July 2021 | VOL. 20, NO. 7| www.McGowan.pitt.edu
Pitt-UPMC Scientists Are Inventing mRNA Vaccine to Cure Liver Failure

Today, the only cure for end-stage liver disease is an organ transplant – an expensive treatment, and the demand for which outweighs the supply. Nearly 12,000 people in the U.S. are waiting for a liver as of July 2021.
But what if the same technology as the Moderna and Pfizer COVID-19 vaccines could prompt a diseased liver to cure itself, eliminating the need for a transplant?
University of Pittsburgh School of Medicine and UPMC physician-scientists have done just that in rats – and now they’ve set their sights on humans.
“What we’ve discovered could transform organ transplantation,” said Alejandro Soto-Gutiérrez, MD, PhD, associate professor of pathology at Pitt. “We’re talking about, essentially, a vaccine that might bring patients back from terminal liver failure, no surgery needed.”
Dr. Soto-Gutierrez partnered with UPMC transplant surgeon Ira Fox, MD – both members of the Pittsburgh Liver Research Center and the McGowan Institute for Regenerative Medicine – to explore gene expression and the possibility of “reprogramming” failing liver cells.
Over the past several years, they zeroed in on a transcription factor called P1-HNF4 alpha, which acts as a main control panel, regulating much of the gene expression in liver cells. The researchers found that when P1-HNF4 alpha was turned off in rats, they’d experience liver failure, but re-expression of the transcription factor restored liver function.
Then, the team corroborated that P1-HNF4 alpha was the best target for end-stage liver disease in humans by analyzing nearly 100 failing livers donated by UPMC transplant patients.
In their most recent study – published in Hepatology Communications – the researchers describe the results of treating cells from failing human livers in the lab with a messenger RNA (mRNA) vaccine that delivered instructions to turn the transcription factor back on. The treatment worked.
“There are a lot of genes in liver cells that depend on transcription factors to perform all the essential functions of the liver,” said Dr. Soto-Gutierrez. “When supplemented with our mRNA treatment, all those functions came back in a matter of about 24 hours. Basically, what we believe we have is a new way to treat chronic diseases.”
The clinical research team is meeting with investors and pharmaceutical companies to develop partnerships to translate their findings into clinical trials. If all goes well, Dr. Soto-Gutierrez estimates they could begin human clinical trials within two years.
RESOURCES AT THE MCGOWAN INSTITUTE
August Histology Special – Alcian Blue and PAS Staining
The combination of the Alcian blue and PAS stains can be used to distinguish neutral mucins from acid mucins. Mucins are a key component in lubrication, cell signaling, and forming chemical barriers. Using Alcian blue (pH 2.5), acid mucins will be stained a deep blue. The subsequent application of the PAS technique will stain the neutral mucins a bright magenta. Tissues or cells that contain both neutral and acidic mucins will be stained a dark blue or purple color.
The combined Alcian blue/PAS technique is the most sensitive and comprehensive method for detection of mucins, since all mucins should react regardless of their charge nature.
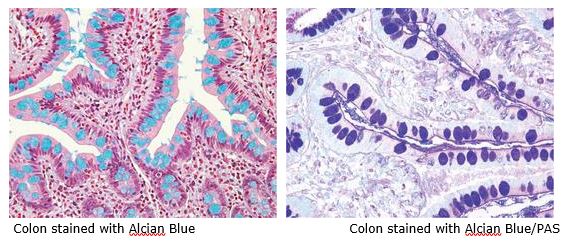
You will receive 30% off Alcian Blue, PAS, or combination Alcian Blue/PAS staining in August when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
Modeling Women’s Health After Childbirth

Childbirth is a momentous and fraught time. For women, childbirth is one of the most significant biomechanical events in life – in terms of forces and motion, the body during childbirth is a structure stressed to extremes.
For bioengineer Steven Abramowitch, PhD, that is not a cold description. He studies damage to women’s pelvises due to pregnancy and delivery – damage that may only manifest decades after the birth. His team relies on resources in modeling structures of the pelvis that indicate potential health problems. His work includes support from McGowan Institute for Regenerative Medicine affiliated faculty member Pamela Moalli, MD, PhD, Professor, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh, Division of Urogynecology & Pelvic Reconstructive Surgery, Magee-Womens Hospital of UPMC, with secondary appointments in the Department of Bioengineering, Clinical and Translational Institute, Adjunct Professor of Bioengineering at Carnegie Mellon University, the Division Director, Urogynecology and Reconstructive Pelvic Surgery, and a Primary Member, Magee-Women Research Institute.
“In the past, this aspect of women’s health has been considered ‘private’ in nature,” Dr. Abramowitch explains. “Women’s health research has always taken a backseat. Pelvic prolapse – when pelvic muscles can no longer hold up organs like the bladder – is more prevalent than conditions like ACL injuries, but no one discusses it. It’s embarrassing. It can lead to symptoms like urinary and fecal incontinence, which folks don’t like talking about.”
Dr. Abramowitch is associate professor of bioengineering at the Swanson School of Engineering and the Clinical and Translational Science Institute. His research into pelvic health disorders resulting from childbirth increasingly relies on computational models developed with CRC resources.
Computer modeling is better than studying animal models for a simple reason – animals are generally quadrupeds. Gravity does not affect their organs the same ways it affects humans. Bipedal humans inherited an evolutionary conflict between efficiently walking upright and efficiently delivering babies.
“Women’s bodies in childbirth are really at the limits of what the tissues can withstand,” Dr. Abramowitch explains. “Childbirth is associated with a lot of soft tissue injuries, many of them unrecognized because they are internal. A muscle tear or nerve damage is not obvious to the obstetrician delivering the baby.”
The injuries appear later in life, sometimes driven by a secondary event or in a degenerative process taking place over time. Modeling similarities and differences among a range of pelvic shapes, Dr. Abramowitch believes, can provide insight into the potential for injury.
Dr. Abramowitch’s team uses two methods to model pelvises – finite element analysis and statistical shape modeling of the pelvis and bones of the hip, sacrum, and coccyx, based on MRI scans.
Finite element analysis essentially breaks the complex whole of a pelvis into manageable geometric points. Collectively the discrete points create a picture of the entire system. More points create a more complete picture – and call for more computer memory and processing power.
Statistical shape modeling looks at those discrete geometric points across a population to build a statistical model of how those geometries are distributed, the goal being to analyze the same corresponding points. The geometric shapes that have been broken into small pieces are reassembled and compared – again calling for a lot of advanced computing resources.
“With the models we can say what pelvic geometries are associated with certain types of birth outcomes,” explains Dr. Abramowitch. “We can input those geometries into our models to find underlying mechanisms that might be associated with particular outcomes.”
PhD candidate Megan Routzong is part of Dr. Abramowitch’s team and lead author on a 2020 paper in the journal Computer Methods and Programs in Biomedicine – “Pelvic floor shape variations during pregnancy and after vaginal delivery.” Dr. Moalli is a co-author on this study.
Ms. Routzong tells the story. “When I started this work, some family asked: ‘Why are you studying childbirth? Women have been giving birth for centuries.’ I ask, did they enjoy that experience? How many women still die from that experience or have a severe quality-of-life loss?”
“The idea behind the research is that if we define an average or typical anatomy, then the more easily a clinician would be able to identify someone whose anatomy tends toward a disease state. The question is which anatomical factors – computationally we refer to them as geometries – correlate or correspond with predicted negative vaginal birth outcomes like excessive stretching in a particular region of a muscle.”
The team creates a segmented, partial pelvis shape and a template shape and tries to fit the template shape to the segmented shape, to fill in the missing data. When a woman gets an ultrasound during pregnancy, it would be possible to use what is visible in the ultrasound field of view – which is smaller than an MRI field of view – to make predictions about how the rest of her pelvic shape might affect the delivery. The goal is reducing the amount of data need to make predictions, ultimately to create a tool that clinicians could use for every pregnancy.
Ms. Routzong works closely with graduate student Liam Martin, who performs much of the computation on Dr. Abramowitch’s team. Mr. Martin explains, “The goal of the statistical shape modeling of the geometries of the pelvises is to understand more patterns of differences than could be explained by random noise. On the images of the pelvises the shapes are shaded between bright and dark colors. Closer to blue or black is the mean shape, and the brighter colors show high variability.”
“With scans of 24 pelvic geometries, we derived three average pelvises based on smoothing – an algorithm that deforms the geometries into a normal shape, average shape, and template shape. That makes it very easy to calculate an average or point-to-point comparison. Theoretically the points are in the same places and represent the same point on the same structure for every subject shape, so it allows for a lot of comparisons.”
Dr. Abramowitch considers the potential impact of the work.
“I’m a trained bioengineer, so these problems are very interesting to me from that perspective. But they are quality of life issues. Women are deeply affected. The long-term impact of pelvic damage is not just a casual part of the aging process, like wrinkles or walking slower. These can be really catastrophic, life-altering conditions.”
Gamifying Vision: How Mobile Gaming Is Tracking Vision Degradation in Patients

Allison Proffitt, for Bio-IT World, recently reported how innovative vision restoration/care and video gaming have merged. Her article features her conversation with Edouard Gasser, CEO of Tilak, who speaks of his partnership with McGowan Institute for Regenerative Medicine affiliated faculty member José-Alain Sahel, MD, Chair and Distinguished Professor of the Department of Ophthalmology at the University of Pittsburgh School of Medicine, director of the UPMC Eye Center, and the Eye and Ear Foundation Endowed Chair of Ophthalmology.
Mr. Gasser hails from the video game industry. Before cofounding Tilak he was Studio Director at Gameloft Madrid, a company developing and editing videogames. While Mr. Gasser brings the video game expertise, his co-founder, Dr. Sahel brings the medical expertise.
In 2016, Mr. Gasser and Dr. Sahel met, and Dr. Sahel pitched his idea for a gaming tool to more frequently monitor his patients who had chronic diseases of aging. The result of that meeting was the first Tilak Healthcare product: OdySight, an app Mr. Gasser describes as a highly engaging, clinically validated, medical mobile game to help monitor patients with chronic eye diseases of aging such as AMD and diabetic retinopathy.
The app doesn’t diagnose eye disease and playing the games doesn’t claim to improve visual acuity. Instead, OdySight is available by prescription only for current patients and uses the phone’s camera and patient input to flag changes in patient vision.
Patients are introduced to the app by their retinal specialist who prescribes OdySight through a unique code that launches a secure download from the Apple or Google app store. The doctor puts in the patient baseline based on treatment thus far, and the first use of the OdySight app—1 to 3 minutes—usually takes place in the doctor’s office. That first use combined with the doctor’s entered data calibrates the app’s baseline visual acuity for the patient.
From there, the patient plays OdySight games regularly to continue tracking visual trends. A user session consists of two parts: medical modules and dynamic puzzle games. The two clinically validated medical modules launch first when a user opens the app. One measures visual acuity; the other is an Amsler grid test, a tool for flagging problem spots in the field of vision. Completing the medical modules earns patients “energy” to play games: “a fantastic adventure composed of dynamic puzzles where you have to recompose an image,” Mr. Gasser explained.
For doctors, OdySight is designed to be easy to incorporate into their clinical routines and not overly invasive. Doctors have a patient dashboard; color codes group users as inactive, active and well, or active and requiring medical attention. Tilak is wary of over-notifying doctors, Mr. Gasser said, and tries to limit doctor emails to twice a week.
One missed test within the medical module doesn’t raise red flags, but several bad results indicate a trend and trigger an alert to both the patient and the doctor.
“When there is a drop in visual acuity, usually what doctors say is you need to go see your ophthalmologist within 10-14 days. When you look at the lack of resources that you have in terms of number of ophthalmologists, and how hard it is to access treatment, you realize that having a tool that helps you monitor those changes is critical to optimizing the healthcare process,” Mr. Gasser explains.
COVID’s Effects on Kids

An article in The Atlantic on the effects of COVID-19 on children included input from McGowan Institute for Regenerative Medicine affiliated faculty member Ivet Bahar, PhD, Distinguished Professor, the John K. Vries Chair, and the Founding Chair in the Department of Computational & Systems Biology at the University of Pittsburgh School of Medicine. Dr. Bahar’s work, which appeared in the PNAS in September 2020, was referenced as a part of the author’s research. Roxanne Khamsi wrote the story for The Atlantic.
Ms. Khamsi reports on the occasionally fatal, COVID-related condition known as multisystem inflammatory syndrome in children (MIS-C). When kids first started showing signs of MIS-C in early 2020—rash or conjunctivitis; low blood pressure; diarrhea or vomiting; etc.—doctors guessed it was an inflammatory disease that occurs most often in toddlers called Kawasaki disease. Now most experts believe it’s a separate condition, affecting kids at an average age of 8. No more than a few hundred children in the U.S. have died from COVID-19 during the pandemic—compared with more than half a million deaths overall—but more than 4,000 have developed MIS-C, and doctors still don’t have foolproof ways to cure it. But a handful of scientists think they’ve found important clues about what drives MIS-C. The disease, they say, may have something to do with a dangerous condition most commonly associated with tampon use.
Dr. Bahar’s lab found a resemblance between the SARS-CoV-2 spike protein, which allows the pandemic virus to infect human cells, and the Staphylococcus superantigen toxin. “This level of similarity, both in sequence and structure with the bacterial toxin, tells us for sure that this segment of spike has the same behavior,” Dr. Bahar told Ms. Khamsi.
The abstract from Dr. Bahar’s PNAS paper entitled, “Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation,” follows:
Multisystem Inflammatory Syndrome in Children (MIS-C) associated with COVID-19 is a newly recognized condition in children with recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. These children and adult patients with severe hyperinflammation present with a constellation of symptoms that strongly resemble toxic shock syndrome, an escalation of the cytotoxic adaptive immune response triggered upon the binding of pathogenic superantigens to T cell receptors (TCRs) and/or major histocompatibility complex class II (MHCII) molecules. Here, using structure-based computational models, we demonstrate that the SARS-CoV-2 spike (S) glycoprotein exhibits a high-affinity motif for binding TCRs, and may form a ternary complex with MHCII. The binding epitope on S harbors a sequence motif unique to SARS-CoV-2 (not present in other SARS-related coronaviruses), which is highly similar in both sequence and structure to the bacterial superantigen staphylococcal enterotoxin B. This interaction between the virus and human T cells could be strengthened by a rare mutation (D839Y/N/E) from a European strain of SARS-CoV-2. Furthermore, the interfacial region includes selected residues from an intercellular adhesion molecule (ICAM)-like motif shared between the SARS viruses from the 2003 and 2019 pandemics. A neurotoxin-like sequence motif on the receptor-binding domain also exhibits a high tendency to bind TCRs. Analysis of the TCR repertoire in adult COVID-19 patients demonstrates that those with severe hyperinflammatory disease exhibit TCR skewing consistent with superantigen activation. These data suggest that SARS-CoV-2 S may act as a superantigen to trigger the development of MIS-C as well as cytokine storm in adult COVID-19 patients, with important implications for the development of therapeutic approaches.
Ms. Khamsi concludes, that for now, the fact that SARS-CoV-2 might function as a superantigen in children (as well as some adults) underscores a risk that may be growing. Youngsters who are not yet immunized against COVID-19 would be the most important beneficiaries of a better understanding of this link.
BNP Test: Critical for PPCM Patients

Peripartum cardiomyopathy (PPCM) is a relatively rare but serious complication of pregnancy in which the heart muscle weakens. The source of the condition is unknown, but researchers believe it impacts at least one in 2,000 live births in the US. Greater risk for the disease is associated with preeclampsia, twin or multiple births, older maternal age, some genetic mutations, and ethnicity. Sarah Yahr Tucker reported on PPCM for Popular Science.
McGowan Institute for Regenerative Medicine affiliated faculty member Dennis McNamara, MD, is a cardiologist, Director of the Center for Heart Failure Research at the University of Pittsburgh Medical Center, and a leading PPCM researcher who Ms. Tucker spoke with for her article.
The risk of developing PPCM for black pregnant people is considered at least double that of white people, and some studies have found rates up to 16 times higher. Although the majority of patients eventually recover, a significant number have permanent heart damage and need lifelong medication, implanted cardiac devices, or even transplants. For others, the diagnosis results in tragedy. PPCM is one of the leading causes of maternal mortality in the US, listed by the Centers for Disease Control and Prevention as the primary cause of death from one week to one year after delivery.
Because PPCM is considered rare, doctors aren’t watching for it, which ironically may mean the actual number of cases is higher than we know. According to a report from the California Pregnancy-Associated Mortality Review Committee, more than 50 percent of PPCM fatalities in the state between 2002-2007 were only diagnosed through autopsy records, and so not recorded as an official cause of death.
At the same time, PPCM is only one element of a larger problem. Maternal mortality is declining worldwide, but US rates have nearly doubled in the past two decades, making it the only developed nation seeing an increase. The US now has the highest maternal mortality rate of any G7 country, and the numbers continue to rise—from 658 deaths in 2018 to 754 in 2019. Serious pregnancy complications, known as severe maternal morbidity, are also rising, affecting more than 50,000 individuals every year. The impact on people of color has been especially grave. Black and indigenous people are currently dying from pregnancy-related causes at two or three times the rate of white people.
The CDC has named “missed or delayed diagnosis” as a key factor behind maternal deaths, and cardiologists and OB-GYNs admit that PPCM is often misdiagnosed or ignored. Symptoms like shortness of breath, extreme fatigue, and swelling in the feet and legs can mimic normal pregnancy discomfort, causing patients and doctors to miss the warning signs. But many survivors say that health care providers dismissed their concerns and neglected to order cardiac testing, including a simple blood test that can indicate heart failure. The brain natriuretic peptide (BNP) test measures a hormone secreted by the heart in response to pressure or enlargement, which means it can detect conditions like PPCM. The cost of the test ranges from about $15–$250 depending on insurance coverage, in line with many basic blood tests. These missed opportunities are especially critical for PPCM patients, as their hearts can grow weaker by the day. Research has shown that the earlier people are diagnosed with PPCM, especially during late pregnancy or the first month after delivery, the more likely they are to recover.
This dangerous lack of awareness among physicians was mentioned in a 2019 practice bulletin on pregnancy and heart disease from the American College of Obstetricians and Gynecologists (ACOG), which notes that the “rising trend” in maternal cardiovascular disease appears related directly to diseases like PPCM. The bulletin estimates that if more maternity care providers considered cardiac issues, “a quarter or more of maternal deaths could be prevented.” ACOG’s assessment echoes the opinion of the CDC, which has stated that about 60 percent of all maternal deaths are, in fact, preventable. And in the case of PPCM, at least, doctors may be ignoring an obvious solution.
“The BNP test is a fantastic test that is completely underutilized in pregnancy,” says Lisa Levine, MD, an OB-GYN, maternal fetal medicine specialist, and director of the Pregnancy and Heart Disease Program at Penn Medicine. “It has a very good negative predictive value. Meaning, if someone comes in with symptoms, and their BNP is negative, it is very unlikely that they would have cardiomyopathy or any heart condition.”
Dr. McNamara, a Professor of Medicine at the University of Pittsburgh, says the issue is not only doing more BNP tests but also correctly interpreting the results. BNP levels can become moderately elevated during pregnancy and for other health reasons, but a “sky high” BNP almost always means heart failure. Dr. McNamara feels that widespread BNP testing would undoubtedly lead to more cases being detected. “In part because of the test,” he says, “but that also assumes that both women and healthcare providers are thinking about the diagnosis, and that’s half the battle.”
Research Offers New Insight for Treating Aggressive Form of Breast Cancer

An international team of researchers—including McGowan Institute for Regenerative Medicine affiliated faculty member Ioannis Zervantonakis, PhD—led by California State University, Northridge (CSUN) biology professor Jonathan Kelber, PhD, has identified, for the first time, that the PEAK1 gene can promote cancer metastasis and therapy resistance in aggressive HER2-positive breast cancers.
Their report, published in the Nature journal Oncogene, identifies PEAK1 upstream and downstream factors that create a “shield,” which blunts the effectiveness of FDA-approved treatments for this aggressive malignancy.
In 2015, Dr. Kelber’s CSUN research group was also the first to report that PEAK1 played a critical cancer “support wall” function by mediating the earliest stages of breast cancer metastasis—notably, that initial work attributed a tumor-promoting function to PEAK1 from within the cancer cells themselves.
“In any cancer, there are tumor cells and non-tumor, tumor-associated cells,” Dr. Kelber said. “For the first time, we have been able to demonstrate that PEAK1 also promotes breast cancer progression from within the non-tumor cell population of the cancer—specifically, from within fibroblast cells. We also found that PEAK1 controls the ability of these fibroblasts to produce a protein hormone known as activin-A, which then causes neighboring breast cancer cells to become resistant to the HER2-targeting drug known as lapatinib (TykerbTM). We were able to identify three new populations of HER2-positive breast cancer cells that emerge in response to PEAK1/activin-A signaling during the course of lapatinib treatment — markers that improve the overall fitness of the cancer cells and prevent lapatinib from working properly.”
HER2-positive breast cancer is one of the most aggressive forms of breast cancer.
As a side note, it was Dr. Kelber’s PhD advisor, the late Wylie Vale, PhD, a Salk Institute Professor and a world-renowned expert on brain hormones, who discovered/characterized activin-A and the first transmembrane serine/threonine kinase receptors for the TGFbeta superfamily of protein hormones to which activin-A belongs.
A major priority of the National Institutes of Health–National Cancer Institute’s Cancer MoonshotSM initiative is to develop ways to overcome cancer’s resistance to therapy. In this regard, Dr. Kelber noted that targeted therapy resistance is a major concern in patients with HER2-positive breast cancer.
“It’s promising that patients with this type of breast cancer have options other than non-specific chemotherapies,” he said. “The downside is that these therapies don’t always work. Findings from this study, however, suggest that therapy combinations incorporating inhibitors of these fibroblast and tumor cell pathways will exploit vulnerabilities in these “shields” to restore therapeutic efficacy.”
Dr. Kelber said the findings may also have implications well beyond HER2-positive breast cancer since therapy resistance is a significant clinical problem for many cancer types.
While this line of inquiry formally began in Dr. Kelber’s CSUN Developmental Oncogene Laboratory in 2017 by former graduate student Sarkis Hamalian, the first piece of data collected that was included in the manuscript was collected by Dr. Kelber’s first CSUN graduate student, Megan Agajanian, back in 2014. The international nature of this collaboration is underscored by the fact that data for this manuscript were also collected with Dr. Zervantonakis, currently an Assistant Professor in the University of Pittsburgh Bioengineering Department and a member of the UPMC Hillman Cancer Center, during Dr. Kelber’s 2017 ASCB-MAC-sponsored visiting professorship at the Harvard Ludwig Center with Joan Brugge, PhD; and with Jonathan D. Humphries, PhD, during Dr. Kelber’s 2019-20 Fulbright Cancer Research UK scholarship to work at the University of Manchester Wellcome Centre for Cell Matrix Research with Martin Humphries, PhD.
Current Kelber Lab postdoctoral fellow Farhana Runa and undergraduate Joshua Gamez played instrumental roles in identifying the functional connection between PEAK1 and activin-A, as well as establishing clinical significance for PEAK1 expression in the cancer-associated fibroblast compartment of HER2-positive breast cancer cells.
Dr. Runa noted that lapatinib resistance is a common challenge to HER2-positive metastatic breast cancer.
“Our understanding of the molecular mechanisms of lapatinib resistance is not well defined,” she said. “For me, the most interesting part of the project was to explore whether activin-A is necessary to drive PEAK1-dependent MSC-induced lapatinib resistance in HER2-positive breast cancer cells. This work is distinctive and significant in addressing outstanding questions about heterogeneity within the breast cancer microenvironment and which potential targets may aid in developing efficacious treatments for HER2-positive metastatic breast cancer.”
“There’s much more to explore beyond the HER2-positive breast cancer microenvironment,” Dr. Runa continued. “Our findings that PEAK-1 drives activin-A-dependent expansion of breast cancer cells may help to define more breast cancer subtypes in the future and illuminate new mechanisms for treating the disease.”
Mr. Gamez added that, for him, “the most interesting things about working on this project were to explore the correlation between cytokine activin-A and PEAK1, as well as uncovering the clinical significance of PEAK1 expression in the stroma of HER2-positive breast cancer and how this connection leads to lapatinib resistance in these tumor cells.”
“This report is important, as the findings have implications for potential targets that may pave the path towards developing treatments and targeted therapy for patients with HER2-positive metastatic breast cancer as well as other subtypes of breast cancer,” he said. “This report is also significant in further developing our understanding of the microenvironment surrounding HER2-positive breast cancer cells—this understanding can help us identify any additional signaling pathways that lead these cells to become resistant to lapatinib or other treatments approved against this malignancy.”
Illustration: McGowan Institute for Regenerative Medicine.
Divers Have Brain Oxygen Levels Even Lower Than Seals During Deep Dives

Elite freedivers who dive unaided in open sea, have brain oxygen levels even lower than seals during their deepest dives, new research at the University of St. Andrews has found.
The divers, who reached depths of 107 meters (351 feet), had brain oxygen levels that would be expected to normally induce unconsciousness and had heart rates as low as those of seals, whales, and dolphins while in the water.
The new findings, published in Philosophical Transactions of the Royal Society B, are helping scientists understand the physiology of marine mammals and could help find new ways to treat human cardiac patients as well as increase the safety of freedivers. McGowan Institute for Regenerative Medicine affiliated faculty member Jana Kainerstorfer, PhD, Associate Professor, Department of Biomedical Engineering and Carnegie Mellon Neuroscience Institute, Carnegie Mellon University, is a co-author on the study.
It could provide information on how freedivers have conditioned themselves to tolerate bouts of extremely low oxygen and brain oxygen delivery to help understand how pre-treatment (pre-conditioning) for surgical procedures could be carried out.
It might be possible to develop these surgical procedures to improve protection of the brain and heart during cardiac surgery, and for post-conditioning therapeutic intervention after events such as a cardiac event.
Project Leader Professor Erika Schagatay, of Mid Sweden University, who has researched freediving for three decades, said: “Before now, understanding the effects on these exceptional divers’ brains and cardiovascular systems during such deep dives, and just how far these humans push their bodies, was not possible, as all research was done during simulated dives in the lab.
“The diver can reach a point where hypoxic (low oxygen) blackout occurs, and the diver then needs to be rescued. One of the main aims of the research is to warn the diver and safety personnel of an imminent blackout.”
Using a device that works in a similar way to a smartwatch – using light emitting LEDs in contact with the skin to measure heart rate, blood volume and oxygen levels in the brain – the team from the University of St. Andrews, Mid Sweden University, Carnegie Mellon University and University of Tokyo created a system that could be worn by the world’s best freedivers during their dives.
The wearable human biomedical technology can measure the physiology of these elite athletes on dives up to depths of at least 107 meters (351 feet).
Elite human freedivers achieve some of the most exceptional feats of human endurance, in what is one of the world’s most extreme sports. Making dives lasting more than four minutes and reaching depths of more than 100 meters on a single breath-hold, freedivers push the limits of what the human body can tolerate.
Lead researcher Dr. Chris McKnight, of the Sea Mammal Research Unit (SMRU) at the University of St. Andrews, said: “The divers showed exceptional physiological responses during their dives.
“We measured heart rates as low as 11 beats per minute and blood oxygenation levels, which are normally 98 per cent oxygenated, drop to 25 per cent, which is far beyond the point at 50 per cent at which we expect people to lose consciousness and equivalent to some of the lowest values measured at the top of Mount Everest.”
An existing, non-invasive human bio-medical technology device, using near-infrared spectroscopy (NIRS), developed by Dutch collaborators Artinis Medical Systems, was adapted by researchers at the University of St. Andrews to withstand the extreme pressure of deep dives in the open ocean.
Dr. Kainerstorfer, who is leading the Biophotonics Lab at Carnegie Mellon University, said: “NIRS is a powerful tool which has extensively been used for measuring brain function in healthy subjects as well as clinical populations.
“Recent advances in miniaturizing NIRS devices have enabled measurements of brain function in more natural environments. The application of NIRS to study diving physiology is particularly exciting and will help us understand how brain function can be maintained under such extreme environmental conditions.”
Lead engineer on the project Steve Balfour of SMRU Instrumentation at the University of St. Andrews, said: “It’s fantastic to be involved in such an exciting and challenging engineering project.
“To see the end-product descending to such depths and returning unique data makes the sleepless nights worth it.”
Dr. McKnight added: “Beyond the exceptional physiological responses that freedivers display and the extremes they can tolerate, they may be a very informative physiological group. Their physiological reactions are so unique and the conditions they’re exposed to are not easily replicated, so they offer a unique way of understanding how the body responds to low blood oxygen, low brain oxygenation and severe cardiovascular suppression.
“Our instrument now allows us to study unique physiological responses while these incredible athletes do their maximal performances.”
ECM a Part of VML Treatment
In its July 2021 edition, Healthcare Radius e-magazine covered the acellular biologic scaffold treatment for volumetric muscle loss (VML) research led by McGowan Institute for Regenerative Medicine Deputy Director Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery and Director of the Center for Pre-Clinical Tissue Engineering within the Institute. The article (pages 30-34) explained how VML is a severe and debilitating clinical medical problem and how Dr. Badylak’s team is helping to solve that problem.
The current standard of care does not address underlying muscle strength deficits, nor does it address restoring muscle function. However, participants in Dr. Badylak’s clinical trial have shown significant improvement in strength and range of motion and evidence for skeletal muscle regeneration.
“Previously, there was no effective treatment for these patients, but this approach holds significant promise,” said Dr. Badylak. “This approach could be a game-changer and not just an incremental advance.”
For the Muscle Tendon Tissue Unit Repair and Reinforcement Reconstructive (MTURR) Reconstructive Surgery Research Study, which was sponsored by the U.S. Department of Defense, 11 men and 2 women who had lost at least 25 percent of leg or arm muscle volume and function first underwent a customized regimen of physical therapy for 4 to 16 weeks.
Lead study surgeon, McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh, then surgically implanted a “quilt” of compressed ECM sheets designed to fill in their injury sites. Within 48 hours of the operation, the participants resumed physical therapy for up to 24 additional weeks.
By 6 months after implantation, patients showed an average improvement of 37.3 percent in strength and 27.1 percent in range of motion tasks compared with pre-operative performance numbers. CT or MRI imaging also showed an increase in post-operative soft tissue formation in all 13 patients.
“For well-selected patients with this type of loss, we now have a treatment available to help improve their function,” Dr. Rubin said.
The new data builds upon a previous Pitt study that showed damaged leg muscles grew stronger and showed signs of regeneration in three out of five men whose old injuries were surgically implanted with ECM derived from pig bladder. Those patients also underwent similar pre- and post-operative physical therapy.
The recent results included more patients with varying limb injuries; used three different types of pig tissues for ECM bioscaffolds; investigated neurogenic cells as a component of the functional remodeling process; and included CT and MRI imaging to evaluate the remodeled muscle tissue.
“The three different types of matrix materials used all worked the same, which is significant because it means this is a generic property of these materials and gives the surgeons a choice for using whichever tissue they like,” Dr. Badylak said. “Prior to the surgery, each patient went through physical therapy focused on getting them to the point where they couldn’t get any better. We then started active rehab within 48 hours after surgery, which proved to be critically important for these patients.”
This work was published in npj Regenerative Medicine.
In another study published in the Journal of Immunology and Regenerative Medicine, Dr. Badylak and his team showed that cellular communication occurs using nanovesicles, extremely tiny fluid-filled sacs that bud off from a cell’s outer surface and allow cells to communicate by transferring proteins. Researchers had yet to identify them in solid body tissues.
“We always thought exosomes are free floating, but recently wondered if they are also present in the solid ECM and might facility the cellular communication that is critical to regenerative processes,” said Dr. Badylak. To explore this possibility, his team used specialized proteins to break up the ECM.
Researchers then exposed two different cell types—immune cells and neuronal stem cells—to isolated matrix-bound vesicles, and found that they caused both cells types to mimic their normal regrowth behaviors.
“Sure enough, we found that vesicles are embedded within the ECM. In fact, these bioscaffolds are loaded with these vesicles. This study showed us that the matrix-bound vesicles are active, can influence cellular behavior, and are possibly the primary mechanism by which bioscaffolds cause tissue regrowth in the body,” Dr. Badylak explained.
Other McGowan Institute affiliated faculty members involved in these works include George Hussey, PhD, Hēth Turnquist, PhD, Fabrisia Ambrosio, PhD, MPT, Neill Turner, PhD, and Michael Boninger, MD.
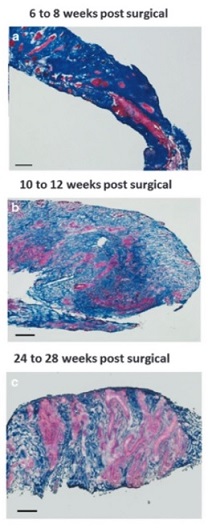
Illustration: Massons trichrome staining of human muscle biopsies shows islands of skeletal muscle present at 6-8 weeks, 10-12 weeks, and 24-28 weeks post-surgery, respectively. (Figure 2, npj Regenerative Medicine; 1, Article number: 16008 (2016).)
Missing Bile Ducts Offer Clues to Mechanism of Liver Injury

Scientists from the University of Pittsburgh School of Medicine described a new phenomenon in which the deletion of a single gene involved in liver embryogenesis completely wipes out bile ducts of newborn mice. But despite a major defect in their bile excretion system, those animals don’t die immediately after birth. Rather, they survive for up to eight months and remain physically active, if small and yellow-tinted.
Published in the journal Cell Reports, the finding offers clues as to why some patients with cholestasis—or impaired bile secretion due to liver injury—seem to fare far better than others with seemingly identical genetic makeup. Researchers think that the key lies in the novel cell-signaling pathway activated as a way of adapting to the liver injury—and, incidentally, that pathway was never studied in the context of adaptation before.
“Our finding was quite serendipitous,” admits senior author Satdarshan (Paul) Monga, MD, Endowed Research Chair in Experimental Pathology, Vice-Chair of Experimental Pathology, Department of Pathology at the University of Pittsburgh, Professor of Pathology (Division of Experimental Pathology), Professor of Medicine (Division of Gastroenterology, Hepatology & Nutrition), and a faculty member of the McGowan Institute for Regenerative Medicine. “We discovered that mice with a deletion of an embryonic gene YAP1 in the early precursors of liver cells don’t form bile ducts at all. But surprisingly, those animals didn’t die—rather, their livers figured out a way to shunt the excess bile into the bloodstream instead of poisoning themselves.
“The liver couldn’t care less about other organs, it just wants to live,” Dr. Monga added.
Additional McGowan Institute affiliated faculty members on this study include:
- Tirthadipa Pradhan-Sundd, PhD, Assistant Professor of Medicine, University of Pittsburgh, Division of Hematology/Oncology
- George Michalopoulos, MD, PhD, Professor and Chairman of the Department of Pathology at the University of Pittsburgh
- Simon Watkins, PhD, Founder and Director of the Center for Biologic Imaging at the University of Pittsburgh, a member of the Pittsburgh Cancer Institute, and Distinguished Professor and Vice Chairman within the Department of Cell Biology
The liver is vital for our body’s survival. It serves more than 500 essential functions a day, acting like a giant synthetic, metabolic and purification plant that clears out toxic chemicals from our bloodstream—from alcohol to excessive amounts of fats, which get broken down by soap-like bile acids in the intestine.
But the bile itself is toxic, too. The bile ducts, which collect secreted bile acids and deliver them to the gut where they help with digestion are important for ensuring that the liver doesn’t poison itself. Whenever the bile ducts are injured or malformed as a result of genetic defects, the liver function suffers. For example, children with Alagille syndrome, whose bile ducts are underdeveloped or absent because of mutations in embryonic proteins, develop cholestasis, which might result in liver failure, as well as heart disease and bone abnormalities.
Even more puzzling, the scientists observed a phenotype similar to human Alagille-like syndrome in mice lacking YAP1 gene—a signaling molecule widely present in embryonic cells that give rise to liver cells, or hepatocytes, and cells that make up bile ducts, or cholangiocytes. Those animals, scientists say, look yellow and are very small in size, but otherwise survive despite the complete lack of bile ducts.
Rather than being poisoned by bile, the liver of those mice seemingly adapted to the injury by decreasing bile synthesis, making it less toxic, and shunting it to the bloodstream instead of dumping it into the liver indiscriminately.
This newly discovered innate adaptive mechanism might explain why some patients with liver injury develop symptoms of acute liver toxicity later than others. It also might offer a clue to clinicians about new ways of treating those patients in the future.
“The severity of symptoms in patients with genetic defects in the liver varies dramatically,” said first author Laura Molina, PhD, medical student at Pitt. “Our research suggests that YAP1 could be an elusive disease modifier that regulates disease outcomes in these patients. We hope that, by studying the functions of YAP1 and promoting the mechanism of adaptation, we can better understand the liver disease and improve existing treatments.”
Study: Immunocompromised Response to COVID-19 Vaccination

People with conditions that compromise their immune systems exhibit a wide spectrum of antibody responses to COVID-19 vaccination, ranging from only 1 in 5 lung transplant patients having an antibody response to a nearly complete response in patients with well-controlled HIV. The results are part of an interim analysis of a large study on UPMC patients and health care workers.
The study is one of the first to verify that the ability of blood from immunocompromised participants to neutralize the virus closely mirrors their antibody levels measured by a Food and Drug Administration (FDA)-approved test, meaning that antibody testing is a good proxy for neutralizing titers.
In an effort to share crucial information to guide clinical and public health decisions, UPMC and University of Pittsburgh School of Medicine physician-scientists published the findings today in medRxiv, a preprint journal, and announced the results ahead of peer-reviewed publication. McGowan Institute for Regenerative Medicine affiliated faculty members who are co-authors of this article include:
- Derek Angus, MD, MPH, FRCP, Executive Vice President, UPMC, and Chief Innovation Officer; Associate Vice Chancellor for Healthcare Innovation at the University of Pittsburgh Schools of the Health Sciences; and Distinguished Professor and holds the Mitchell P. Fink Endowed Chair in Critical Care Medicine at the University of Pittsburgh, with secondary appointments in Medicine, Health Policy and Management, and Clinical and Translational Science
- Abhinav Humar, MD, Clinical Director of the Thomas E. Starzl Transplantation Institute, Chief, Division of Transplantation in the Department of Surgery at the University of Pittsburgh Medical Center, Professor in the Department of Surgery at the University of Pittsburgh School of Medicine, and a Staff Physician at the Pittsburgh VA Medical Healthcare System
- James Luketich, MD, Henry T. Bahnson Professor of Cardiothoracic Surgery, the Chairman, Department of Cardiothoracic Surgery, the Chief, Division of Thoracic and Foregut Surgery, the Director, Thoracic Surgical Oncology, the Co-Director, Lung Cancer Center, the Associate Director of Surgical Affairs, University of Pittsburgh Cancer Institute, and the Director, Mark Ravitch/Leon C. Hirsh Center for Minimally Invasive Surgery
- Alan Wells, MD, DMS, Thomas J. Gill III Professor of Pathology and the Executive Vice-Chairman of the Department of Pathology at the University of Pittsburgh with secondary appointments in Bioengineering and Computational & Systems Biology, and a Staff Physician at the Pittsburgh VA Medical Healthcare System
“Our study highlights the urgent need to optimize and individualize COVID-19 prevention in patients with immunocompromising conditions and have other treatments—such as monoclonal antibodies—available should vaccination fail,” said lead author Ghady Haidar, MD, UPMC transplant infectious diseases physician and assistant professor in Pitt’s Division of Infectious Diseases. “Given the Centers for Disease Control and Prevention’s recommendations permitting vaccinated people to abandon masking and social distancing in most settings, our findings also have implications for public health guidance, since nearly 4% of Americans are immunocompromised.”
Between April 14 and June 14, the UPMC-Pitt team tested blood samples from 107 healthy volunteer health care workers and 489 immunocompromised patients who had completed COVID-19 vaccination as part of the COVID-19 Vaccine in the Immunocompromised Study (CoVICS). The immunocompromised patients included those who had a solid organ transplant, an autoimmune disorder, blood cancer, a solid tumor cancer, or HIV.
Antibodies are proteins that block invading pathogens—such as SARS-CoV-2, the virus that causes COVID-19—from infecting cells. They are produced by the immune system in response to vaccination.
While 98.1% of the health care workers produced antibodies after vaccination, only 37.2% of the vaccinated solid organ transplant patients were positive for antibodies; 54.7% of the blood cancer patients; 82.4% of those with solid tumor cancer; and 83.8% of patients with autoimmune disorders. In contrast, 94.6% of patients with HIV made antibodies.
Among patients with solid organ transplants, lung transplant patients had a particularly poor response to vaccination, with only 22.2% producing antibodies. Liver transplant patients fared best, with 60.6% of study participants producing antibodies after vaccination. Patients who received their transplant less than a year ago were less likely to respond to vaccination than those transplanted earlier.
In addition, cancer patients receiving radiation therapy and patients taking certain medications—such as antimetabolites for transplant and anti-CD20 monoclonal antibodies for autoimmune disorders—were more likely not to produce antibodies after COVID-19 vaccination.
The researchers then took blood sera, the amber-colored, protein-rich liquid that separates out when blood coagulates, from a subset of the participants—30 health care workers and 36 immunocompromised patients—and tested it for its ability to neutralize SARS-CoV-2 pseudovirus. They found that the ability of the participants’ sera to neutralize the virus correlated strongly with their antibody levels measured by an independent FDA-approved test.
“This is important because we’ve seen several studies indicating that immunocompromised people are less likely to produce antibodies in response to COVID-19 vaccination,” said senior author John Mellors, MD, chief of the Division of Infectious Diseases at UPMC and Distinguished Professor of Medicine at Pitt. “And we can assume this means they’re less likely to be able to fight the virus, but until we were able to test for virus neutralization, that was only an assumption. Our results give us more confidence in saying that people who do not produce antibodies truly are at greater risk of COVID-19 infection and that the level of antibodies produced is a proxy for ability to neutralize the virus.”
However, the physician-scientists stressed that the immune system has more tools than antibodies at its disposal, including T-cells and other immune cells, which may provide some level of immunity. So, while it is concerning when someone gets a negative antibody test, it is still possible for the immune system to fight off the virus, even if vaccination does not produce antibodies, Dr. Mellors said.
The Centers for Disease Control and Prevention, FDA, American Society of Transplantation, and several national cancer associations, among others, recommend against routine antibody testing after vaccination, and UPMC currently does not offer it outside of a research study or clinical indication.
“At this point, clinical advice does not change for immunocompromised people, whether an antibody test is positive or negative,” said Dr. Haidar. “They should still wear a mask in public, practice social distancing, get vaccinated and encourage those around them to be vaccinated. A positive antibody test does not give us certainty that they are protected against the virus, and the risk of COVID-19 causing serious complications and death still exists.”
Nanotechnology May Help Improve Organ Transplantation Outcomes

A nanoparticle technology created at Duquesne University for oxygen delivery could enable the preservation of organs prior to transplantations, according to a new study from McGowan Institute for Regenerative Medicine affiliated faculty members located at Duquesne and the Wake Forest Institute of Regenerative Medicine.
“One of the issues with transplants involves providing adequate oxygen to preserve tissues until the transplantation can take place,” said Vijay Gorantla, MD, PhD, associate professor at Wake Forest and a co-author of the 2019 study in Nanomedicine. “This has traditionally been done with the use of whole blood, but has several limitations, including stability, infectious risk and blood shortages.”
The Duquesne nanotechnology, the first to be created using pharmaceutical quality by design standards, uses nanoparticles as oxygen carriers. The goal is to improve the viability and function of organs or transplanted tissues, said Jelena Janjic, PhD, associate professor of pharmaceutics at Duquesne and founder and co-director of the university’s Chronic Pain Research Consortium.
“We have developed a stable, cost-effective nanomedicine that can carry oxygen to tissues, replacing the need for whole blood,” she said. “It also reduces the post-transplantation chance for infection.”
Dr. Janjic, who created the first inflammatory pain nanomedicine that directly targets the pain source, added that nanoparticles also performed well during the ischemic time involving transplantations.
“Usually, several hours elapse between the donor recovery and transplantation of an organ in the body,” Dr. Gorantla said. “This means that the tissue must remain healthy until the medical team and patient are ready for the transplantation. The oxygen delivery provided through nanoparticles helps to ensure tissue preservation.”
One of the benefits of the quality by design process is that the nanoparticles can be produced on a large scale, ensuring that supplies are readily available, Dr. Janjic said. She and Eric Lambert, a Duquesne pharmacy graduate student, recently published this work in Scientific Reports.
“Several technologies for oxygen delivery for organ preservation have been attempted over the past several decades,” Dr. Gorantla said. “This technology is very unique and cutting edge and has a real chance to help improve patient outcomes.”
In the future, the researchers hope to investigate how the technology may be able to deliver medicine to transplanted organs, such as anti-rejection drugs and immunosuppressive treatments required by patients.
Drs. Janjic, Gorantla and Mr. Lambert co-authored a paper on the oxygen delivery nanoparticles in a special issue of the journal Nanomedicine. The research is supported by a grant from the United States Air Force.
The study exemplifies Duquesne’s commitment to extensive research that expands student horizons and benefits society at large.
Illustration: McGowan Institute for Regenerative Medicine.
International Research Provides Insight into How Fibrosis Can Start and Become Devastating in Different Body Tissues

As a result of the COVID-19 pandemic, universities worldwide have been forced to reduce in-person interactions, altering how we connect and work with one another. The transition to a “virtual world” has been eye-opening, proving to many that research and innovation can continue, even in a remote setting.
For McGowan Institute for Regenerative Medicine affiliated faculty member Kris Dahl, PhD, professor of chemical engineering at Carnegie Mellon University (CMU), the pandemic has in part created both international and interdisciplinary research opportunities. In conjunction with the CMU Portugal Program, Dr. Dahl teamed up with João Sanches, PhD, associate professor at the Institute for Systems and Robotics (ISR), to co-advise Portuguese master’s student Maria Teresa Parreira on the analysis of data obtained during a study considering cellular monolayers of epithelium.
Epithelial layers line most of the exposed regions in our bodies, such as the lungs, digestive tract, genital tract, and to some degree, even our skin. When cells in the epithelial layer stiffen, it is often a sign that they have become cancerous or fibrotic. In a lab at CMU, Dr. Dahl and her group stiffened single cells within epithelial monolayers and examined the morphology and motion of surrounding cells. The data was collected in the form of images, then sent to Dr. Sanches and Ms. Parreira, who conducted morphometric analysis and temporal tracking to determine the results.
“We’ve always analyzed our data with respect to standard analysis techniques, but João and his team were able to show us brand-new techniques that we hadn’t used before,” said Dr. Dahl. “Everything was automated, and they had some very clever ideas on how to analyze the shape of these cells over time.”
Predictably, the stiffened cell was significantly altered; however, Dr. Dahl was surprised to find drastic changes in the cells radially outward from the stiff cell. The study revealed that cells are highly sensitive to the presence of mechanically impaired neighbors, leading to a generalized loss of coordination in collective cell migration. Normal cells within the monolayer appear to be dependent on the distance to the impaired cells, pointing to compensatory behavior in response to force transmission imbalances in the monolayer. The results suggest long-range impacts of single-cell defects related to tissue dysfunction and provide insight into how fibrosis can start and become so devastating in different body tissues.
“Many areas of the body have the ability to regenerate, but these areas lose that ability once they become fibrotic,” said Dr. Dahl. “By understanding how fibrosis is initiated, we can work on developing ways to interrupt that process.”
Dr. Dahl will present the team’s research at the upcoming European Society for Clinical Hemorheology and Microcirculation, International Society of Clinical Hemorheology, and International Society of Biorheology Joint Meeting on July 4-7, 2021.
In the future, Dr. Dahl hopes to build upon this research by collaborating with the University of Pittsburgh Medical Center (UPMC) to understand how cells react to stents placed in the body. When a patient experiences coronary artery issues, physicians perform a procedure called angioplasty to open narrow or blocked blood vessels that supply blood to the heart. Immediately following the surgery, a stiff metal mesh tube known as a stent is placed inside the blood vessel to keep it open, allowing blood to continue flowing. Due to a stent’s stiff properties, there is reason to believe they may significantly impact surrounding cells.
This paper was published in Cytoskeleton in 2021. Other authors include Kirill Lavrenyuk, doctoral student in Carnegie Mellon and the University of Pittsburgh’s joint Molecular Biophysics and Structural Biology Program.
UPMC, Pitt Pediatricians Make Rare Disease Breakthrough

It was a nagging mystery: A rare-disease expert at UPMC Children’s Hospital of Pittsburgh had found a successful treatment for two of the deadliest symptoms of one of the more common classes of rare diseases diagnosed by newborn screenings, but one symptom—painful episodes of muscle breakdown that land victims in intensive care—persisted.
The scientists’ paper in the journal Clinical & Translational Immunology announced that they’ve gotten to the bottom of the self-destructive syndrome and have a good lead on a treatment.
“These episodes looked a lot like inflammatory muscle disease, but usually that is persistent and doesn’t wax and wane, so it wasn’t a perfect fit,” said senior author Jerry Vockley, MD, PhD, chief of Genetic and Genomic Medicine and director of the Center for Rare Disease Therapy at UPMC Children’s and professor of pediatrics and human genetics at the University of Pittsburgh School of the Health Sciences. “Still, I couldn’t shake the thought that there was some inflammatory link, so we tested patient blood samples. Sure enough, when the episodes were happening, certain inflammatory markers were high, and when the patients were well, they were lower. Knowing this will allow us to try to figure out why this inflammation is happening and prevent it.”
Very long chain acyl-CoA dehydrogenase deficiency—VLCADD—is a genetic disease where a mutation prevents an enzyme from breaking down fatty acids into energy. It afflicts about one in 40,000 people but is part of a class of diseases that affect four times that number, making it one of the more common of the rare genetic diseases that U.S. doctors now universally test for in newborns.
If untreated, the disease can cause heart failure and low blood sugar—two life-threatening conditions. When coupled with a modified diet, a recently approved drug that Dr. Vockley was involved in developing can manage those symptoms. But patients still experience rhabdomyolysis—muscle breakdown—indicating that the disease acts through another pathway.
With his hunch that inflammation might be behind the rhabdomyolysis, Dr. Vockley consulted with Abbe de Vallejo, PhD, associate professor of pediatrics, immunology and rheumatology in Pitt’s School of Medicine and director of the Flow Cytometry Core Facility at the John G. Rangos Sr. Research Center located at UPMC Children’s. Dr. de Vallejo is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
Their teams tested patient blood samples that Dr. Vockley had stored from his previous research and obtained additional samples from new patients. One patient—Bella Linz, 15, of Meadville, Pa.—had particularly persistent bouts of rhabdomyolysis requiring week-long hospitalizations more than a dozen times per year. She consented to give blood samples over several years and participate in the study. Regular infusions of an anti-inflammatory medication kept her from needing to be hospitalized for nearly 10 months, and then only irregularly since.
“Bella is a very empathetic child, very caring,” said her mother, Carrie Linz. “So, when she was going through trying out the different medications and the pain that came with the shots, we’d talk about the other children this would help—how what she was doing would benefit other kids, quicker and for a longer period of time. That helped us find purpose.”
The team found that when the patients were experiencing episodes of rhabdomyolysis, they were having what’s known as a “cytokine storm”—when various inflammatory molecules are produced by immune cells in excessive quantities, leading them to attack the body. Even when they weren’t having these episodes, the patients had elevated inflammatory markers, though at lower numbers than when they were symptomatic.
Still, the solution isn’t so clear-cut, Dr. de Vallejo said.
“The cytokines activate cells, but those cells aren’t following the rules,” he said. “In the immune system, cytokines and cells ‘talk’ to each other to regulate and counter-regulate their actions accordingly. But that’s not happening the way you’d expect in VLCADD patients. It’s paradoxical and is our next challenge. If we can find the disconnect, we may be able to learn what is triggering the inflammatory response and stop it from happening altogether.”
In the meantime, the team also is exploring the off-label and compassionate use of certain anti-inflammatory medications to treat acute, symptomatic rhabdomyolysis and prevent it from occurring.
Pitt Scientists Who Regrew Retina Cells to Restore Vision in Tiny Fish Set Their Sights on Humans

For centuries, people thought that blindness was incurable – once the darkness sets in, it never retreats. Unlike in fish and frogs, the human retina doesn’t regenerate, and the vision loss caused by damage to cells in the back of the eye – be it genetic or physical – can rarely be fixed. But new research suggests that regrowing the retina may not be science fiction after all.
In a paper published in the Proceedings of the National Academy of Sciences, scientists at Pitt’s Department of Ophthalmology found that immune cells in the eyes of a zebrafish are indispensable for the regeneration of the retinal pigment epithelium, or RPE – a thin layer of flat, darkly colored cells that maintain the health of the light-sensing cells of the retina. In the future, the researchers hope to harness that feature to regenerate the RPE and restore sight in humans.
“We are finally beginning to understand the mechanism of retina and RPE regeneration,” said senior author Jeff Gross, PhD, the E. Ronald Salvitti Chair in Ophthalmology Research in the Department of Ophthalmology, University of Pittsburgh School of Medicine, and the Vice-Chair and Director of Research; the Director of the Louis J. Fox Center for Vision Restoration and of the Ocular Development, Disease, and Regeneration Laboratory at the University of Pittsburgh School of Medicine; and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “Even though there is a lot of work yet to be done, we are standing at the frontier of biology.”
It might come as a surprise to many, but the retina of zebrafish – those tiny blue-striped creatures so common in fish tanks in our homes, offices, and classrooms – is uncannily similar to the human retina, both in structure and in function.
Just like the human eye, the eye of a zebrafish is lined with RPE cells that serve many important functions, providing nutrients to the retina and maintaining the integrity, structure and function of cones and rods – the light-sensing cells that allow perception of colors and shades of gray.
In fact, RPE cells are so important that their damage causes injury to the light-sensing cells in turn. “Simply put, RPE cells are fascinating,” said lead author Lyndsay Leach, PhD, a research scientist at Pitt.
The scientists discovered that the immune system is intimately intertwined with the process of RPE regeneration in zebrafish. Using various genetic and imaging techniques, the researchers noticed that regeneration requires the activation of different immune cells, and that manipulating the immune pathway can determine if the regenerative process will succeed or fail.
The researchers hope to use that new knowledge to explore the possibility of reawakening the intrinsic but long-hibernating capacity of the human RPE cells to regenerate.
“We hope to leverage what we learned about RPE regeneration in zebrafish and use that information to develop new therapies in the human eye,” said Dr. Gross.
Perhaps, one day, the long-lost secrets of evolution can be used to cure human blindness.
AI in Critical Illness: Emergence and Emergent Issues

The International Conference on Complex Acute Illness (ICCAI) is the annual meeting of the Society for Complex Acute Illness (SCAI), which, since its inception over 15 years ago, has successfully bridged the domains clinical practice of critical care medicine and quantitative approaches to understanding critical illness, with a strong translational focus. On the methodological side, it has emphasized eclecticism, covering, through its interdisciplinary membership, modeling approaches ranging from pure mechanistic differential equations models and agent-based models to purely data-driven machine learning techniques. The meeting has a lot of local leadership: Drs. Gilles Clermont, Ruben Zamora, and Yoram Vodovotz are members of the organizing committee.
This program will be presented remotely. For additional information and the program, please see www.iccai.org.
AWARDS AND RECOGNITION
Dr. Stephen Badylak: Award Winning Paper

Each year the Annals of Biomedical Engineering journal recognizes the article published in the journal that has the most citations for all papers published in the preceding year. For 2020, the winning paper is “Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior” by Madeline C. Cramer and Stephen F. Badylak, DVM, PhD, MD. Dr. Badylak is a Deputy Director of the McGowan Institute for Regenerative Medicine, a Professor in the Department of Surgery, and Director of the Center for Pre-Clinical Tissue Engineering within the Institute. The award will be presented during the Biomedical Engineering Society (BMES) annual meeting, October 2021, with a $500 honorarium for the award.
The abstract of the winning paper follows:
Biologic scaffold materials composed of allogeneic or xenogeneic extracellular matrix (ECM) are commonly used for the repair and remodeling of injured tissue. The clinical outcomes associated with implantation of ECM-based materials range from unacceptable to excellent. The variable clinical results are largely due to differences in the preparation of the material, including characteristics of the source tissue, the method and efficacy of decellularization, and post-decellularization processing steps. The mechanisms by which ECM scaffolds promote constructive tissue remodeling include mechanical support, degradation and release of bioactive molecules, recruitment and differentiation of endogenous stem/progenitor cells, and modulation of the immune response toward an anti-inflammatory phenotype. The methods of ECM preparation and the impact of these methods on the quality of the final product are described herein. Examples of favorable cellular responses of immune and stem cells associated with constructive tissue remodeling of ECM bioscaffolds are described.
Congratulations, Dr. Badylak and Ms. Cramer!
Dr. Yoram Vodovotz Named Editor-in-Chief of Frontiers in Systems Biology

McGowan Institute for Regenerative Medicine faculty member Yoram Vodovotz, PhD, Professor in the Department of Surgery with secondary appointments in the Department of Computational & Systems Biology, the Department of Bioengineering, the Department of Immunology, the Department of Communication Science and Disorders (of the School of Health and Rehabilitation Science), and the Clinical and Translational Science Institute, and the Director of the Center for Inflammation and Regeneration Modeling at the McGowan Institute, is the Founding Field Chief Editor (editor-in-chief) of the Open Access journal, Frontiers in Systems Biology. The Scope and Mission of this journal are:
Research in biology and biomedicine is tackling more complex questions than ever before, due to the seemingly intractable nature of the urgent questions affecting us all. Addressing these issues requires an integrated, interdisciplinary approach, and yet much of research in the field of biology – systems biology included – remains entrenched in silos based on distinct disciplines and methods. Five sections currently comprise the Frontiers in Systems Biology ecosystem:
- Multiscale Mechanistic Modelling will highlight advances in computational modeling across biological scales
- Data and Model Integration will feature the emerging field of ‘omics integration as well as studies in which data-driven and mechanistic models combine to drive novel insights
- Translational Systems Biology and In Silico Trials will integrate disease modeling, quantitative systems pharmacology, and systems medicine to drive novel insights from bench to bedside
- Integrative Genetics and Genomics will be focused on the application of systems biology to gain insights into the genome
- Integrative Systems Immunology will highlight systems-level studies of immunology and inflammation
These sections are viewed as integral parts of a whole. The goal at Frontiers in Systems Biology is to live up to the promise of this discipline to integrate theory, experimentation, and practical application in an ethical and sustainable context.
Dr. Freddie Fu Receives EFORT Recognition for Contributions to the International Orthopaedic Community
 McGowan Institute for Regenerative Medicine faculty member Freddie Fu, MD, Professor and Chair of Orthopaedic Surgery at the University of Pittsburgh School of Medicine and orthopaedic surgeon at UPMC, was awarded honorary membership from The European Federation of National Associations of Orthopaedics and Traumatology (EFORT)—the platform organization linking Europe’s national orthopaedic associations aimed at promoting the exchange of scientific knowledge and experience with a particular emphasis on education and research.
McGowan Institute for Regenerative Medicine faculty member Freddie Fu, MD, Professor and Chair of Orthopaedic Surgery at the University of Pittsburgh School of Medicine and orthopaedic surgeon at UPMC, was awarded honorary membership from The European Federation of National Associations of Orthopaedics and Traumatology (EFORT)—the platform organization linking Europe’s national orthopaedic associations aimed at promoting the exchange of scientific knowledge and experience with a particular emphasis on education and research.
EFORT hosts an annual congress, during which a world-renowned leader in orthopaedic surgery is selected to present the Michael Freeman Honorary Lecture—this year, Dr. Fu was selected to speak.
In recognition of Dr. Fu’s popularity among orthopaedic surgeons and expertise in knee anatomy—particularly the ACL— he gave the lecture entitled, “Is The Latest Always The Greatest?” The presentation discussed innovation and the development of new technologies as necessary factors to advance healthcare and dissected the history of past innovations to reveal that many have been ineffective and at times harmful.
During the live lecture broadcast, EFORT leadership recognized Dr. Fu’s contributions to the international orthopaedic community with the EFORT Honorary Award and Membership. Dr. Fu was one of three distinguished surgeons to receive this honor, along with Dr. Gilles Walch (France) and Dr. Reinhard Windhager (Austria).
Over the duration of his career, Dr. Fu has been honored with more than 260 professional awards and honors, has delivered over 1,200 national and international presentations, co-authored 173 book chapters, wrote over 675 peer-reviewed articles, and edited 30 major orthopaedic textbooks. In 2019, the University of Pittsburgh was named the number one university in the world for ACL publications over a 40-year period, and Dr. Fu was named the most published author with 378 publications on the ACL.
Congratulations, Dr. Fu!
Dr. Rory Cooper Receives IEEE Honor

McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, FISA & Paralyzed Veterans of America Professor and Distinguished Professor of the Department of Rehabilitation Science & Technology, Professor of Bioengineering, Physical Medicine & Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh, Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories, is the recipient of the IEEE 2022 Award for Biomedical Engineering. He received this honor “for extensive contributions to wheelchair technology that have expanded mobility and reduced secondary injuries for millions of people with disabilities.”
Dr. Cooper notes, “Thank you for helping to make it possible for me to be the recipient of the 2022 award (medal) for Biomedical Engineering. It was a long team effort starting many years ago with Boy Scouts, SLOSH, US Army, VA GI-Bill, Cal Poly, Paralympics, UCSB, CSUS, and the University of Pittsburgh and VA. As well as many people along the way – then and now. Also, a special thanks to Paralyzed Veterans of America and to my family (especially my wife, Rosi) for always believing in me. This award is shared by my students (past and present), family, friends, and colleagues within the Human Engineering Research Laboratories.”
Each year the IEEE Awards Board recommends a select group of recipients to receive IEEE’s most prestigious honors. These are the men and women whose exceptional achievements and outstanding contributions have made a lasting impact on technology, society, and the engineering profession.
Dr. Victoria Webster-Wood Receives NSF 2021 CAREER Award

McGowan Institute for Regenerative Medicine affiliated faculty member Victoria Webster-Wood, PhD, an Assistant Professor of Mechanical Engineering with a courtesy appointment in Biomedical Engineering at Carnegie Mellon University, received a National Science Foundation (NSF) CAREER Award for her work on robotic design. Her research will focus on creating actuators for biohybrid robots using living muscle and controlling these actuators with neurons. Dr. Webster-Wood’s project uses animal-inspired designs to improve robotic mobility. She hopes that muscle-based robots will be more adaptable and self-healing. Her award also describes outreach programs to increase numbers of underrepresented groups in robotics.
An NSF CAREER Award is given to people early in their careers who are believed to play a part in furthering their area of science. The awards support their research and educational goals.
Congratulations, Dr. Webster-Wood!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#223 –– Dr. Samira Kiani discusses her research in gene editing as well as her work with the organization, Tomorrow.Life.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Edgar N. Tafaleng, Amitava Mukherjee, Aaron Bell, Kazutoyo Morita, Jorge Guzman-Lepe, Nils Haep, Rodrigo M. Florentino, Ricardo Diaz-Aragon, Carla Frau, Alina Ostrowska, Joshua R. Schultz, Paolo G. V. Martini, Alejandro Soto-Gutierrez, Ira J. Fox
Title: Hepatocyte Nuclear Factor 4 alpha 2 Messenger RNA Reprograms Liver-Enriched Transcription Factors and Functional Proteins in End-Stage Cirrhotic Human Hepatocytes
Summary: The only definitive therapy for end-stage liver disease is whole-organ transplantation. The success of this intervention is severely limited by the complexity of the surgery, the cost of patient care, the need for long-term immunosuppression, and the shortage of donor organs. In rodents and humans, end-stage degeneration of hepatocyte function is associated with disruption of the liver-specific transcriptional network and a nearly complete loss of promoter P1-driven hepatocyte nuclear factor 4-alpha (P1-HNF4α) activity. Re-expression of HNF4α2, the predominant P1-HNF4α, reinstates the transcriptional network, normalizes the genes important for hepatocyte function, and reverses liver failure in rodents. In this study, we tested the effectiveness of supplementary expression of human HNF4α2 messenger RNA (mRNA) in primary human hepatocytes isolated from explanted livers of patients who underwent transplant for end-stage irreversibly decompensated liver failure (Child-Pugh B, C) resulting from alcohol-mediated cirrhosis and nonalcoholic steatohepatitis. Re-expression of HNF4α2 in decompensated cirrhotic human hepatocytes corrects the disrupted transcriptional network and normalizes the expression of genes important for hepatocyte function, improving liver-specific protein expression. End-stage liver disease in humans is associated with both loss of P1-HNF4α expression and failure of its localization to the nucleus. We found that while HNF4α2 re-expression increased the amount of P1-HNF4α protein in hepatocytes, it did not alter the ability of hepatocytes to localize P1-HNF4α to their nuclei. Conclusion: Re-expression of HNF4α2 mRNA in livers of patients with end-stage disease may be an effective therapy for terminal liver failure that would circumvent the need for organ transplantation. The efficacy of this strategy may be enhanced by discovering the cause for loss of nuclear P1-HNF4α localization in end-stage cirrhosis, a process not found in rodent studies.
Source: Hepatology Communications. 01 July 2021. Online ahead of print.
GRANT OF THE MONTH
PI: Kacey Marra
Title: Development of an Implantable Medical Device for Human Extremity Nerve Injuries
Description: Arm, leg, and nerve-related injuries are among the most common injuries sustained by our brave Warfighters. Battlefield extremity injuries are often more severe than civilian, thus highlighting a need for therapies that treat extensive peripheral nerve injuries. Most challenging of these are the healing of long-gap peripheral nerve injuries (i.e., nerve gaps greater than 1 inch). When there is a long-gap nerve injury, the current solution is to relocate a nerve from one part of the body to where the nerve gap exists, a surgical practice known as “autograft.” This treatment involves secondary concerns of additional surgical incisions and complications such as infection, prolonged operating times, longer anesthesia burden; and potential effects of permanent numbness where the nerve was removed. Additionally, autografting may not be an option for everyone, as patients with multiple injuries may not have access to acquire a “donor” nerve from an uninjured extremity. There are a few medical devices, known as nerve guides or tubes, that can regrow nerve gaps less than 1 inch long; however, they are not FDA-approved for use in long nerve gaps. Indeed, the need for a commercially available nerve guide to repair peripheral nerve gaps is significant. This need is addressed in our current work of a creative of an off-the-shelf nerve guide, called AxoMax nerve guide. Our patented technology utilizes a tube that can safely break down in the body, and also contains a controlled drug delivery system that gives the cues necessary for the regrowth of nerves over long gaps. We have conducted both large and small animal studies that demonstrate AxoMax can heal these nerve gaps and now plan to safely demonstrate this action in humans. This project addresses the following Topic Area: Sustained-Release Drug Delivery, within this Area of Encouragement: Research into techniques to provide sustained release of drugs in tissue repair applications, such as bone or nerve regeneration.
This project will allow the team of doctors and scientists to use an established current Good Manufacturing Practices (cGMP) laboratory at the Mayo Clinic to prepare AxoMax guides that will be used in future clinical trials. In order to test the nerve guides in humans, we will need to get regulatory approval from the FDA. In order to obtain FDA approval, we need to develop a reproducible fabrication protocol for large inner diameter AxoMax nerve guides of varying length. We have created an automated machine that we have been using to reproducibly manufacture nerve guides. This machine can be transferred to the Mayo Clinic’s cGMP facility for the large-scale production of nerve guides. In Aim 1, we will validate the machine using the aforementioned mechanical characterization, biocompatibility assays, and release kinetics to be performed on the guide. We will complete cGMP scale-up and process validation and manufacture nerve guides at the cGMP facility for biocompatibility tests, sterilization validation and packaging validation, and shelf-life tests. In Aim 2, we will validate the design process for large nerve guides following FDA regulations and assemble a Design History File. There are numerous issues to address prior to applying for regulatory approval. This includes such quality management activities like meeting design inputs with design processes and design outputs, and most importantly, meeting user needs with validation data, thereby necessitating an Investigational Device Exemption (IDE)-based clinical investigation. Thus, in Aim 2 will validate the nerve guide design in the clinical setting, assessing user specification using the larger nerve guides.
We have been developing this off-the-shelf nerve guide for over 10 years and have established the efficacy of the nerve guide in a non-human primate nerve defect model, successfully bridging a 2-inch nerve gap. We are deeply committed to examine the nerve guide in humans immediately following the completion of this 2-year technology development project. Our preclinical data can support basic device functionality and address significant safety concerns for this device. We will further refine and develop the AxoMax nerve guide so that it can be examined in a clinical trial, followed by future commercialization.
Source: DoD CDMRP PRMRP TTDA
Term: July 1, 2021-June 30, 2023
Amount: $2,085,067
