November 2014 | VOL. 13, NO. 11 | www.McGowan.pitt.edu
Team Discovers Stem Cells in the Esophagus—Could Lead to New Models to Study Esophageal Disease
 Despite previous indications to the contrary, the esophagus does have its own pool of stem cells, said senior investigator Eric Lagasse, PharmD, PhD, associate professor of pathology, Pitt School of Medicine, and director of the Cancer Stem Cell Center at the McGowan Institute for Regenerative Medicine, and researchers from the University of Pittsburgh School of Medicine in an animal study published online in Cell Reports. The findings could lead to new insights into the development and treatment of esophageal cancer and the precancerous condition known as Barrett’s esophagus.
Despite previous indications to the contrary, the esophagus does have its own pool of stem cells, said senior investigator Eric Lagasse, PharmD, PhD, associate professor of pathology, Pitt School of Medicine, and director of the Cancer Stem Cell Center at the McGowan Institute for Regenerative Medicine, and researchers from the University of Pittsburgh School of Medicine in an animal study published online in Cell Reports. The findings could lead to new insights into the development and treatment of esophageal cancer and the precancerous condition known as Barrett’s esophagus.
According to the American Cancer Society, more than 18,000 people will be diagnosed with esophageal cancer in the U.S. in 2014 and almost 15,500 people will die from it. In Barrett’s esophagus, the lining of the esophagus changes for unknown reasons to resemble that of the intestine, though gastro-esophageal reflux disease or GERD is a risk factor for its development.
“The esophageal lining must renew regularly as cells slough off into the gastrointestinal tract,” said Dr. Lagasse. “To do that, cells in the deeper layers of the esophagus divide about twice a week to produce daughter cells that become the specialized cells of the lining. Until now, we haven’t been able to determine whether all the cells in the deeper layers are the same or if there is a subpopulation of stem cells there.”
The research team grew pieces or “organoids” of esophageal tissue from mouse samples, and then conducted experiments to identify and track the different cells in the basal layer of the tissue. They found a small population of cells that divide more slowly, are more primitive, can generate specialized or differentiated cells, and have the ability to self-renew, which is a defining trait of stem cells.
“It was thought that there were no stem cells in the esophagus because all the cells were dividing rather than resting or quiescent, which is more typical of stem cells,” Dr. Lagasse noted. “Our findings reveal that there indeed are esophageal stem cells, and rather than being quiescent, they divide slowly compared to the rest of the deeper layer cells.”
In future work, the researchers will examine human esophageal tissues for evidence of stem cell dysfunction in Barrett’s esophagus disease.
“Some scientists have speculated that abnormalities of esophageal stem cells could be the origin of the tissue changes that occur in Barrett’s disease,” Dr. Lagasse said. “Our current and future studies could make it possible to test this long-standing hypothesis.”
Upcoming Events
Computational Modeling: From Clinical Needs to Bench Results
 December 15, 2014; 8 AM – 4 PM
December 15, 2014; 8 AM – 4 PM
Room S100-Biomedical Science Tower
Yoram Vodovotz, PhD
The workshop is designed to bring together leading local academic physicians, translational researchers, and members of industry in order to share advances in the clinically-driven applications of computational modeling. Participants will be exposed to cutting-edge presentations regarding advances in computational modeling in biomarker discovery, personalized diagnostics, and drug design.
The program includes:
- Morning Keynote: Translational Systems Biology: Roadmap for the Future of Biomedical Research
- Session I: An Overview of Clinical Questions and Computational Successes
- Session II: Computational Modeling for Pre-Clinical Targets
- Session III: Interdisciplinary collaborations and practical considerations
- Afternoon Keynote: Computational biology and personalized medicine at Pitt and UPMC
Seating is LIMITED; please register HERE to reserve your place for this exciting program.
RESOURCES AT THE MCGOWAN INSTITUTE
December Special at the Histology Lab
Ho Ho Herovici!!
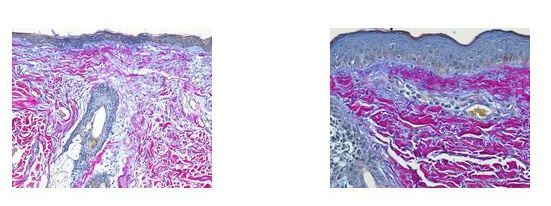
The Herovici combination of picro methyl blue or aniline blue and picro acid fuchsin in proper proportion, colors Type I collagen, mature dense collagen red, while reticulum and newly formed Type III collagen, stains blue.
The holidays are a magical time for collagen of all ages! Bring your samples to the McGowan Histology Lab and receive a 30% discount on all Herovici Stains for the entire month of December.
Happy Holidays Collagen! Let’s paint the town Red… or Blue.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or call 412-624-5265
As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
Pitt, Carnegie Mellon Engineers Develop New Method to Explore Mechanical Communication Between Cells
 When the body forms new tissues during the healing process, cells must be able to communicate with each other. For years, scientists believed this communication happened primarily through chemical signaling. Now researchers at Carnegie Mellon University and the University of Pittsburgh have found that another dimension – mechanical communication – is equally if not more crucial. The findings, published in the Proceedings of the National Academy of Sciences, could lead to advancements in treatments for birth defects and therapies for cancer patients.
When the body forms new tissues during the healing process, cells must be able to communicate with each other. For years, scientists believed this communication happened primarily through chemical signaling. Now researchers at Carnegie Mellon University and the University of Pittsburgh have found that another dimension – mechanical communication – is equally if not more crucial. The findings, published in the Proceedings of the National Academy of Sciences, could lead to advancements in treatments for birth defects and therapies for cancer patients.
“It’s like 19th century scientists discovering that electricity and magnetism were the same force,” said McGowan Institute for Regenerative Medicine affiliated faculty member Lance Davidson, PhD, associate professor and Wellington C. Carl Faculty Fellow of Bioengineering at the University of Pittsburgh, who co-led the study. “The key here is using mechanical engineering tools and frameworks to reverse-engineer how these biological systems work, thereby giving us a better chance to develop methods that affect this cellular communication process and potentially treat various diseases related to tissue growth.”
“We answered this very important biological question by building a new tool that enabled us to see these mechanical processes at the cellular level,” said McGowan Institute for Regenerative Medicine affiliated faculty member Philip LeDuc, PhD, professor of Mechanical Engineering at Carnegie Mellon, who co-led the study with Dr. Davidson. The researchers developed a microfluidic control system that delivers chemicals at extremely low flow rates over very small, specific areas, such as integrated collections of individual cells. They hypothesized that in addition to using chemical signals to communicate with each other, embryonic or regenerative cells also used mechanical processes – pushing and pulling on each other – to stimulate and respond.
“In order to identify these mechanical processes, we really had to control small parts of a multi-cellular tissue, which today’s technology can finally allow us to do,” Dr. Davidson explained. For example, a tissue sample 2 millimeters across may contain up to 8,000 cells. The microfluidic device enables researchers to “touch” as few as three or four and view the mechanical processes using a high resolution laser scanning microscope to view proteins moving in cells.
“We proved that mechanical processes are absolutely important along with chemical,” Dr. LeDuc said. When the researchers disabled the mechanical connections between the cells using microfluidics, the ability of cells to communicate with each other dropped substantially. Although the cells communicated through chemical signaling as well, the cells’ mechanical connections – their ability to push and pull on each other – were dominant in transmitting the signals.
Understanding this additional dimension could impact future research in tissue regeneration, from embryonic development to healing to cancer growth.
“If you are dealing with someone who has a birth defect, and their heart didn’t form correctly, the question is how do you target it?” Dr. LeDuc asked. “This discovery leads us to believe there is a mechanical way to influence tissue development and one day help the cells better communicate with each other to heal the body.”
Creating Medical Devices with Dissolving Metal
 University of Pittsburgh researchers recently received another $1.5 million from the National Science Foundation to continue a combined multi-university, private-industry effort to develop implantable medical devices made from biodegradable metals.
University of Pittsburgh researchers recently received another $1.5 million from the National Science Foundation to continue a combined multi-university, private-industry effort to develop implantable medical devices made from biodegradable metals.
Body-degradable metals—usually magnesium based—are not new, having been originally considered in the late 19th century. “But,” says McGowan Institute for Regenerative Medicine director William Wagner, PhD, deputy director of the project and a principal investigator, “the question comes when you start to design medical devices for a specific application and a clinical partner says, ‘We want that to be gone in a month, or a month-and-a-half, or we want that to be there for a year.’” Then you have to figure out how to meet those specifications, he says.
To that end, the University of Pittsburgh team as well as collaborators at the University of Cincinnati (UC) and North Carolina Agricultural and Technical State University (N.C. A&T) are creating new alloys and new manufacturing processes that suit clinical demands. The consortium seeks to design devices that can adapt to changes in a patient’s body and dissolve once healing has occurred, reducing the follow-up procedures and potential complications of major orthopedic, craniofacial, and cardiovascular procedures, and sparing millions of patients worldwide added pain and medical expenses.
Thus far, the consortium has created novel screws and plates for facial reconstruction, a stent to be used in kidney dialysis, a nerve guide, and a ring that will assist in pulling together and healing ruptured ligaments. The group has also created a tracheal stent for pediatric patients whose tracheas are underdeveloped at birth and prone to collapse. Once the stent is implanted, Dr. Wagner—professor of surgery, bioengineering, and chemical engineering in Pitt’s School of Medicine and Swanson School of Engineering—says it would dissolve, obviating the need for a second procedure on the young patient.
The universities have also involved several private enterprises in the project, including InCube Labs, nanoMag-Thixomat, ACell, OrthoKinetic Technologies, Fort Wayne Metals, General Nano, and IonBond.
Grant funding has also allowed Pitt to help N.C. A&T establish the first degree-granting program in bioengineering at a Historically Black College and University with the assistance of faculty members in Pitt’s Department of Bioengineering and McGowan Institute for Regenerative Medicine. The program currently offers bachelor’s and master’s degrees and ultimately aims to offer a PhD.
“And there has been a lot of student exchange between the universities,” Dr. Wagner says. “It has been a good exercise for the trainees involved, as it spans the individual universities and reaches into active collaborations.”
In addition to undergraduate and graduate degrees, the research team, via the grant, has advanced STEM education to K-12 students in the Greensboro, North Carolina, Cincinnati, Ohio, and Western Pennsylvania regions by converting its work into a curriculum for aspiring engineers, with particular attention given to underrepresented groups. K-12 teachers are also invited to spend summer rotations in faculty-member laboratories to further broaden the transfer of scientific knowledge from the research effort.
Unraveling Traumatic Brain Injuries
 University of Pittsburgh researchers—including McGowan Institute for Regenerative Medicine affiliated faculty members Stephen Wisniewski, PhD, senior associate dean and co-director of the Epidemiology Data Center at the University of Pittsburgh Graduate School of Public Health, and David Okonkwo, MD, PhD, associate professor of neurological surgery and clinical director of the Brain Trauma Research Center at Pitt’s School of Medicine—are key players in a national “dream team” that seeks to identify the best biological and imaging markers of traumatic brain injury (TBI) to improve the ability of clinical trials to find effective treatments for the condition, which annually affects 2.5 million people in the U.S., including athletes and soldiers.
University of Pittsburgh researchers—including McGowan Institute for Regenerative Medicine affiliated faculty members Stephen Wisniewski, PhD, senior associate dean and co-director of the Epidemiology Data Center at the University of Pittsburgh Graduate School of Public Health, and David Okonkwo, MD, PhD, associate professor of neurological surgery and clinical director of the Brain Trauma Research Center at Pitt’s School of Medicine—are key players in a national “dream team” that seeks to identify the best biological and imaging markers of traumatic brain injury (TBI) to improve the ability of clinical trials to find effective treatments for the condition, which annually affects 2.5 million people in the U.S., including athletes and soldiers.
The $17 million initiative, called the TBI Endpoints Development (TED) Award, is funded by the U.S. Department of Defense (DOD) and includes many universities, the U.S. Food and Drug Administration (FDA), companies, and philanthropies. It is overseen by the University of California, San Francisco (UCSF).
“This project is going to redefine how we measure the outcomes for traumatic brain injury studies,” said TED investigator Dr. Wisniewski. “We need a more robust, detailed way to determine what challenges a person faces when he suffers a traumatic brain injury, and that is what we’re setting out to accomplish with this ambitious study.”
Under Dr. Wisniewski’s leadership, Pitt Public Health will run the data analysis for the project, meaning the school will compile data from previous studies and analyze it to see what existing methods for measuring traumatic brain injuries prove most promising. That information will be used as a launch point for clinical evaluation in real-life situations.
Dr. Okonkwo is co-leading the second branch of the project to test those findings through the previously announced $18.8 million National Institutes of Health (NIH) project called Transforming Research and Clinical Knowledge in TBI, or TRACK-TBI.
The project is specifically designed to overcome the difficulty in demonstrating the effectiveness of TBI drugs and medical devices by actively involving the FDA in clinical-trial design from the outset. It also fosters collaboration between the DOD, the NIH, foundation-funded research networks, industry co-sponsors such as General Electric, and patient advocacy groups to try to develop procedures, outcomes measures, and standards for interpreting clinical data.
“TBI is really a multifaceted condition, not a single event,” said UCSF neurosurgeon Geoffrey T. Manley, MD, PhD, principal investigator for the new award and chief of neurosurgery at San Francisco General Hospital and Trauma Center, a UCSF partner hospital. “TBI lags 40 to 50 years behind heart disease and cancer in terms of progress and understanding of the actual disease process and its potential aftermath. More than 30 clinical trials of potential TBI treatments have failed, and not a single drug has been approved.”
New Processes Could Provide Personalized Pain Treatment
 As reported by Karen Ferrick-Roman, Duquesne University Fall 2014 Magazine, pain costs Americans up to $635 billion each year for medical treatment and in lost productivity, says the Institute of Medicine of the National Academies. That hefty price tag is equivalent to the first 10 years of spending for homeland security, illustrating a nationwide problem of giant proportions. Today, an estimated 116 million Americans live with chronic pain.
As reported by Karen Ferrick-Roman, Duquesne University Fall 2014 Magazine, pain costs Americans up to $635 billion each year for medical treatment and in lost productivity, says the Institute of Medicine of the National Academies. That hefty price tag is equivalent to the first 10 years of spending for homeland security, illustrating a nationwide problem of giant proportions. Today, an estimated 116 million Americans live with chronic pain.
With the goal to introduce new ideas into pain research and bring people together across disciplines who typically would not work on pain or even collaborate, the Chronic Pain Research Consortium was co-founded on May 16, 2011, at Duquesne University by McGowan Institute for Regenerative Medicine affiliated faculty members Jelena Janjic, PhD, Assistant Professor of Pharmaceutics in the Graduate School of Pharmaceutical Sciences and Mylan School of Pharmacy at Duquesne University, and John Pollock, PhD, Full Professor of Biological Science at Duquesne University, out of love and passion for patient care, multidisciplinary science, and a holistic view of life and medicine. Since that time, Duquesne University scientists study all aspects of chronic pain, from molecular mechanisms to mind-body interaction and psychological impact of pain on relationships and quality of life. The team includes pharmacists, nurses, physical and occupational therapists, neurobiologists, neuropharmacologists, molecular biologists, medicinal chemists, and pharmacuetical scientists.
Most pain studies focus only on biology or behavior, drug delivery or microscopic cell anatomy, but the research of Drs. Janjic and Pollock incorporates each of these areas to personalize pain treatment. Their methods could enable doctors to pinpoint where pain is originating, then provide medication to that precise location—allowing a smaller dose of medication to be effective in curtailing pain while creating few side effects. This could be a breakthrough for treating pain, Dr. Pollock explains, because soreness in one location might actually be caused by a pinched nerve or issue elsewhere.
“The process relies on the interplay between the immune system and the nervous system to work,” he says.
The first step is to envision the pain with Drs. Janjic and Pollock’s non-invasive fluorescence imaging and MRI imaging achieved in collaboration with Carnegie Mellon University, pinpointing the origins of pain-inducing inflammation. Next, researchers specifically locate the immune cells involved in pain, then target them with medication.
Dr. Janjic has pioneered targeted drug delivery to treat pain, developing nanodroplets that, when injected intravenously, accumulate at the inflamed areas. This process could have a huge impact for people with inflammation-related pain, including osteoporosis and arthritis.
“We’re helping to fulfill a completely unmet need in pain research,” says Dr. Janjic.
UPMC Voice Center: A Unique Resource
 Dedicated to the evaluation and care of voice disorders, the UPMC Voice Center offers a wide range of services and is a unique resource for both the general public and those who use their voices professionally. Its mission is to prevent injury and rehabilitate patients who have voice problems and to provide cutting edge research. The Voice Center is staffed by a select group of medical experts—including McGowan Institute for Regenerative Medicine affiliated faculty member Clark Rosen, MD, director of the UPMC Voice Center and professor of otolaryngology in the University of Pittsburgh School of Medicine—who utilize state-of-the-art technology, including a voice analysis laboratory, to provide patients with a wide range of medical, surgical, and behavioral treatments for all voice disorders.
Dedicated to the evaluation and care of voice disorders, the UPMC Voice Center offers a wide range of services and is a unique resource for both the general public and those who use their voices professionally. Its mission is to prevent injury and rehabilitate patients who have voice problems and to provide cutting edge research. The Voice Center is staffed by a select group of medical experts—including McGowan Institute for Regenerative Medicine affiliated faculty member Clark Rosen, MD, director of the UPMC Voice Center and professor of otolaryngology in the University of Pittsburgh School of Medicine—who utilize state-of-the-art technology, including a voice analysis laboratory, to provide patients with a wide range of medical, surgical, and behavioral treatments for all voice disorders.
Voice disorders are often signs of other medical problems. In order to offer a truly comprehensive approach to the diagnosis and treatment of even the most complex voice disorders, the Voice Center staff consults with specialists in gastroenterology, internal medicine, neurology, psychiatry, psychology, physical therapy, and other disciplines.
To treat voice disorders, the Voice Center offers a combination of behavioral, medical, and surgical approaches:
- Thyroplasty, arytenoid adduction, and vocal cord augmentation—to restore your voice following vocal cord paralysis
- Vocal cord microsurgery—specialized microsurgery to treat vocal cord lesions such as polyps, cysts, and scars
- BOTOX® injections of vocal cords—to reduce tight, squeezed phonation and voice breaks caused by spasmodic dysphonia or essential tremor
- Laryngeal reconstruction—to repair a narrowed or injured throat or windpipe
- Laryngeal microspot laser surgery—a precise laser treatment of vocal cord problems such as bleeding
- Voice therapy—to rehabilitate and maximize the physiology of voice production
- Singing voice therapy—to rehabilitate the injured singing voice
The Voice Center also provides voice health care services for professional and amateur vocal performance groups such as the Pittsburgh Opera as well as performing arts centers at regional high schools and universities. To provide these health care services, the Voice Center at UPMC Mercy brings together two teams of surgeons and voice therapists. Their goal is to keep professionals like singers, teachers, and public speakers out of the operating room. And Dr. Rosen says Pittsburgh is once again leading the way.
“That combination of having those two disciplines…is unique in Western PA and is very rare throughout the world,” said Dr. Rosen.
AWARDS AND RECOGNITIONS
Dr. Anna Balazs Recognized by Pittsburgh WCC for Excellence in the Chemical Sciences
 McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, was selected as this year’s recipient of the Greater Pittsburgh Area Women Chemists Committee’s (WCC) Award for Excellence in the Chemical Sciences. The award “recognizes the achievements of female chemists and chemical engineers in the greater Pittsburgh area who have a record of accomplishment in their field.” According to the committee’s chair, Dr. Balazs’ packet was “nothing short of extraordinary.” Dr. Balazs was recognized at the Awards Banquet of the 2014 American Chemical Society Central Regional Meeting (CERM 2014) held in Pittsburgh on October 30, 2014.
McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, was selected as this year’s recipient of the Greater Pittsburgh Area Women Chemists Committee’s (WCC) Award for Excellence in the Chemical Sciences. The award “recognizes the achievements of female chemists and chemical engineers in the greater Pittsburgh area who have a record of accomplishment in their field.” According to the committee’s chair, Dr. Balazs’ packet was “nothing short of extraordinary.” Dr. Balazs was recognized at the Awards Banquet of the 2014 American Chemical Society Central Regional Meeting (CERM 2014) held in Pittsburgh on October 30, 2014.
Dr. Balazs is a Distinguished Professor of Chemical Engineering and the Robert v. d. Luft Professor, Department of Chemical & Petroleum Engineering, University of Pittsburgh. She also serves as an Adjunct Professor in Pitt’s Department of Chemistry.
Dr. Balazs received a MS from the Massachusetts Institute of Technology (MIT) and went on to earn her PhD from the same university. Her postdoctoral research was completed at Brandeis University, MIT, and the University of Massachusetts. She has also held the position of visiting professor at the Scripps Research Institute in Southern California, the University of Texas at Austin, and Oxford University in the United Kingdom.
The research interests of Dr. Balazs center on statistical, mechanical, and computer modeling of complex chemical systems and developing theories for the properties of polymer blends and the behavior of polymers at surfaces and interfaces.
Book Release
 Congratulations to McGowan Institute for Regenerative Medicine faculty member Yoram Vodovotz, PhD, a co-author of the recently released book, “Translational Systems Biology: Concepts and Practice for the Future of Biomedical Research.” The text provides a foundational description of the barriers facing biomedical research today and the immediate future, and how these barriers could be overcome through the adoption of a robust and scalable approach that will form the underpinning of biomedical research for the future. Dr. Vodovotz is a Professor in the Department of Surgery with secondary appointments in the Departments of Computational & Systems Biology, Bioengineering, Immunology, Communication Science and Disorders, and the Clinical and Translational Science Institute. He also is the Director of the Center for Inflammation and Regenerative Modeling at the McGowan Institute.
Congratulations to McGowan Institute for Regenerative Medicine faculty member Yoram Vodovotz, PhD, a co-author of the recently released book, “Translational Systems Biology: Concepts and Practice for the Future of Biomedical Research.” The text provides a foundational description of the barriers facing biomedical research today and the immediate future, and how these barriers could be overcome through the adoption of a robust and scalable approach that will form the underpinning of biomedical research for the future. Dr. Vodovotz is a Professor in the Department of Surgery with secondary appointments in the Departments of Computational & Systems Biology, Bioengineering, Immunology, Communication Science and Disorders, and the Clinical and Translational Science Institute. He also is the Director of the Center for Inflammation and Regenerative Modeling at the McGowan Institute.
 The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#141 – Mr. Patrick Cantini is the Director of Strategic Scientific Collaborations for the McGowan Institute for Regenerative Medicine. Mr. Cantini discusses the results of recent military-related studies.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
Picture of the Month
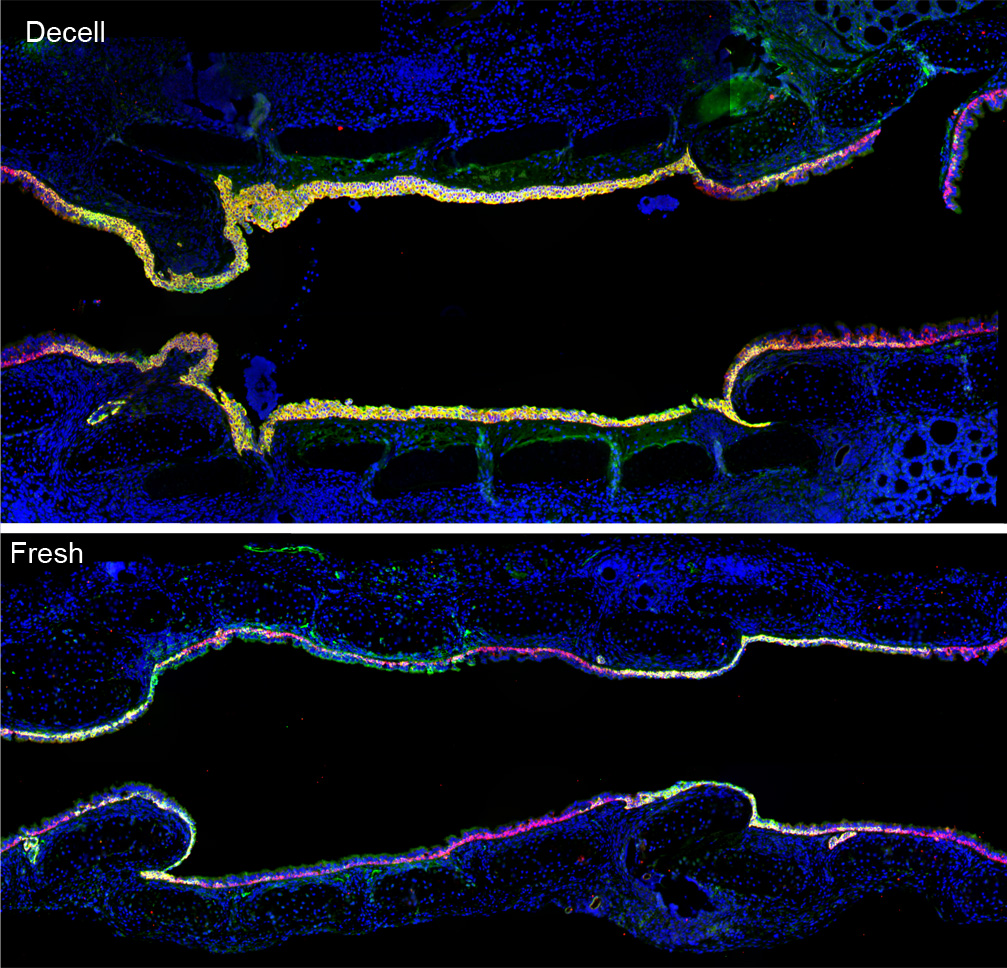
Image adapted from Kutten JC, McGovern D, Hobson CM, Luffy SA, Nieponice A, Tobita K, Francis RJ, Reynolds SD, Isenberg JS, Gilbert TW. “Decellularized Tracheal Extracellular Matrix Supports Epithelial Migration, Differentiation, and Function.” Tissue Engineering Part A, 2014 [epub ahead of print]. doi:10.1089/ten.tea.2014.0089.
