March 2023 | VOL. 22, NO. 3| www.McGowan.pitt.edu
22nd McGowan Institute for Regenerative Medicine Scientific Retreat

With over 200 in attendance, the McGowan Institute for Regenerative Medicine held its 2023 Scientific Retreat on March 6-7, 2023.
The participation and contributions of the guests and external collaborators – along with McGowan Institute for Regenerative Medicine affiliated faculty and trainees – provided for insightful topics for discussion. The program chair was Bryan Brown, PhD, associate professor Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute.
Program Highlights
The Director’s State of the Institute Address/Retreat Kickoff was presented by McGowan Institute Director William Wagner, PhD, Distinguished Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh.
The sessions addressed:
- Computational Biology in Regenerative Medicine
- Multi-scale Analysis of Inflammatory Microenvironments
- Bioelectronics: Materials and Interfaces
- Organ-on-a-Chip Models and Organoids for Disease Modeling and Drug Development
- Regenerating Diversity, Equity, Inclusion and Accessibility (DEIA) in Regenerative Medicine
- Advances in Organ Transplantation Strategies
- Pediatric Device Innovators Forum: Patient Matched Devices for Pediatrics: A Case Study
In addition to the sessions, a Lunch Session was held on both days. On Monday, Keynote Speaker Marc Giulianotti, Senior Manager of Space Biomanufacturing at Sierra Space, gave a lunchtime presentation on “Exploring Beyond our Planet: Leveraging Platforms in LEO for the Development of Biomedical Technologies.”
On Tuesday, several faculty members presented updates and opportunities at the McGowan Institute. These updates addressed:
- PITT Nanofabrication and Characterization Facility – Medical Applications
- New Imaging Technologies at the Center for Biologic Imaging
- Overview of the Center for Military Medicine Research and the Federal Omnibus-IV Program
- Immunologic Monitoring and Cellular Products Laboratory (IMCPL): A New Bridge for Cellular Medicine at University of Pittsburgh
- How to Commercialize Your Discoveries: Introduction to the Office of Innovation and Entrepreneurship
Poster Session
 The poster session was effective in introducing the focus of the Retreat and interests of the faculty and the guests. McGowan Institute faculty member Andrew Duncan, PhD, Associate Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. The winners of the poster session were:
The poster session was effective in introducing the focus of the Retreat and interests of the faculty and the guests. McGowan Institute faculty member Andrew Duncan, PhD, Associate Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. The winners of the poster session were:
Outstanding Poster Awards:
Meagan J. Makarczyk
An Innervated Synovium-Cartilage Chip for Modeling Joint Inflammation and Associated Pain
Mentor: Hang Lin, PhD
Department of Orthopaedic Surgery
University of Pittsburgh
Alexis Nolfi
Therapeutic Use of an Interleukin-4 Eye Drop in a Rabbit Model of Dry Eye Disease: A Pilot Study
Mentor: Bryan Brown, PhD
Dept. of Bioengineering
University of Pittsburgh
Matthew Poskus
Mathematical Modeling of Fibroblast-Mediated Drug Resistance in HER2+ Breast Cancer
Mentor: Ioannis Zervantonakis, PhD
Dept. of Bioengineering
University of Pittsburgh
Sierra R. Wilson
Diploid hepatocytes resist acetaminophen-induced acute liver injury and drive compensatory regeneration
Mentor: Andrew Duncan, PhD
Department of Pathology
University of Pittsburgh
Merit Poster Awards:
Isabelle Chickanosky
Modeling Endometriosis Angiogenesis in Endometriotic Conditions
Mentor: David Vorp, PhD
Dept. of Engineering
University of Pittsburgh
Bryant Fisher
Inhibition of Vasa Vasorum Angiogenesis Induces Thoracic Aortic Aneurysm
Mentor: Julie Phillippi, PhD
Dept. of Cardiothoracic Surgery and Bioengineering
University of Pittsburgh
Mohamed S. Ibrahim
Advancements in Cardiovascular Stent Technology: Introducing a Nanostructured Electronic Stent for Continuous Monitoring of Restenosis
Mentor: Youngjae Chun, PhD
Department of Industrial Engineering
University of Pittsburgh
Dorota Jazwinska
Modulating mesothelial cell motility and contractility alters ovarian cancer metastatic potential
Mentor: Ioannis Zervantonakis, PhD
Dept. of Bioengineering
University of Pittsburgh
Brandon M. Lehrich
β-Catenin Activation Promotes B-cell Exclusion in the Hepatocellular Carcinoma Microenvironment
Mentor: Satdarshan (Paul) Singh Monga, MD
Department of Pathology
University of Pittsburgh
Lauren L. Luciani
Mathematical Model of Influenza Infection in Juvenile Mice Suggests Increased Production of Type 1 Interferon by Infected Cells is Associated with Severe Infection
Mentor: Jason Shoemaker, PhD
Department of Chemical and Petroleum Engineering
University of Pittsburgh
Miranda Poklar
3D Bioprinting of Functional iPSC Derived Islet Organoids and Human Islets in Hydrogel Constructs
Mentor: Ipsita Banerjee, PhD
Department of Chemical and Petroleum Engineering
University of Pittsburgh
Hannah Yankello
Exploring monocyte and trophoblastic signaling and their role in pre-eclampsia
Mentor: Elizabeth Wayne, PhD
Department of Biomedical Engineering and Chemical Engineering
Carnegie Mellon University
Outstanding Poster Award ($200) and Merit Poster Awards ($150). Congratulations to all!
See the full Retreat Program here.
A special thank you is extended to the retreat committee (Bryan Brown-Chair, Patrick Cantini, Andy Duncan, Rebecca Bauroth, Lainey Becker, Katy Wharton, Jamelle Price, and John Murphy) and to all who made this year’s Retreat a success!
Photo Credit: Jamelle Price.
RESOURCES AT THE MCGOWAN INSTITUTE
April Histology Special – Toluidine Blue
Mast cells play a key role in the inflammatory process.
A mast cell is a myeloid derived cell and is part of the immune system. Mast cells contain distinctive granules rich in histamine and heparin. Although best known for their role in allergy and anaphylaxis, mast cells play an important protective role as well, being intimately involved in angiogenesis, defense against pathogens, and wound healing.
Toluidine blue is one of the most common stains for acid mucopolysaccharides and glycoaminoglycans, both of which are components of mast cells granules.
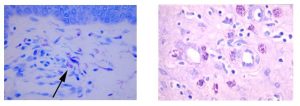
You’ll receive 25% off Toluidine Blue Staining in April when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: Please contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
Dr. Steffi Oesterreich Among Four UPMC/Pitt Scientists to Receive Funding for Critical Research

McGowan Institute for Regenerative Medicine affiliated faculty member Steffi Oesterreich, PhD (pictured), Professor of Pharmacology and Chemical Biology at the University of Pittsburgh Cancer Institute, is among the four leading UPMC/Pitt breast cancer scientists receiving funding for research from the Breast Cancer Research Foundation (BCRF).
In partnership with her husband, Adrian V. Lee, PhD, the Oesterreich/Lee lab researches the molecular basis of breast cancer development and its resistance to therapy. They aim to improve precision medicine and outcomes for breast cancer patients.
BCRF has given Drs. Lee and Oesterreich more than $200,000 annually for a total of $5 million in funding from BCRF.
“This funding is essential for us to make progress in understanding the unique features of ILC and to develop precision medicine approaches to this subtype of breast cancer,” said Dr. Lee.
Dr. Oesterreich added, “Without BCRF’s support of our early work on ILC, we would not have been able to establish the larger lobular research program that we now have. Also, the flexibility of the funds allows us to make changes in research approaches often required in response to new findings.”
Dr. Oesterreich also serves on BCRF’s Scientific Advisory Board, whose aim is to seek out promise and potential in individuals with the goal of bringing the world closer to prevention and cure.
Other UPMC scientists who were awarded funding are Wendie Berg, MD, PhD and Norman Wolmark, MD.
BCRF’s mission is to prevent and cure breast cancer by advancing the world’s most promising research.
Congratulations, Dr. Oesterreich!
Delivering mRNA to the Pancreas

Messenger RNA (mRNA) therapies have so much clinical potential for vaccination, protein replacement, gene editing, immunotherapies, and tissue regeneration, but it is a challenge to deliver to organs other than the liver, spleen, and lungs. McGowan Institute for Regenerative Medicine affiliated faculty member Kathryn Whitehead, PhD (pictured), associate professor of chemical and biomedical engineering in the College of Engineering at Carnegie Mellon University, is working to change that.
“We’ve created technology that delivers to a very important organ,” Dr. Whitehead said.
mRNAs are a type of single-stranded RNA involved in protein synthesis. Their role is to carry protein information from the DNA in a cell’s nucleus to the cell’s cytoplasm. To do this, mRNA needs a nanoparticle delivery vehicle to protect it.
Dr. Whitehead’s lab aims to alter the chemistry of nanoparticles so that they can reroute them to organs where nanoparticles don’t naturally accumulate, such as the pancreas.
“In the last couple of years, it’s become increasingly clear in the field, by my own group and others, that when you change the chemistry of the particle, you are then changing the types of proteins in the blood that adhere to the surface of the particle. Those unique sets of proteins can guide the particles to different places,” Dr. Whitehead said.
Dr. Whitehead’s team recently published a paper in the journal Science Advances that details a new nanoparticle that they developed. This nanoparticle can deliver mRNA to the pancreas by using intraperitoneal injection.
“This unexpected delivery to the islets would enable treatments for several very serious conditions, including Type 1 diabetes and a type of pancreatic cancer that originates within these islets,” Dr. Whitehead said.
The hope is that one day this technology can be used in alpha-to-beta transdifferentiation as a treatment for Type 1 diabetes. The goal is to use mRNA to reprogram alpha cells to produce insulin. If mRNAs can be delivered to the pancreas, then it could lead to more effective and less arduous treatments to patients.
The abstract of this paper reads:
Systemic messenger RNA (mRNA) delivery to organs outside the liver, spleen, and lungs remains challenging. To overcome this issue, we hypothesized that altering nanoparticle chemistry and administration routes may enable mRNA-induced protein expression outside of the reticuloendothelial system. Here, we describe a strategy for delivering mRNA potently and specifically to the pancreas using lipid nanoparticles. Our results show that delivering lipid nanoparticles containing cationic helper lipids by intraperitoneal administration produces robust and specific protein expression in the pancreas. Most resultant protein expression occurred within insulin-producing β cells. Last, we found that pancreatic mRNA delivery was dependent on horizontal gene transfer by peritoneal macrophage exosome secretion, an underappreciated mechanism that influences the delivery of mRNA lipid nanoparticles. We anticipate that this strategy will enable gene therapies for intractable pancreatic diseases such as diabetes and cancer.
Congratulations, Dr. Whitehead!
New Treatment Strategies for Improved Pain Relief

Although neurostimulation therapies, such as dorsal root ganglion stimulation and spinal cord stimulation, have shown promising results in the treatment of chronic pain, the mechanisms underlying their efficacy are largely unknown. The research team, which includes co-principal investigator Douglas Weber, PhD (pictured), Professor of Mechanical Engineering and Neuroscience at Carnegie Mellon University and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, is proposing parallel and complementary experiments using innovative behavioral, electrophysiological, imaging, and computational modeling techniques to address this lack of knowledge. The results of these studies will provide novel insights on the mechanisms fundamental to the effectiveness of these neurostimulation therapies and potentially offer new treatment strategies for improved pain relief.
The National Institute of Neurological Disorders and Stroke funded this 3-year project entitled “Defining Mechanisms of Pain Relief Associated with Dorsal Root Ganglion and Spinal Cord Stimulation,” which began on September 17, 2022.
The abstract of this project follows:
Chronic pain is a debilitating condition for which there is a pressing need for safe, effective treatments as is highlighted by the ongoing opioid crisis. One potential solution to this problem is the use of neuromodulatory devices such as spinal cord stimulators and dorsal root ganglion stimulators that are currently approved by the FDA for the treatment of pain. While these devices have consistently generated promising results, they are only used in a fraction of chronic pain patients as an intervention of last resort. One of the reasons that these devices are not used more broadly is because their mechanism of action is largely unknown. Here we propose a series of parallel and complementary experiments to investigate the mechanisms of pain relief for spinal cord (SCS) and dorsal root ganglion (DRGS) stimulation, where conventional stimulation parameters will be used for DRGS, and conventional, burst and kilohertz (kHz) stimulation will be used for SCS. We will focus on the two prevailing models used to explain the therapeutic efficacy of these devices: 1) Gate-control, wherein stimulation of large diameter afferent fibers activates inhibitory interneurons that block nociceptive signals from reaching the brain; and 2) T-junction filtering, wherein stimulation of sensory neurons leads to conduction failure at the T-junction in nociceptive fibers in the DRG, blocking nociceptive inputs from reaching the spinal cord. Experiments designed to test these models are described under four Specific Aims in mice and rats with and without chronic pain induced with chronic compression of the DRG, and in a human DRG nerve preparation. In Aim 1 we will determine which neurons (primary afferent and/or dorsal horn) are activated by SCS and DRGS with 2P-calcium imaging in mice, and single unit recording in rats and human tissue. We will use the data generated at an unprecedented level of detail to refine computational models of the cells and circuits engaged by these devices. In Aim 2 we will confirm the efficacy of DRGS and SCS neuromodulation on simple reflexive responses and complex nociceptive behaviors in rats and mice. The timing and relative efficacy of the site and parameters of neuromodulation will inform computational models and mechanistic studies. In Aim 3 we will directly test the gate-control model using a novel strategy of 2P Ca2+ imaging in mice enabling identification of subpopulations of inhibitory, excitatory, and projection neurons in the same preparation. This will be combined with single unit and MEA recordings from rats and mice, all done in an iterative fashion with computational modeling. In Aim 4 we will test the T-junction filtering model with direct measurements of transmission efficiency in rat and human tissue. Pharmacological studies and computational modeling will investigate specific mechanisms responsible for this filtering. The results from these conceptually and technically innovative studies will provide a major advance in our understanding of the mechanisms underlying the effectiveness of these neuromodulatory devices and potentially offer new treatment strategies for improved pain relief.
Congratulations, Dr. Weber!
A New Way to Measure Brain Pressure

Monitoring intercranial brain pressure (ICP) is complicated and invasive. Testing includes placing a probe through the skull for several days. This is why the procedure is often reserved for patients who are most at risk for serious brain injury.
The procedure can be risky, but, if left untreated, elevated ICP can be fatal.
“The problem with invasive testing, other than the fact that it is painful, is that it can be associated with complications, such as infection, damage to the brain and bleeding. Those things are not extremely common, but they are common enough that it limits when we choose to measure brain pressure to circumstances where the risk is justified by the potential benefit,” said Dr. Michael McDowell, assistant professor of neurological surgery at the University of Pittsburgh School of Medicine.
Dr. McDowell collaborates with McGowan Institute affiliated faculty member Jana Kainerstorfer, PhD (pictured), CMU associate professor of biomedical engineering. Together, they created a device that can be attached to the patient’s forehead either using a piece of adhesive or a headband. This device eliminates the need for a more intrusive procedure or incision.
In as little as 60 seconds, the device is able to transmit near infrared light, which detects the light that bounces off of the brain through the skin and skull. This allows the sensor to provide information about the speed of blood flow in the brain by looking at the amount of light that returns and combining that information with the patient’s heart rate and blood pressure.
The study involved 15 children from the pediatric intensive care unit at UPMC Children’s Hospital of Pittsburgh. It found that the device closely matches the more invasive procedure.
The results of a preliminary study were published in the Journal of Neurosurgery in the paper, “Clinical Translation of Noninvasive Intracranial Pressure Sensing with Diffuse Correlation Spectroscopy.”
The abstract reads:
Objective: Intracranial pressure (ICP) is an important therapeutic target in many critical neuropathologies. The current tools for ICP measurements are invasive; hence, these are only selectively applied in critical cases where the benefits surpass the risks. To address the need for low-risk ICP monitoring, the authors developed a noninvasive alternative.
Methods: The authors recently demonstrated noninvasive quantification of ICP in an animal model by using morphological analysis of microvascular cerebral blood flow (CBF) measured with diffuse correlation spectroscopy (DCS). The current prospective observational study expanded on this preclinical study by translating the method to pediatric patients. Here, the CBF features, along with mean arterial pressure (MAP) and heart rate (HR) data, were used to build a random decision forest, machine learning model for estimation of ICP; the results of this model were compared with those of invasive monitoring.
Results: Fifteen patients (mean age ± SD [range] 9.8 ± 5.1 [0.3-17.5] years; median age [interquartile range] 11 [7.4] years; 10 males and 5 females) who underwent invasive neuromonitoring for any purpose were enrolled. Estimated ICP (ICPest) very closely matched invasive ICP (ICPinv), with a root mean square error (RMSE) of 1.01 mm Hg and 95% limit of agreement of ≤ 1.99 mm Hg for ICPinv 0.01-41.25 mm Hg. When the ICP range (ICPinv 0.01-29.05 mm Hg) was narrowed on the basis of the sample population, both RMSE and limit of agreement improved to 0.81 mm Hg and ≤ 1.6 mm Hg, respectively. In addition, 0.3% of the test samples for ICPinv ≤ 20 mm Hg and 5.4% of the test samples for ICPinv > 20 mm Hg had a limit of agreement > 5 mm Hg, which may be considered the acceptable limit of agreement for clinical validity of ICP sensing. For the narrower case, 0.1% of test samples for ICPinv ≤ 20 mm Hg and 1.1% of the test samples for ICPinv > 20 mm Hg had a limit of agreement > 5 mm Hg. Although the CBF features were crucial, the best prediction accuracy was achieved when these features were combined with MAP and HR data. Lastly, preliminary leave-one-out analysis showed model accuracy with an RMSE of 6 mm Hg and limit of agreement of ≤ 7 mm Hg.
Conclusions: The authors have shown that DCS may enable ICP monitoring with additional clinical validation. The lower risk of such monitoring would allow ICP to be estimated for a wide spectrum of indications, thereby both reducing the use of invasive monitors and increasing the types of patients who may benefit from ICP-directed therapies.
Newly Issued Patents for McGowan Institute Affiliated Faculty Members

During the months of January and February 2023, the following patents were issued based on research work conducted by McGowan Institute for Regenerative Medicine affiliated faculty members. Their newly issued patents include:
Patent No. 11,548,939
Therapeutic Combinations Using IGF1R Pathway Inhibitors, And Methods to Predict Anti-IGF1R Therapeutic Efficacy
Adrian Lee; Steffi Oesterrich; Jennifer Xavier; Alison Nagle
Patent No. 11,559,612
Use of Self-Assembled Alkysilane Coatings for Drug Delivery Applications
Elia Beniash; Avinash Jagannath Patil
Patent No. 11,564,854
Wheelchair Pressure Ulcer Risk Management Coaching System and Methodology
Sathish Sundaram; Rory Cooper; Rosemarie Cooper; Garrett Grindle; Chengshiu Chung
Patent No. 11,575,127
High Capacity, Air-Stable, Structurally Isomorphous Lithium Alloy Multilayer Porous Foams
Prashant Kumta; Prashanth Hanumantha; Pavithra Shanthi; Moni Datta; Oleg Velikikhatnyi
Patent No. 11,576,887
Nitro-Oleic Acid Controlled Release Platform to Induce Regional Angiogenesis in Abdominal Wall Repair
Antonio D’Amore; Marco Fazzari; Bruce Freeman; William Wagner
Patent No. 11,583,613
Hydrogel Systems for Skeletal Interfacial Tissue Regeneration Applied to Epiphyseal Growth Plate Repair
Juan Taboas; Jingming Chen
Patent No. 11,591,564
Peptide Conjugated Hydrogel Substrate for the maintenance and Expansion of Human Pluripotent Stem Cells
Ipsita Banerjee; Prashant Kumta; Thomas Richardson
Patent No. 11,590,162
Biodegradable, Antioxidant, Thermally Responsive Injectable Hydrogel for Treatment of Ischemic Cardiomyopathy
William Wagner; Yang Zhu; Yasumoto Matsumura; Munugesan Velayutham
Patent No. 11,590,266
Biodegradable Iron-Containing Compositions, Methods of Preparing and Applications Thereof
Prashant Kumta; Oleg Velikokhatnyi; Moni Datta; Sung Jae Chung; Da-Tren Chou; Dae Ho Hong; Partha Sasha
Patent No. 11,589,906
Biodegradable, Magnesium-Containing Bone Screws, Methods for Their Preparation and Medical Applications Thereof
John Holmes; Alejandro Almarza; William Chung; Sarah Henderson
Patent No. 11,594,753
Cathodes and Electrolytes for Rechargeable Magnesium Batteries and Methods of Manufacture
Prashant Kumta; Partha Saha; Moni Datta; Ayyakkannu Manivannan
Congratulations on these successful developments!
Illustration: U.S. Patent and Trademark Office logo.
AWARDS AND RECOGNITION
Dr. Prashant Kumta Named Distinguished Professor of Bioengineering

McGowan Institute for Regenerative Medicine affiliated faculty member Prashant Kumta, PhD, the Edward R. Weidlein Chair at the University of Pittsburgh Swanson School of Engineering and professor in the Departments of BioEngineering, Chemical and Petroleum Engineering, and Mechanical Engineering and Materials Science, has been named Distinguished Professor of Bioengineering by the University of Pittsburgh. This is the highest honor that the University of Pittsburgh bestows upon faculty members. It “honors extraordinary, internationally recognized scholarly attainment.”
“I was absolutely delighted and thrilled to learn that I would be receiving this honor,” Dr. Kumta said. “I couldn’t have done it without the help and support of everyone who contributed, including my students, postdocs, research faculty, colleagues, and, of course, those who set this in motion by nominating me.”
Dr. Kumta has enjoyed a long career, which included initiating new programs in ceramics and electrochemically active systems as well as the tissue engineering initiative which led to the Bone Tissue Engineering Center (BTEC) and, ultimately, the Biomedical Engineering Department. Dr. Kumta was nominated for his research in energy storage and biomaterials. This research combines engineering and biotechnology to create materials that have real-world impact.
“Professor Kumta has had, and continues to have, a distinguished academic career. His rare expertise of working at the intersection of energy storage/conversion science and technology, biomaterials/biotechnology, and bioengineering places him in a class of his own,” said McGowan Institute faculty member Sanjeev Shroff, PhD, Interim U.S. Steel Dean of Engineering. “I am delighted that Pitt has bestowed on him this well-deserved honor in recognition of his outstanding research, educational, mentoring, and professional service contributions, and I am proud to be his colleague.”
Dr. Kumta lists this achievement as the third major milestone in his academic career. The first milestone was receiving tenure at Carnegie Mellon University while the second was receiving an offer to come to the University of Pittsburgh as the Edward R. Weidlein Endowed Chair Professor.
“This isn’t the end. I see this as a source of inspiration to continue to help the School in whatever way I can,” Dr. Kumta said. “I hope we can keep on climbing. I don’t look at this honor as an endpoint, but as an inspiration for more good things to come.”
Dr. Kumta is also the recipient of the Research Initiation Award from the National Science Foundation in 1993, Fellow of the American Ceramic Society and the American Institute of Medical and Biological Engineers and has been continuously listed in Who’s Who in Science and Engineering and Who’s Who in America, among other prestigious publications. He is the author or co-author of more than 333 refereed journal publications with a Google H-index of 70 and was the editor-in-chief of Materials Science and Engineering B Advanced Solid-State Functional Materials, an international journal. In 2012, Dr. Kumta was listed in Forbes India as 1 of the top 18 Indian scientists doing cutting edge work.
Congratulations, Dr. Kumta!
Dr. Thomas Rando Receives the 2023 ISSCR Achievement Award

Thomas Rando, MD, PhD, director of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, has received the International Society for Stem Cell Research (ISSCR) 2023 Achievement Award. He is scheduled to present his research on June 17, 2023, at the ISSCR 2023 Boston + Virtual event.
“Dr. Rando is a trailblazer,” said ISSCR President Haifan Lin, PhD. “His work has had broad impact on the fields of stem cell biology, the biology of aging, and regenerative medicine. It is an honor to recognize Tom, a pioneer in our field, with the 2023 ISSCR Achievement Award.”
The prize is given in recognition of “the transformative body of work of an investigator that has had a major impact on the field of stem cell research or regenerative medicine.”
Dr. Rando’s lab focuses on muscle stem cell biology, muscle stem cell aging, muscular dystrophies, tissue engineering, and basic muscle cell biology.
“I am honored beyond words to receive this award from my valued colleagues at ISSCR,” said Dr. Rando. “The recognition by one’s peers is what makes this so special.”
Dr. Rando is a renowned neurologist and stem cell biologist whose research has yielded critical insights into stem cell function and tissue repair. His laboratory’s groundbreaking studies in mice found that old tissues could be rejuvenated by exposure to young blood. This work has formed the basis of an expanding area of aging research and led to clinical trials of novel therapies for Alzheimer’s disease and other neurodegenerative conditions.
Founded in 2002, the ISSCR mission is to promote excellence in stem cell science and applications to human health. They envision a world where stem cell science is encouraged, ethics are prioritized, and discovery improves understanding and advances human health.
Congratulations Dr. Rando!
PUBLICATION OF THE MONTH
Author: Fuat Baris Bengur, Chiaki Komatsu, Caroline Nadia Fedor, Shawn Loder, Jocelyn S Baker, Aanchal Totwani, Zhazira Irgebay, W Vincent Nerone, Mario G Solari, Kacey G Marra
Title: Biodegradable Nerve Guide with Glial Cell Line-Derived Neurotrophic Factor Improves Recovery After Facial Nerve Injury in Rats
Summary: Background: Bioengineered nerve guides with glial cell line-derived neurotrophic factor (GDNF) support recovery after facial nerve injury by acting as regenerative scaffolds. Objective: To compare functional, electrophysiological, and histological outcomes after repair of rat facial nerve transection in control, empty nerve guide, and nerve guide with GDNF conditions. Methods: Rats underwent transection and primary repair of the buccal branch of the facial nerve and were divided into (1) transection and repair only, (2) transection and repair augmented with empty guide, (3) transection and repair augmented with GDNF-guide groups. Weekly measurements of the whisking movements were recorded. At 12 weeks, compound muscle action potentials (CMAPs) at the whisker pad were assessed, and samples were collected for histomorphometric analysis. Results: Rats in GDNF-guide group displayed the earliest peak in normalized whisking amplitude. CMAPs were significantly higher after GDNF-guide placement. Mean fiber surface area of the target muscle, axonal count of the injured branch, and the number of Schwann cells were highest with GDNF guides. Conclusion: The biodegradable nerve guide containing double-walled GDNF microspheres enhanced recovery after facial nerve transection and primary repair.
Source: Facial Plast Surg Aesthet Med. 2023 Mar 6. Online ahead of print.
GRANT OF THE MONTH
PIs: Joerg Gerlach and Pablo Sanchez
Title: Incorporating Hepatic Cell Function into Lung ex vivo Lung Perfusion for Transplant Preservation
Description: In contrast with other organs, preservation times of lung grafts for transplantation are limited to no more than 8 hours with standard cold preservation. Declining quality with extending preservation times increases the risk of primary graft dysfunction, a risk factor for developing chronic rejection, which could account for the low 5-year survival rate (50%) following lung transplantation (LTx). Ex Vivo Lung Perfusion (EVLP) is expected to improve lung preservation and transplant outcomes by providing normothermic circulation and ventilation. A problem is that current EVLP is self-limited by normothermia-activated lung metabolism. The accumulation of toxic metabolites leads to a proinflammatory state, activating Receptor of Advanced Glycation End-Products (RAGE) and nuclear factor (NF)-kB mechanisms, which in turn upregulate proinflammatory cytokine signaling. Experimentally, cross-circulation of a whole swine with EVLP by others demonstrated improved lung resuscitation, but the exact contributors to this improvement remain unclear. Own in vitro preliminary data suggest a role for hepatic function in enhancing endothelial preservation. In previous work, we have maintained normal hepatic detoxification, synthesis, and regulation in in vitro circuits using liver cell bioreactors (BRx). We hypothesize first that the liver function in the swine cross-circulation model played a major role in enhancing EVLP and tissue viability. We also hypothesize that a hepatocyte BRx can partly substitute for whole-swine liver function on EVLP and maintain the observed improvements lung preservation and LTx outcomes. Our Specific Aim is to provide proof-of-principle for the ability of hepatocyte BRx to enhance lung graft preservation. We will incorporate a hepatic BRx in our established EVLP circuits and demonstrate its effect on short-term LTx in experimental rat models. We will repeat these experiments using a cadaveric human EVLP model. We will conduct comprehensive phenotypic, transcriptional, and functional endpoint assessments on lung tissue and hepatic cells in BRx, such as RAGE and NF-kB. If successful, our technology will provide the means to change the current state of EVLP for lung preservation and allow lungs to be maintained longer outside the donor body with less cellular injury. Ultimately, our work will address current limitations in LTx, including increasing viable donor organ transportation time and distance, helping reduce LTx waiting lists, and improving post-transplant patient survival.
Source: National Heart, Lung, and Blood Institute
Term: 04/01/2023 – 01/31/2024
Amount: $238,500
