address chronic allograft vasculopathy and fibrosis, also called chronic rejection. This is an immunosuppressant-resistant disease that develops in almost half of heart transplants after ten years and remains a leading cause of recipient death one-year post-transplant. The blockage of graft blood vessels and progressive graft scarring causing chronic rejection may reflect failed tissue repair by the recipient’s immune system. These studies will pair precise transgenic mice transplant models with cutting-edge technologies to provide previously unattainable insights into how innate and adaptive alloresponses may dysregulate repair processes to cause chronic heart transplant rejection.
The project abstract reads:
Early graft injury is the consequence of unavoidable transplant-associated events, such as donor brain death, ischemia/reperfusion injury, and surgical trauma. Recipient responses to graft alloantigens will also produce graft damage throughout the life of the graft. These injuries and stresses cause the release of self-molecules containing damage-associated molecular patterns (DAMPs). It is well appreciated that DAMPs support pro-inflammatory responses when they are sensed by cells of the innate immune system, particularly monocytes and macrophages.
Preclinical rodent heart transplant studies reveal that these released DAMPs initiate and propagate alloresponses and targeting inflammatory DAMPs, or the signaling cascades they activate, reduce alloimmunity and improve outcomes after transplantation. Yet, our recent research has provided compelling evidence that these graft injuries also release reparative DAMPs (rDAMPs), such as Interleukin-33 (IL-33), which limits local inflammation and initiates immune-mediated tissue repair after heart transplant.
By assessing pediatric heart transplant recipient samples and using a preclinical mouse heart transplant model, we identified that IL-33 is upregulated in graft stromal cells and limits the development of allograft vasculopathy and graft fibrosis that later culminates in chronic rejection (CR). Our published and preliminary data suggest that IL-33 mediates this protection by targeting infiltrating monocytes and macrophages, as well as regulatory T cells (Tregs), to limit the generation of pro-inflammatory macrophages and then coordinate a Treg and reparative macrophage-mediated response to injury. While these data are encouraging, our studies also suggest that the processes that initiate repair of early tissue damage may become dysregulated over the graft’s life.
In preliminary data, we show that vessel-associated Tregs become pathologic when their sustained secretion of repair factors in response to IL-33 promotes the proliferation of local fibroblasts contributing to CR. In addition to having their functions programmed by local rDAMPs, graft infiltrating recipient monocytes recognize allogenic molecules causing them to mature into pro-inflammatory myeloid cells that stimulate T cell proliferation and IFNγ production in the graft. We have established that monocytes and macrophages recognize and develop allospecific cytotoxicity and memory to MHCI antigens via paired immunoglobulin-like receptors-A.
These data lead us to HYPOTHESIZE that rDAMP signals initiating the repair of tissue damage early after heart transplantation become dysregulated around the vasculature due to a sustained inflammatory response by alloreactive immune cells. We will test this hypothesis in two aims: In AIM 1, we will define how rDAMP and immune cell interactions evolve in heart transplant microenvironments during CR development. In AIM 2, we will establish if innate alloimmunity prevents effective injury resolution and repair after heart transplantation.
Congratulations, Dr. Turnquist!
RESOURCES AT THE MCGOWAN INSTITUTE
September Histology Special – Safranin O Staining
Safranin O staining is used for the detection of cartilage, mucin, and mast cell granules on formalin-fixed, paraffin-embedded tissue sections or frozen sections. The cartilage and mucin will be stained orange to red, and the nuclei will be stained black. The background is stained bluish green.
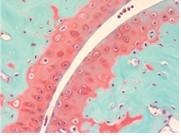
You’ll receive 30% off your Safranin O staining in September when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
UPCOMING EVENTS
Save the Date! 2023 McGowan Institute Scientific Retreat

The venue for the 2023 Retreat is the University Club, and the dates are March 6 & 7, 2023.
The program committee for the 2023 McGowan Institute Scientific Retreat, under the leadership of Bryan Brown, PhD, has begun to formulate the program and is requesting your suggestions for session topics.
The committee is also seeking to learn of possible interest by individuals in organizing a session. Please submit your program suggestions here.
9th Annual International Symposium on Regenerative Rehabilitation

The McGowan Institute for Regenerative Medicine invites you to join us for the 9th Annual International Symposium on Regenerative Rehabilitation, to be held October 27-29 in Austin, TX! Now in its 9th year, this symposium is the largest medical and scientific conference specific to Regenerative Rehabilitation in the world and brings together renowned experts in the fields of regenerative medicine and rehabilitation. This year’s featured speakers include, Theresa A. Jones, PhD (University of Texas at Austin), Karunesh Ganguly, MD, PhD (University of California, San Francisco), Conor Walsh, PhD (Harvard University), William R. Wagner, PhD (University of Pittsburgh), and José del R. Millán, PhD (University of Texas at Austin).
Join us in discovering integrated methods to enhance tissue healing and regeneration through mechanotransduction and applied biophysics, as well as how these approaches relate to the application of clinically available rehabilitation approaches. Cutting-Edge Research, Trainee Opportunities, Travel Awards, Diversity Grants, Networking and More!
View the agenda and register here.
SCIENTIFIC ADVANCES
Newly Issued Patents for McGowan Institute Affiliated Faculty Members

During the months of May-June-July 2022, the following patents were issued based on research work conducted by McGowan Institute for Regenerative Medicine affiliated faculty members. Their newly issued patents include:
U.S. Patent No. 11,376,349
Biodegradable Iron-Containing Compositions, Methods of Preparing and Applications Thereof
Prashant Kumta; Oleg Velikokhatnyi; Moni Datta; Sung Jae Chung; Da-Tren Chou; Dae Ho Hong; Partha Saha
U.S. Patent No. 11,384,400
Methods of Treating Cancers Containing Fusion Genes
Jianhua Luo; Zhangui Chen; Yanping Yu; George Michalopoulos; Joel Nelson
U.S. Patent No. 11,382,862
Gel for Treating Periocular and/or Orbital Pathologies and Conditions
Morgan Fedorchak; Jenny Yu; Michael Washington
U.S. Patent No. 11,389,566
Method and Composition for Treating Inflammatory Bowel Disease Without Colectomy
Timothy Keane Jr.; Stephen Badylak; Marc Ramer
U.S. Patent No. 11,389,569
Biodegradable, Porous, Thermally Responsive Injectable Hydrogel as Soft Tissue Defect Filler
William Wagner; Yang Zhu; Hongbin Jiang; Hideyoshi Sato; Stephen Badylak
U.S. Patent No. 11,395,853
Biomimetic Drug Delivery of an Immunomodulatory Agent for the Treatment of Ocular Conditions
Michelle Ratay; Andrew Glowacki; Morgan Fedorchak; Steven Little; Stephen Balmert
U.S. Patent No. 11,317,955
Magnesium Enhanced/Induced Bone Formation
Sayuri Smith; Andrew Brown; Amy Chaya; Charles Sfeir
U.S. Patent No. 11,318,103
Treating Soft Tissue Via Controlled Drug Release
Peter Alexander; Riccardo Gottardi; Patrick Bianconi; Steven Little
U.S. Patent No. D950,690
Toilet Seat Wrap
Rory Cooper; Rosemarie Cooper
U.S. Patent No. 11,339,362
Organ Chip to Model Mammalian Joint
Hang Lin; Peter Alexander; Riccardo Gottardi; Rocky Tuan
U.S. Patent No. 11,338,017
Small Peptide Compositions and Uses Thereof
Cecelia Yates-Binder; Jesse Jaynes
U.S. Patent No. 11,338,077
Adjustable Liposuction Cannula
Peter Rubin; Arielle Richter; Kacey Marra; Stephen Holland; Jessica Huynh; Mark Gartner
U.S. Patent No. 11,338,058
Injectable Peripheral Nerve Specific Hydrogel
Bryan Brown; Jonathan Cheetham
U.S. Patent No. 11,337,799
Stentless Biopolymer Heart Valve Replacement Capable of Living Tissue Regeneration
Yasumoto Matsumura; William Wagner; Vinay Badhwar; Antonio D’Amore
U.S. Patent No. 11,317,852
Medical Device for Diagnosing Pressure Ulcers
Sanna Gaspard; Mel Siegel; Todd M. Przybycien; James F. Antaki; David M. Brienza; Mark B. Friedman
U.S. Patent No. 11,367,189
Method for Object Detection Using Hierarchical Deep Learning
Daniel Clymer; Jonathan Cagan; Philip LeDuc
U.S. Patent No. 11,406,736
Vascular Extracellular Matrix Hydrogel
Stephen Badylak; George Fercana Jr.; Thomas Gleason; Julie Phillippi
Congratulations on these successful developments!
Illustration: U.S. Patent and Trademark Office logo.
Developing Next-Generation AAV Gene Therapy Vectors for Ocular Diseases
Avista Therapeutics, which recently launched as a spinout of leading health system University of Pittsburgh Medical Center (UPMC), aims to develop innovative gene therapies for rare ophthalmic conditions. The new company announced a partnership with Roche to develop novel AAV gene therapy vectors for the eyes.
The partnership aims to apply Avista’s single-cell adeno-associated virus (AAV) engineering (scAAVengr) platform technology to develop intravitreal AAV capsids matching a capsid profile defined by Roche. Under the terms of the partnership, Roche has the right to evaluate and license novel capsids from Avista, and will be responsible for conducting preclinical, clinical, and commercialization activities for gene therapy programs using these novel capsids, which will be distinct from Avista’s internal pipeline.
“We are excited to enter into this collaboration with Roche, a global leader in health care,” said Robert Lin, PhD, Chief Executive Officer of Avista Therapeutics. “This collaboration will complement our in-house pipeline and will accelerate the delivery of transformative therapies to patients.”
Avista will receive an upfront payment of $7.5 million and, if successful, is eligible to receive additional payments during the research phase of the partnership, as well as clinical and sales milestone payments and royalties for resulting products with a total potential deal value that may exceed $1 billion.
Built on the research of leading experts in viral vector development and clinical ophthalmology, Avista was founded by Leah Byrne, PhD, Assistant Professor of Ophthalmology at the University of Pittsburgh School of Medicine, and expert in AAV vector generation; José-Alain Sahel, MD, Chair of the Department of Ophthalmology at the University of Pittsburgh School of Medicine, who is known worldwide for his expertise in vision restoration techniques; and, Paul Sieving, MD, PhD, internationally recognized physician-scientist and former director of the National Eye Institute. Both Drs. Byrne and Sahel are affiliated faculty members of the McGowan Institute for Regenerative Medicine.
Avista’s computationally guided, in vivo scAAVengr platform leverages a high-throughput approach with built-in quantitative validation of novel cell-specific AAVs, enabling the rapid translation of transformative gene therapies to the clinic for diseases impacting people’s vision. The company will develop a proprietary pipeline built on a toolkit of AAV variants that can target gene delivery to individual retinal cell types.
“Traditional therapies for retinal dystrophies address only symptoms and complications, neglecting the underlying biology of the diseases, and while current vector technologies hold promise, they have been greatly limited in their ability to target key cell types across the retina,” added Dr. Lin, who is also a vice president at UPMC Enterprises, the innovation, venture capital, and commercialization arm of UPMC. “Avista was founded to solve this problem, and our innovative scAAVengr platform allows us to deliver gene therapy payloads through intravitreal injection to treat a full range of retinal diseases with reduced immunogenicity.”
Avista received $10 million in seed funding and years of foundational support from UPMC Enterprises, which invests in translational science with the potential to radically transform health care.
“UPMC is thrilled to support the launch of Avista, a team with unmatched expertise in AAV technology and clinical ophthalmology who are addressing a diverse group of blinding disorders that have a profound impact on patients’ quality of life,” said Jeanne Cunicelli, MBA, President of UPMC Enterprises. “We believe that Avista is uniquely positioned to address the high unmet medical need in inherited retinal diseases.”
Illustration: Avista Therapeutics.
New Study Highlights Differences in Neuropathic Pain Relief for Men and Women

Research at Duquesne University using a nanomedicine that targets neuropathic pain at its source found that while males and females develop pain-like behavior to the same degree, the nanomedicine provided less relief for females.
The study, published in Scientific Reports (Nature), tested male and female rats using a nonsteroidal anti-inflammatory drug (NSAID) nanoemulsion, which delivers a low-dose medication to the site of injury. It found that male rats experienced neuropathic pain relief for five days, while female rats experienced only one day of relief from the same dose.
The study marks the latest findings of the university’s Chronic Pain Research Consortium (CPRC), a multidisciplinary collaboration by Jelena Janjic, PhD (founder/co-director, pictured top), and John Pollock, PhD (co-director, pictured bottom). A key component of this study is the application of the first inflammatory pain nanomedicine created by Dr. Janjic, which directly targets the source of pain.
“One of the goals of this research is to eventually develop personalized treatments for pain,” said Dr. Janjic, associate pharmacy professor who co-authored the study. “Discovering more about the differences in pain response between males and females at the molecular level serves as a first step toward designing such treatments.”
One of the possible reasons for the differences in the drug’s effectiveness may be related to how immune systems function in slightly different ways in males and females, said Dr. Pollock, biological sciences professor.
“Every time we look carefully, we find there are subtle differences in the underlying physiology of females versus males,” said Dr. Pollock, who co-authored the study. “Normally, how the body responds to injury and pain progresses with time, hopefully shifting to an immune response that supports healing and tissue regeneration. What we see in these rats is that the processes have several sex-specific differences that need to be clarified so we can provide the best pain relief therapy for humans.”
The study focused on macrophages (also known as white blood cells), which contribute to neuroinflammatory pain by activating an enzyme known as cyclooxygenase-2 (COX-2). The Duquesne researchers suspected that honing-in on the differences in macrophage activity could provide insight about pain response between males and females.
“We saw that pain sensitivity was linked to the number of macrophages at the site of injury,” said Brooke Deal, Duquesne graduate student researcher and co-author of the study. “The less macrophages at the injury site, the more pain relief.”
The findings also demonstrate that COX-2 activation may have different levels of importance in the neuroinflammatory response depending on sex; this could mean that there are other immunological systems at work, Ms. Deal added.
The use of nanomedicines to treat pain is a future game-changer, Dr. Janjic said, noting that the nanoemulsion both treats pain and allows researchers to track the location of the immune cells in vivo.
“Imaging with nanomedicines can provide us with key information for the future,” she said. “We can learn which medicine works better not only for men and women, but individual patients, as their bodies undergo biological changes during the course of their lives.”
Model (Virus) Behavior

Avian influenza viruses, or “bird flu,” normally infects, well, birds. But when the virus does infect humans, it can cause more severe illness than other similar influenza viruses.
Research led by the University of Pittsburgh’s Jason Shoemaker, PhD (pictured), uses computational modeling to try to understand the body’s immune response to avian flu. His latest work, published in the journal Viruses, finds that the levels of interferon may be responsible for its more severe presentation—and may also be the key to treating it.
“It’s hard to see what’s happening in the human body when it’s infected with a virus, but our computational modeling can help us understand the immune system’s reaction, and where we might be able to help it do a better job,” said Dr. Shoemaker, who is an assistant professor of chemical and petroleum engineering at the Swanson School of Engineering and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “We need more modeling to really understand what happens to high-risk individuals when they’re infected to make them high-risk. Then we can figure out how to better treat them.”
Dr. Shoemaker and his coauthors Emily Ackerman and Jordan Weaver, graduate students in Shoemaker’s ImmunoSystems Lab, used data from mice infected with either H5N1, the high-pathogenic (or disease-causing) avian flu, or H1N1, the low-pathogenic swine flu. They then used an engineering-based approach to model and predict virus replication and key immune responses based on the mice’s infections, including the levels of interferon and immune cell activity.
By exploring the different biological responses, the researchers were able to determine that the production rate of interferon drives the strain-specific immune responses observed in the mice. In other words, the high viral load and the resulting interferon production by cells in the lungs after H5N1 infection seems to be the main reason for differing infection outcomes.
“This modeling provides more evidence for the theory that interferon is induced earlier and more severely by the high-pathogenic H5N1 strain than by other influenza viruses,” said Dr. Shoemaker. “Interferon then appears to be a main factor in determining how severe the infection will be and explains the distinctive immune response we see in H5N1 infections.”
Though the recent paper did not specifically look at SARS-CoV-2, the virus that causes COVID-19, the findings could still give researchers a path to developing better treatments. The group’s modeling work has revealed other factors that may be at play, as well. For example, the lab is also using agent-based modeling to understand why women often experience a more severe immune response to the flu.
Company Launches with $55 Million Seed to Reprogram Biology by Writing and Delivering Big DNA

McGowan Institute for Regenerative Medicine affiliated faculty member Joseph Glorioso III, PhD (pictured), is a Professor in the Department of Microbiology and Molecular Genetics at the University of Pittsburgh School of Medicine and holds a secondary appointment in the Department of Human Genetics, University of Pittsburgh Graduate School of Public Health. He is also a co-founder of the genome writing company, Replay, where he is the inventor of the company’s synHSV™ technology and is the Senior Advisor for Gene Therapy Programs.
Replay aims to define the future of genomic medicine through reprogramming biology by writing and delivering big DNA. Replay has assembled a toolkit of disruptive platform technologies – including a high payload capacity HSV platform, a hypoimmunogenic platform, and a genome writing platform – to address the scientific challenges currently limiting clinical progress and preventing genomic medicine from realizing its full potential. The company’s hub-and-spoke business model separates technology development within Replay from therapeutic development in product companies, which leverage the technology platforms. For example, Replay’s synHSV™ technology, a high payload capacity HSV vector capable of delivering up to 30 times the payload of AAV, is utilized by Replay’s four gene therapy product companies, bringing big DNA treatments to diseases affecting the skin, eye, brain, and muscle. The company has, additionally, established an enzyme writing product company engaging its evolutionary inference machine learning and genome writing technology to optimize functionality. Replay is led by a world-class team of academics, entrepreneurs, and industry experts.
Read more…
Building a Better Blood Bank—in Kenya

Trauma surgeons and obstetricians in the United States count on a blood banking system that works—the right amount and type, adequately screened for disease, and delivered where it’s needed, when it’s needed. Experience a hemorrhage during childbirth or a car crash and your doctor can transfuse what you need to survive the crisis.
“As a trauma surgeon, I need blood right now and blood appears,” says McGowan Institute for Regenerative Medicine affiliated faculty member Juan Carlos Puyana, MD (pictured), a Pitt professor of surgery, critical care medicine, and clinical translational science and director of Pitt’s Global Health-Surgery program. “Sadly, the only time we may run out of blood is when caring for victims of a mass shooting event.”
For patients and doctors in the low- and lower-middle income countries of sub-Saharan Africa, blood shortages are a fact of life—and death. In Kenya, the National Blood Transfusion Service estimates need at some 500,000 units annually. Yet blood drives yield less than a third of that target, leading to many preventable deaths each year from postpartum hemorrhage, injury, and other forms of acute hemorrhage, as well as from malarial anemia and sickle cell disease.
With funding from the National Heart Lung and Blood Institute’s (NHLBI) BLOODSAFE program, a team of Pitt engineers and doctors has spent the last two years helping their collaborators across Kenya lay the groundwork for better blood banking systems.
Bopaya Bidanda, PhD, the Ernest Roth Professor of Industrial Engineering in the Swanson School, leads the engineering team. “Bopaya’s expertise on process, supply chain, figuring out how to deal with bottlenecks—from vaccine delivery systems to airports, even Nordstrom’s inventory supply—made him an ideal partner,” says Dr. Puyana. “Whether blood or shoes, he would figure out how to make the system better.”
Dr. Bidanda has a long history of applying his expertise to the thorny problems of society, including an ambitious project currently underway in rural Tuver, India, to combine green job development and fossil-free, off-grid power to help a village become self-sustaining and access telemedicine to improve their health. “We picked a village as far away as you can get,” says Dr. Bidanda. “It’s a five-hour drive from the nearest airport.”
In 2017, Pitt Chancellor Pat Gallagher invited Dr. Bidanda, who serves on the advisory board of the College of Engineering Universidad de los Andes in Bogota, to travel with a university delegation to explore partnership opportunities in Colombia.
For two years, Drs. Puyana and Bidanda bided their time, considering and rejecting opportunities that just weren’t quite right. Then in 2019, the NHLBI posted its call for proposals to address the continuum from donation to transfusion in the blood supply for sub-Saharan Africa. Dr. Puyana contacted Dr. Bidanda. Each contacted collaborators with whom he’d worked in Kenya. Their networks connected them with the Center for Public Health and Development (CPHD), a Kenyan NGO focused on public health, and Pratap Kumar, an MD, neuroscientist, and health economist at Strathmore University in Nairobi. Dr. Kumar is co-principal investigator of the project with Dr. Puyana.
Their team would grow to reflect the complexity of the problem they intended to tackle. Swanson School Industrial Engineering faculty Jayant Rajgopal, PhD, and Bo Zeng, PhD, and graduate student Yiqi Tian joined in. So did critical care physicians, healthcare entrepreneurs, obstetrician-gynecologists, social scientists, transfusion medicine specialists, and trauma surgeons throughout Kenya and the United States. Dr. Puyana credits Dr. Bidanda and his team with mastering the medical jargon in short order and tying the team together. “The engineers have become the highway through which we communicate.”
The Team submitted a proposal Pathways for Innovation in Blood Transfusion Systems in Kenya (PITS Kenya). The proposal was one of just three the NHLBI funded in July 2020. (The others are based in Malawi and Ghana.) The first order of business for the PITS Kenya team was re-tooling their pre-pandemic proposal—initially conceived with a heavy in-person component—to barrel ahead by email, phone, and videoconference. Dr. Bidanda and his team conducted extensive interviews with their Kenyan team members and built detailed process maps to chart the many steps by which blood flows from donor to patient. Sage Open Medicine published their first report in November 2021. Later that month, Pitt representatives traveled to Kenya to refine their process maps.
When the Pitt contingent returned to Kenya in February 2022, both Drs. Bidanda and Puyana made the trek, visiting the three project sites selected by their Kenyan collaborators. Each has unique challenges and opportunities, from the extreme isolation of the semi-nomadic Turkana people in the northwest to verdant Siaya, a short drive from Lake Victoria, and semi-urban Nakuru, an agricultural district northwest of Nairobi.
Using data collected by Kenyan researchers, Dr. Bidanda and his team have built a discrete event simulation model to identify bottlenecks and game out interventions. Users can modify such parameters as the number of blood drives per month, the yield of donors from each blood drive, the transfusion transmitted infection (TTI) rates, hospital capacity, the number of patients, even the amount of blood each patient requires. Already, PITS Kenya has identified a series of interventions slated to begin this summer, pending approval from the NHLBI. The simulations have also helped team members launch side projects, including an analysis of ways to optimize blood screening and the development of new pathology tools to aid in the process. “Intellectual curiosity is the main thing,” says Dr. Bidanda. “As long as you’re curious, these projects keep coming and coming.”
Protein Homeostasis in a Frontotemporal Dementia

Funded by the National Institute on Aging, the 2-year project entitled, “Protein homeostasis in a frontotemporal dementia iPSC model,” was granted to McGowan Institute for Regenerative Medicine affiliated faculty member Charleen Chu, MD, PhD (pictured), the A. Julio Martinez Chair in Neuropathology in the Department of Pathology, University of Pittsburgh School of Medicine. The project began on August 1, 2022.
The valosin-containing protein (VCP) functions as a regulatory hub for multiple facets of protein homeostasis. Mutations in VCP cause familial neurodegeneration with features of frontotemporal dementia and musculoskeletal disease. Dr. Chu’s team will create new pluripotent cellular models derived from patient fibroblasts to study which of the major VCP functions are altered by a disease-causing mutation in human brain cells.
The project abstract reads:
Maintaining protein homeostasis is a particular challenge for neurons, in which a high demand for protein synthesis, folding and transport represents a constant source of stress. Evidence of protein mishandling is observed in nearly all neurodegenerative diseases including the AD-related dementias (ADRD). The AAA-ATPase valosin-containing protein (VCP) plays a central role in maintaining multiple aspects of protein homeostasis. Mutations in VCP have been linked to several forms of frontotemporal lobar degeneration with or without concurrent motor dysfunction and musculoskeletal disease.
Yet there is limited understanding of how these mutations affect different aspects of VCP function in neurons. The VCP-T262A mutation causes familial frontotemporal dementia with aphasia and parkinsonism. Preliminary studies in primary neurons transfected with VCP-T262A reveal deficits in dendritic arborization, and patient fibroblasts bearing the endogenous mutation exhibit disrupted secretory function.
Reagents to create human neurons and other relevant cell types bearing the endogenous VCP-T262A mutation are critically needed to study pathophysiological mechanisms of disease. In this exploratory project, we will create isogenic pairs of iPSC lines expressing T262A vs. wild type VCP. We will differentiate to cortical neurons to study the effects of this mutation upon ER stress, autophagy and Golgi markers compared to iPSC-derived cells with a mutation in a different VCP functional domain. Our long-range goals are to understand how alterations in VCP contribute to synaptic loss so that this mechanistic understanding can be translated into new therapeutic approaches.
Congratulations, Dr. Chu!
Dr. Kurt Weiss and Pittsburgh Cure Sarcoma

McGowan Institute for Regenerative Medicine affiliated faculty member Kurt Weiss, MD (pictured), Associate Professor of Orthopaedic Surgery in the University of Pittsburgh School of Medicine Department of Orthopaedic Surgery, Division of Musculoskeletal Oncology, is a childhood sarcoma survivor whose life work is now focused on researching treatments for and healing the patients with sarcoma. He is also a co-founder of the local nonprofit 501c3 organization, Pittsburgh Cure Sarcoma.
The mission of Pittsburgh Cure Sarcoma is to raise both awareness of childhood and adult sarcoma and fund resources for sarcoma research in Pittsburgh and throughout the sarcoma community. Past funds received have been used to support six wet labs across the University of Pittsburgh, Children’s Hospital of Pittsburgh of UPMC, Allegheny Health Network, and West Virginia University Medicine. Pittsburgh Cure Sarcoma races funded ten $50,000 sarcoma research grants through the Sarcoma Foundation of America.
CBS Pittsburgh/KDKA recently caught up with Dr. Weiss and Mark Goodman, MD (retired), a duo that went from patient and doctor to colleagues fighting for a cure for sarcoma. Watch their video here.
Did you know that:
- Sarcoma is a cancer of the connective tissue that can strike at any age. Sarcomas are relatively more common among children.
- Children under the age of 20 who are battling sarcoma make up about 15%-20% of childhood cancers. While rare in adults (1% of all cancers) sarcoma are common in children making up 15-20% of all pediatric cancers.
- Each year there are approximately 15,000 new patients and approximately 6,000 deaths as a result of sarcomas.
For more information on how you can help and/or participate in Pittsburgh Cure Sarcoma fund-raising efforts, please visit the organization’s website here.
First Patient Enrolled in a Phase II Clinical Trial of CardiolRx(TM) for Treatment of Acute Myocarditis

Myocarditis is an acute inflammatory condition of the heart muscle (myocardium) characterized by chest pain, impaired cardiac function, atrial and ventricular arrhythmias, and conduction disturbances. Although the symptoms are often mild, myocarditis remains an important cause of acute and fulminant heart failure and is a leading cause of sudden cardiac death in people under 35 years of age.
McGowan Institute for Regenerative Medicine affiliated faculty member Dennis McNamara, MD (pictured), is a Professor of Medicine at the University of Pittsburgh, Director of the Center for Heart Failure Research at the University of Pittsburgh Medical Center, and Chair of the Cardiol Therapeutics Inc. study steering committee of a Phase II Clinical Trial of CardiolRx(TM) for Treatment of Acute Myocarditis. He said, “We have long suspected that it is the response to injury that needs to be addressed to improve outcomes in myocarditis. Given its impact limiting these inflammatory mechanisms, we believe cannabidiol has the potential to truly benefit patients with this condition. I am pleased this important milestone has now been achieved and the ARCHER study, designed to investigate CardiolRx’s therapeutic potential in myocarditis, is formally underway.”
ARCHER has received regulatory clearance in multiple jurisdictions, including IND authorization from the FDA, and is expected to enroll 100 patients at major cardiac centers in North America, Europe, Latin America, and Israel. The primary endpoints of the trial, which will be evaluated after 12 weeks of double-blind therapy, consist of the following cardiac magnetic resonance imaging measures: left ventricular function (ejection fraction and longitudinal strain) and myocardial edema/fibrosis (extra-cellular volume), each of which has been shown to predict long-term prognosis of patients with acute myocarditis.
Some myocarditis patients proceed to develop chronic dilated cardiomyopathy which continues to be the leading indication for cardiac transplantation. Although viral infection is the most common cause of myocarditis, the condition can also result from administration of therapies used to treat several common cancers, including chemo-therapeutic agents and immune checkpoint inhibitors. Myocarditis has also been described as a complication of COVID-19 and, more recently, has been reported as a rare complication associated with certain vaccines for COVID-19. Acute myocarditis should be managed with guideline directed therapies for heart failure, arrhythmia, and conduction disturbances; however, there is no uniformly accepted treatment for the underlying inflammatory processes associated with this condition.
CardiolRx believes there is a significant opportunity to develop an important new therapy for acute myocarditis that would also be eligible for designation as an orphan drug in the United States. The U.S. Orphan Drug Designation program was created to provide the sponsor of a drug significant incentives, including seven-year marketing exclusivity and exemptions from certain FDA fees, to develop treatments for diseases that affect fewer than 200,000 people in the U.S. (73,000 myocarditis cases are estimated in the U.S. annually).
Mother’s Milk Cells Key to Novel Infant Disease Therapy

Breast milk has long been considered an ideal way to feed a growing baby. Full of nutrients that are easily digested, it assists in brain growth, improved eyesight, and the development of the infantile gastrointestinal, nervous, and immune systems. Now, researchers at Carnegie Mellon University (CMU) are exploring ways to utilize the unique properties of breast milk to develop a novel approach to infant disease therapy.
After giving birth to her daughter in 2016, McGowan Institute for Regenerative Medicine affiliated faculty member Kathryn Whitehead, PhD (pictured), associate professor of chemical and biomedical engineering in the College of Engineering at CMU, grew curious about the properties of breast milk. As she began researching the topic, she learned of the millions of living cells present in every milliliter.
“When a child consumes breast milk, they are also consuming their mother’s cells,” Dr. Whitehead said.
Although human cells are one of the least studied components of breast milk, previous work has demonstrated their remarkable properties. These properties include traveling out of the gastrointestinal tract and integrating into an infant’s tissue, where the cells can proliferate and remain into adulthood.
“This is an amazing phenomenon,” Dr. Whitehead said. “As a drug delivery research group, we spend a lot of time thinking about how to transport proteins and other therapeutic molecules out of the gastrointestinal tract. These cells may inspire us as to how to do that.”
Dr. Whitehead believes that if her lab can figure out how these cells achieve transport through the body, they could potentially be used as drug delivery vehicles for therapeutic purposes, something that has never been done before.
Her group’s first paper on the topic, now published in the journal Science Advances, takes the first important steps toward this goal. Here, Dr. Whitehead’s group characterizes the cells in mature stage breast milk at the protein, gene, and transcriptome levels, providing a genetic fingerprint through which to track maternal cells in the infant’s body.
These results will pave the way for Dr. Whitehead’s long-term vision of genetically engineering the cells to orally treat infants with medicines and vaccines or to tolerize them to allergens.
“Our approach will be noninvasive both for the mother and the baby,” Dr. Whitehead said.
Now that Dr. Whitehead’s lab has identified the maternal cellular components of milk, they will focus on tracking the different types of maternal cells as they move through the infant. It is possible that only certain cell types (e.g., lactocytes or immune cells) will have the transport and medicine production abilities to achieve Dr. Whitehead’s long-term vision. Once these optimal maternal cells are identified, the team will then engineer those cells for therapeutic purposes.
Illustration: Carnegie Mellon University.
AWARDS AND RECOGNITION
Dr. Sanjeev Shroff Named Interim Dean of Pitt’s Swanson School of Engineering

McGowan Institute for Regenerative Medicine faculty member Sanjeev Shroff, PhD (pictured), Distinguished Professor of and the Gerald E. McGinnis Chair in Bioengineering and the Chair of the Department of Bioengineering, has agreed to serve as Interim Dean of the University of Pittsburgh Swanson School of Engineering. Dr. Shroff came to Pitt from the University of Chicago in 2000 and is deeply respected by his colleagues at Pitt and in the broader community, as well as by his peers in the field of bioengineering.
Dr. Shroff is a distinguished scholar in cardiovascular physiology and engineering, with special emphasis on cardiac mechano-energetics and cardiovascular structure-function relationships under normal and pathological conditions. His research has been supported by grants from the American Heart Association, National Science Foundation, and the National Institutes of Health. He is also an elected Fellow of the American Physiological Society, the American Institute for Medical and Biological Engineering, the Biomedical Engineering Society, and the International Academy of Medical and Biological Engineering.
“I believe the combination of Sanjeev’s leadership experience, dedication to and knowledge of Pitt and of the Swanson School, and professional expertise makes him the ideal choice for this interim role,” said Ann Cudd, PhD, Provost and Senior Vice Chancellor of Pitt.
“I am both delighted and humbled to accept the role of Interim U.S. Steel Dean of the Swanson School of Engineering, one of the first and finest engineering programs in the U.S. with a rich 176-year history,” said Dr. Shroff. “As Interim Dean, it is my intent to encourage an environment of shared governance among all stakeholders, with the common goal of achieving excellence in both research and education. We have an established foundation of excellence – let us build upon that with our combined knowledge, imagination, and collaboration.”
Congratulations, Dr. Shroff!
Illustration: University of Pittsburgh Swanson School of Engineering.
Dr. Tetsuro Sakai Selected for ILTS Special Interest Groups Steering Committee

Congratulations to McGowan Institute for Regenerative Medicine affiliated faculty member Tetsuro Sakai, MD, PhD (pictured), who was selected as a member of the Steering Committee of the Live Donor Liver Transplantation (LDLT) Special Interest Groups of the International Liver Transplant Society (ILTS) for a three-year term. Dr. Sakai is the only anesthesiology representative among the committee’s nine international LDLT experts.
Dr. Sakai is a Professor of the Department of Anesthesiology and Perioperative Medicine and a Professor of the Clinical Translational Science Institute, University of Pittsburgh School of Medicine. He is also a board-certified staff anesthesiologist (Hepatic and Intestinal Transplantation Anesthesia) in the UPMC Department of Anesthesiology and Perioperative Medicine. Dr. Sakai’s current research interests include education in anesthesiology (resident research education); liver transplantation anesthesiology; and quality improvement in anesthesiology.
The ILTS is a growing organization of approximately 1400 international members belonging to a multidisciplinary cadre of specialists and healthcare providers who have joined to promote the focus of ILTS – the advancement of the science and practice of liver transplantation by sharing of clinical and educational experiences and research at an international level.
The primary goal of the LDLT Special Interest Group (SIG) of the ILTS is education, benchmarking, and best practice establishment in LDLT across the fraternity and across specialties involved in LDLT. The LDLT SIG will lead advances in the field and foster international collaborative scientific efforts.
Congratulations, Dr. Sakai!
Dr. Ipsita Banerjee Receives Faculty Promotion

McGowan Institute for Regenerative Medicine affiliated faculty member Ipsita Banerjee, PhD (pictured), has been promoted to the rank of Professor in the Department of Chemical and Petroleum Engineering, Swanson School of Engineering, University of Pittsburgh. As stated in the letter from Patrick Gallagher, PhD, Chancellor, “Promotion to the highest academic rank recognizes that the promise implicit in the conferral of tenure has been fulfilled. Indeed, your record of accomplishments to date clearly suggests that the years ahead will bring further contributions to your discipline, the University, and to the broader society.”
Dr. Banerjee received her PhD in Chemical and Biochemical Engineering from Rutgers University, from which she won the University and Bevier Fellowship for Excellence in Graduate Research. She did her postdoctoral training in Harvard Medical School/Massachusetts General Hospital before joining the University of Pittsburgh.
Dr. Banerjee’s research interests and collaborations include:
- pluripotent stem cells
- systems modeling of signaling pathways
- biomanufacturing of pluripotent stem cells
- pancreatic islet organoid engineering
- organ engineering
- nanotoxicity evaluation
Dr. Banerjee received the NIH Director’s New Innovator Award, ORAU Ralph E. Powe Junior Faculty Award, and was selected to participate in the NAE Frontiers of Engineering Education Symposium.
Congratulations, Dr. Banerjee!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#236 –– Dr. Alan Wells discusses his research in the conditions of dysregulated responses.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Julie A Phillippi, Elena Aikawa, Josh Hutcheson
Title: Editorial: Exploring the Frontiers of Regenerative Cardiovascular Medicine
Summary: In this issue, a perspective article from investigators of the International Society for Applied Cardiovascular Biology (ISACB) by Hutcheson et al. details significant progress in applying cardiovascular biology in translational directions. A comprehensive retrospective first takes us through the work of international experts since the first human heart transplant in Cape Town, South Africa. This benchmark event in 1967 was commemorated 20 years later when this society was born and then 50 years later in 2017 when scientists reconvened in Cape Town for a celebration and international convention on cardiovascular disease. Motivated by the topics discussed at this commemorative assembly, this comprehensive perspective takes us through the past 50 years, highlighting the pioneering and innovative efforts of the scientific community of clinician, engineer, biologist and industry thought leaders to meet the critical needs of yesterday through interdisciplinary and international cooperation and to address today’s unmet clinical needs for patients with cardiovascular disease. This published Research Topic represents a curated collection of articles that uniquely attack cardiovascular disease from multiple directions, and uniformly embrace a reaffirmed interdisciplinary mission to understand and treat cardiovascular disease through regenerative medicine.
Source: Front Cardiovasc Med. 2019 Feb 26;6:13. doi: 10.3389/fcvm.2019.00013. eCollection 2019.
GRANT OF THE MONTH
PI: Hēth Turnquist, PhD
Title: Immunoregulatory Mechanisms of IL-33 in Heart Transplantation
Description: Early graft injury is the consequence of unavoidable transplant-associated events, such as donor brain death, ischemia/reperfusion injury, and surgical trauma. Recipient responses to graft alloantigens will also produce graft damage throughout the life of the graft. These injuries and stresses cause the release of self-molecules containing damage-associated molecular patterns (DAMPs). It is well appreciated that DAMPs support pro-inflammatory responses when they are sensed by cells of the innate immune system, particularly monocytes and macrophages. Preclinical rodent heart transplant studies reveal that these released DAMPs initiate and propagate alloresponses and targeting inflammatory DAMPs, or the signaling cascades they activate, reduce alloimmunity and improve outcomes after transplantation. Yet, our recent research has provided compelling evidence that these graft injuries also release reparative DAMPs (rDAMPs), such as Interleukin-33 (IL-33), which limits local inflammation and initiates immune-mediated tissue repair after heart transplant. By assessing pediatric heart transplant recipient samples and using a preclinical mouse heart transplant model, we identified that IL-33 is upregulated in graft stromal cells and limits the development of allograft vasculopathy and graft fibrosis that later culminates in chronic rejection (CR). Our published and preliminary data suggest that IL-33 mediates this protection by targeting infiltrating monocytes and macrophages, as well as regulatory T cells (Tregs), to limit the generation of pro-inflammatory macrophages and then coordinate a Treg and reparative macrophage-mediated response to injury. While these data are encouraging, our studies also suggest that the processes that initiate repair of early tissue damage may become dysregulated over the graft’s life. In preliminary data, we show that vessel-associated Tregs become pathologic when their sustained secretion of repair factors in response to IL-33 promotes the proliferation of local fibroblasts contributing to CR. In addition to having their functions programmed by local rDAMPs, graft infiltrating recipient monocytes recognize allogenic molecules causing them to mature into pro-inflammatory myeloid cells that stimulate T cell proliferation and IFNγ production in the graft. We have established that monocytes and macrophages recognize and develop allospecific cytotoxicity and memory to MHCI antigens via paired immunoglobulin-like receptors-A. These data lead us to HYPOTHESIZE that rDAMP signals initiating the repair of tissue damage early after heart transplantation become dysregulated around the vasculature due to a sustained inflammatory response by alloreactive immune cells. We will test this hypothesis in two aims: In AIM 1, we will define how rDAMP and immune cell interactions evolve in heart transplant microenvironments during CR development. In AIM 2, we will establish if innate alloimmunity prevents effective injury resolution and repair after heart transplantation.
Source: National Institutes of Health/National Heart, Lung, and Blood Institute
Term: August 10, 2022 – June 30, 2027
Amount: $610,703 (first year)
