July 2014 | VOL. 13, NO. 7 | www.McGowan.pitt.edu
Stem Cells from Muscle Can Repair Nerve Damage After Injury
 Stem cells derived from human muscle tissue were able to repair nerve damage and restore function in an animal model of sciatic nerve injury, according to McGowan Institute for Regenerative Medicine faculty members Johnny Huard, PhD, Professor in the Departments of Orthopaedic Surgery, Microbiology and Molecular Genetics, Bioengineering, Pathology, Pediatrics, and Physical Medicine and Rehabilitation and the Director of the Stem Cell Research Center, and Donna Stolz, PhD, Associate Director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and Associate Professor in the Departments of Cell Biology and Pathology at the University of Pittsburgh, and researchers at the University of Pittsburgh School of Medicine. The findings, published in the Journal of Clinical Investigation, suggest that cell therapy of certain nerve diseases, such as multiple sclerosis, might one day be feasible.
Stem cells derived from human muscle tissue were able to repair nerve damage and restore function in an animal model of sciatic nerve injury, according to McGowan Institute for Regenerative Medicine faculty members Johnny Huard, PhD, Professor in the Departments of Orthopaedic Surgery, Microbiology and Molecular Genetics, Bioengineering, Pathology, Pediatrics, and Physical Medicine and Rehabilitation and the Director of the Stem Cell Research Center, and Donna Stolz, PhD, Associate Director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and Associate Professor in the Departments of Cell Biology and Pathology at the University of Pittsburgh, and researchers at the University of Pittsburgh School of Medicine. The findings, published in the Journal of Clinical Investigation, suggest that cell therapy of certain nerve diseases, such as multiple sclerosis, might one day be feasible.
To date, treatments for damage to peripheral nerves, which are the nerves outside the brain and spinal cord, have not been very successful, often leaving patients with impaired muscle control and sensation, pain and decreased function, said senior author Dr. Huard.
“This study indicates that placing adult, human muscle-derived stem cells at the site of peripheral nerve injury can help heal the lesion,” Dr. Huard said. “The stem cells were able to make non-neuronal support cells to promote regeneration of the damaged nerve fiber.”
The researchers, led by Dr. Huard and Mitra Lavasani, PhD, first author and Assistant Professor of Orthopaedic Surgery, Pitt School of Medicine, cultured human muscle-derived stem/progenitor cells in a growth medium suitable for nerve cells. They found that, with prompting from specific nerve-growth factors, the stem cells could differentiate into neurons and glial support cells, including Schwann cells that form the myelin sheath around the axons of neurons to improve conduction of nerve impulses.
In mouse studies, the researchers injected human muscle-derived stem/progenitor cells into a quarter-inch defect they surgically created in the right sciatic nerve, which controls right leg movement. Six weeks later, the nerve had fully regenerated in stem-cell treated mice, while the untreated group had limited nerve regrowth and functionality. Twelve weeks later, treated mice were able to keep their treated and untreated legs balanced at the same level while being held vertically by their tails. When the treated mice ran through a special maze, analyses of their paw prints showed eventual restoration of gait. Treated and untreated mice experienced muscle atrophy, or loss, after nerve injury, but only the stem cell-treated animals had regained normal muscle mass by 72 weeks post-surgery.
“Even 12 weeks after the injury, the regenerated sciatic nerve looked and behaved like a normal nerve,” Dr. Lavasani said. “This approach has great potential for not only acute nerve injury, but also conditions of chronic damage, such as diabetic neuropathy and multiple sclerosis.”
Drs. Huard and Lavasani and the team are now trying to understand how the human muscle-derived stem/progenitor cells triggered injury repair, as well as developing delivery systems, such as gels, that could hold the cells in place at larger injury sites.
RESOURCES AT THE MCGOWAN INSTITUTE
August Special at the Histology Lab
There are two ways that a cell can die: necrosis and apoptosis. Necrosis occurs when a cell is damaged by an external force, such as poison, a bodily injury, an infection or ischemia (lack of blood flow/oxygen) such as would occur during a heart attack or stroke.
Apoptosis, on the other hand, is when a cell commits suicide. It is sometimes referred to as programmed cell death.
Apoptosis is a form of cell death that eliminates compromised or superfluous cells (in other words, “maintenance”). It is controlled by multiple signaling and effector pathways that mediate active responses to external growth, survival, or death factors.
One routinely used method for identifying apoptosis via DNA fragmentation is the TUNEL assay. This technique can detect early-stage apoptosis in systems where chromatin condensation has begun and strand breaks are fewer, even before the nucleus undergoes major morphological changes.
The McGowan Institute Histology Core Laboratory is offering 30% off Tunel staining for the month of August with mention of this ad. Contact Lori Walton (perezl@upmc.edu, 412-624-5265) at the McGowan Core Histology Lab and ask about our staining specials.
As always, you will receive the highest quality histology in the quickest turn-around time. Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
Dr. Anna Balazs’ Work Featured on the Cover of Journal of Physical Chemistry Letters
 The research work of McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical Engineering and the Robert v. d. Luft Professor, Department of Chemical & Petroleum Engineering, University of Pittsburgh, was featured on the May 15, 2014, edition cover of the Journal of Physical Chemistry Letters. The cover art corresponds to her paper entitled “Designing Bioinspired Artificial Cilia to Regulate Particle-Surface Interactions.”
The research work of McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical Engineering and the Robert v. d. Luft Professor, Department of Chemical & Petroleum Engineering, University of Pittsburgh, was featured on the May 15, 2014, edition cover of the Journal of Physical Chemistry Letters. The cover art corresponds to her paper entitled “Designing Bioinspired Artificial Cilia to Regulate Particle-Surface Interactions.”
The paper’s abstract reads:
- Biological cilia play a critical role in a stunning array of vital functions, from enabling marine organisms to trap food and expel fouling agents to facilitating the effective transport of egg cells in mammals. Inspired by the performance of these microscopic, hair-like filaments, researchers are synthesizing artificial cilia for use in lab-on-a-chip devices. There have, however, been few attempts to harness the artificial cilia to regulate the movement of particulates in these devices. Here, we review recent computational studies on the interactions between actuated artificial cilia and microscopic particles, showing that these cilia are effective at transporting both rigid and deformable particles in microchannels. The findings also reveal that these beating filaments can be used to separate microparticles based on their size and stiffness. Importantly, these studies indicate that artificial cilia can be used to prevent fouling by a wide variety of agents because they can expel both passive particulates and active swimmers from the underlying surface. These results can help guide experimental efforts to fully exploit artificial cilia in controlling particle motion within fluid environments.
The research interests of Dr. Balazs center on statistical, mechanical, and computer modeling of complex chemical systems and developing theories for the properties of polymer blends and the behavior of polymers at surfaces and interfaces.
Illustration: Journal of Physical Chemistry Letters.
Deep Brain Stimulation for Parkinson’s Disease
 McGowan Institute for Regenerative Medicine affiliated faculty member Mark Richardson, MD, PhD, is an assistant professor, Department of Neurological Surgery, University of Pittsburgh, director, Brain Modulation Laboratory, and the director, Epilepsy and Movement Disorders Surgery Program, both in the Department of Neurological Surgery. Dr. Richardson’s clinical specialization is comprehensive epilepsy surgery and deep brain stimulation (DBS) for movement disorders.
McGowan Institute for Regenerative Medicine affiliated faculty member Mark Richardson, MD, PhD, is an assistant professor, Department of Neurological Surgery, University of Pittsburgh, director, Brain Modulation Laboratory, and the director, Epilepsy and Movement Disorders Surgery Program, both in the Department of Neurological Surgery. Dr. Richardson’s clinical specialization is comprehensive epilepsy surgery and deep brain stimulation (DBS) for movement disorders.
Dr. Richardson started the interventional-MRI DBS program at the University of Pittsburgh Medical Center (UPMC). Today, UPMC is a leader in treating movement disorders such as Parkinson’s disease with DBS, and now offers both standard and MRI-guided asleep DBS, depending on a patient’s condition.
“The problem is, there is a significant population of patients with Parkinson’s who are too anxious, or too symptomatic, or both to undergo awake surgery in the frame,” according to Dr. Richardson.
Surgeons perform the procedure on patients who stay “under” the whole time using customized software and an MRI machine. Surgeons attach an aiming device to the skull and the surgeon maps the trajectory of the electrode in real time.
DBS delivers electrical stimulation to targeted areas in the brain that control movement, blocking the nerve signals that cause abnormal movement. DBS gives significant benefit to about 70 percent of people who undergo the procedure.
$2.9 Million Grant to Improve Brain Implants Received
 Less than 2 years ago, a brain-computer interface designed at the University of Pittsburgh allowed Jan Scheuermann to control a robotic arm solely with her thoughts. Using the arm to bring a chocolate bar to her mouth and taking a bite was a sweet victory for Ms. Scheuermann, who has quadriplegia.
Less than 2 years ago, a brain-computer interface designed at the University of Pittsburgh allowed Jan Scheuermann to control a robotic arm solely with her thoughts. Using the arm to bring a chocolate bar to her mouth and taking a bite was a sweet victory for Ms. Scheuermann, who has quadriplegia.
The feat also was a victory for scientists developing the brain-computer interface technology, which is poised to help other patients with quadriplegia or amputated limbs. Much work still needs to be done to advance the technology for routine medical use, however.
McGowan Institute for Regenerative Medicine faculty member Xinyan “Tracy” Cui, PhD, associate professor of bioengineering in Pitt’s Swanson School of Engineering, was recently awarded a $2.9 million 5-year grant from the National Institutes of Health to move the technology forward. Dr. Cui will focus on the microelectrode arrays, or brain implants, that are used to connect mind and machine. As the primary investigator, she will explore ways to coat the microelectrodes with biological molecules that could not only better maintain the connection between the brain implants and computers that operate devices like robotic arms but also strengthen that connection.
Research has shown that, over time, microelectrode arrays can elicit an inflammatory response and cause damage to neurons, weakening the link. While the harm to the patient isn’t significant, poorer recordings of neural impulses can limit the functionality of the technology and the quality of information reaped by researchers.
“For the first few months, the data are good, but it starts to decline,” she says. “It’s a common trend to see the amplitude of the recorded signal go down, and it becomes lost in the noise. After a year, we lose half the channels.”
“What we hope to do is camouflage [the microelectrode needles] with biochemicals that can escape the immune surveillance response and protect neurons around the electrodes,” Dr. Cui continues. She has high hopes for a cell adhesion molecule called L1, which has shown positive results in animal models. In addition to gaining better understanding of the mechanisms behind L1’s preliminary success, Dr. Cui also plans to pursue and test several other targets.
Dr. Cui’s work could advance not only brain-computer interface technology but also other technologies that use microelectrode arrays to help restore sight, hearing, movement, ability to communicate, and cognitive function.
Dr. Cui’s co-investigators are Pitt School of Medicine faculty member Andrew Schwartz, PhD; McGowan Institute for Regenerative Medicine affiliated faculty member Carl Lagenaur, PhD, associate professor of neurobiology; and Alberto Vazquez, PhD, research assistant professor of radiology, as well as T.K. Kozai, PhD, research assistant professor of bioengineering in Pitt’s Swanson School of Engineering.
Non-Invasive Imaging of the In Situ Restoration of Brain Tissue
 Regenerative medicine is increasingly finding translations from the bench to the bedside. As stem cells are integrated with biomaterials for in situ tissue engineering, the complexity of the procedure is increasing and it is becoming important to monitor how these processes interact over time in vivo. Translation of this non-invasive monitoring into patients requires the development and implementation of appropriate approaches. McGowan Institute for Regenerative Medicine affiliated faculty member Michel Modo, PhD, associate professor, Department of Radiology, University of Pittsburgh, recently received a 3-year, $921,374 grant from the National Institute of Neurological Disorders and Stroke to pursue one approach.
Regenerative medicine is increasingly finding translations from the bench to the bedside. As stem cells are integrated with biomaterials for in situ tissue engineering, the complexity of the procedure is increasing and it is becoming important to monitor how these processes interact over time in vivo. Translation of this non-invasive monitoring into patients requires the development and implementation of appropriate approaches. McGowan Institute for Regenerative Medicine affiliated faculty member Michel Modo, PhD, associate professor, Department of Radiology, University of Pittsburgh, recently received a 3-year, $921,374 grant from the National Institute of Neurological Disorders and Stroke to pursue one approach.
Dr. Modo’s team’s proposal aims to develop chemical exchange saturation transfer, a non-invasive magnetic resonance imaging (MRI) technique, as a core platform to visualize multiple cell types, as well as biomaterials, while maintaining the ability to characterize newly forming tissue with other MRI techniques, such as magnetic resonance spectroscopy, as well as diffusion and perfusion MRI. Very significant technological, as well as neurobiological challenges, however, will be addressed before integration of this multi-parametric MRI into an efficient non-invasive assessment of in situ tissue engineering.
The proposed studies aim to address these challenges and provide a framework within which the team—which includes co-principal investigator McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor of surgery, University of Pittsburgh—can eventually explore the therapeutic potential of this approach. If a newly functional tissue can be generated to replace that which is lost due to the stroke, this approach could indeed dramatically change the long-term outcome after stroke.
Pitt’s Center for Medical Innovation Awards 2014 Round-1 Pilot Funding
 McGowan Institute for Regenerative Medicine affiliated faculty members recently received grants from the University of Pittsburgh’s Center for Medical Innovation (CMI) through its 2014 Round-1 Pilot Funding Program for Early Stage Medical Technology Research and Development.
McGowan Institute for Regenerative Medicine affiliated faculty members recently received grants from the University of Pittsburgh’s Center for Medical Innovation (CMI) through its 2014 Round-1 Pilot Funding Program for Early Stage Medical Technology Research and Development.
CMI, a University Center housed in Pitt’s Swanson School of Engineering (SSOE), supports applied technology projects in the early stages of development with “kickstart” funding toward the goal of transitioning the research to clinical adoption. Proposals are evaluated on the basis of scientific merit, technical and clinical relevance, potential health care impact and significance, experience of the investigators, and potential in obtaining further financial investment to translate the particular solution to healthcare. Since 2011, CMI has awarded over $483,000 in funding to 29 research groups.
“This is our third year of pilot funding, and our leadership team could not be more excited with the breadth and depth of this round’s awardees,” said Alan D. Hirschman, PhD, CMI Executive Director. “This early-stage interdisciplinary research helps to develop highly specific biomedical technologies through a proven strategy of linking UPMC’s clinicians and surgeons with the Swanson School’s engineering faculty.”
The McGowan Institute for Regenerative Medicine affiliated faculty projects are:
Contained Morcellation Device: Award to design, build, and test an improved morcellation device for laparoscopic surgery, preventing the accidental release of cancerous tissue into the body.
- Pamela Moalli, MD, PhD, Associate Professor, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh, Division of Urogynecology & Pelvic Reconstructive Surgery, Magee-Womens Hospital of UPMC
- Alan Rosenbaum, MD, Resident Physician, Obstetrics and Gynecology, Magee-Womens Hospital
- Jeffrey Vipperman, PhD, Associate Professor, Department of Mechanical Engineering & Materials Science, SSOE
Auxetic Structures for Urogynecological Implantations: Award to design, build, and perform in vivo testing of a new class of surgical meshes for treatment of pelvic organ prolapse without the complications of currently marketed devices.
- Steven D. Abramowitch, PhD, Assistant Professor, Department of Bioengineering, SSOE
- Pamela Moalli, MD, PhD, Associate Professor, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh, Division of Urogynecology & Pelvic Reconstructive Surgery, Magee-Womens Hospital of UPMC
Fade to Clear High Visibility Sutures: Award to develop and test a new suture which is highly visible during placement and becomes transparent during patient recovery, thereby improving aesthetic outcomes.
- J. Peter Rubin, MD, Chair of the Department of Plastic Surgery, UPMC Endowed Professor of Plastic Surgery, Professor of Bioengineering at the University of Pittsburgh, Co-Director of the Adipose Stem Cell Center, and Founder and Director of the Center for Innovation in Restorative Medicine in the Department of Surgery at the University of Pittsburgh
- Eric Beckman, PhD, George M. Bevier Professor of Engineering and Co-Director, Mascaro Center for Sustainable Innovation, University of Pittsburgh
PerioMag GBR Barrier Membrane: Award to develop a resorbable barrier membrane for faster and lower cost guided bone regeneration (GBR) in periodontal applications.
- Charles Sfeir, DDS, PhD, Associate Professor at the University of Pittsburgh Department of Oral Medicine and Pathology with secondary appointments within the Departments of Biomedical Engineering and Materials Science and Engineering at Carnegie Mellon University
- Andrew Brown, BS, Graduate Student Researcher, Department of Bioengineering, SSOE
- Steven Little, PhD, Chair, Department of Chemical and Petroleum Engineering and Associate Professor and CNG Faculty Fellow in the Departments of Chemical and Petroleum Engineering, Bioengineering, Immunology, and Ophthalmology, University of Pittsburgh
Surgically Implantable Prosthesis for Prevention of Tracheobronchial Distortion after Lobar Lung Resection: Award to develop a prosthesis to prevent bronchial kinking after removal of one or more lobes of the lung. The device will prevent post-surgical complications that impact recovery and quality of life for lung cancer patients.
- James Luketich, MD, Henry T. Bahnson Professor of Cardiothoracic Surgery, Chief of the Division of Thoracic and Foregut Surgery at the University of Pittsburgh School of Medicine, and Director of the Heart, Lung and Esophageal Surgery Institute at the University of Pittsburgh Medical Center
- William Federspiel, PhD, William Kepler Whiteford Professor in the Department of Bioengineering with secondary appointments in Chemical Engineering and Critical Care Medicine and the Director of the Medical Devices Laboratory at the McGowan Institute
- Arjun Pennathur, MD, Assistant Professor Surgery, Department of Cardiothoracic Surgery, UPMC
- Diane Strollo, MD, Visiting Associate Professor, Department of Cardiothoracic Surgery, UPMC
- Valentino Bianco, DO, Research Fellow, Department of Cardiothoracic Surgery, UPMC
Illustration: Center for Medical Innovation.
Designing Better Vascular Grafts
 Although the development of artificial vascular grafts has advanced, one of the drawbacks is a high rate of aneurysms, which lead to rupture and massive bleeding. However, engineers at the University of Pittsburgh’s Swanson School of Engineering are exploring the use of new polymers in arterial replacements to better prevent aneurysm formation as they remodel into ‘neoarteries.’
Although the development of artificial vascular grafts has advanced, one of the drawbacks is a high rate of aneurysms, which lead to rupture and massive bleeding. However, engineers at the University of Pittsburgh’s Swanson School of Engineering are exploring the use of new polymers in arterial replacements to better prevent aneurysm formation as they remodel into ‘neoarteries.’
Post-doctoral researcher Arturo Valentin, PhD, is the recipient of an American Heart Association Great Rivers Affiliate Postdoctoral Fellowship for “A predictive computational tool for creating tissue engineered arteries” (Award Period: January 1, 2014- December 31, 2015). Dr. Valentin focuses on mechanobiology in the Swanson School’s Department of Mechanical Engineering and Materials Science. Dr. Valentin’s mentors for the fellowship are McGowan Institute for Regenerative Medicine affiliated faculty members Anne M. Robertson, PhD, Professor of Mechanical Engineering and Bioengineering, and Yadong Wang, PhD, the William K. Whiteford Professor of Bioengineering. Paolo Zunino, PhD, Assistant Professor of Mechanical Engineering and Materials Science, is also a faculty mentor.
“The development of artificial vascular grafts holds tremendous promise for the treatment of cardiovascular disease, especially since the ability to transplant healthy grafts is often limited by availability or immune rejection,” Dr. Valentin explained. “Artificial grafts are designed to degrade as the body naturally repairs the artery, but if the graft dissolves too quickly there is a greater risk of aneurysm. We want to determine the exact way in which this degradation rate affects the chemical and mechanical factors which control how living cells transform grafts into neoarteries.”
Dr. Valentin notes that by studying the underlying biological mechanisms at work in neoartery formation, researchers can develop a more effective graft that reduces risk of aneurysms. “This AHA fellowship will support our research to build replacement arteries utilizing advanced synthetic polymers as grafts that better treat patients with peripheral artery disease or who must undergo cardiac bypass surgery.” Dr. Valentin believes that over the next several years his research will move to clinical trials in humans and “ultimately help bring much improved arterial replacements to patients.”
AWARDS AND RECOGNITIONS
Dr. David Hackam Inducted into the Association of American Physicians
 McGowan Institute for Regenerative Medicine affiliated faculty member David Hackam, MD, PhD, has been inducted into the Association of American Physicians (AAP). Dr. Hackam is the Watson Family Professor of Surgery, professor of surgery, cell biology and physiology, and associate dean for medical student research, University of Pittsburgh. He also serves as co-director of the Fetal Diagnosis and Treatment Center of the Children’s Hospital of Pittsburgh of UPMC. His research focuses on understanding the gastrointestinal mechanisms of conditions such as Crohn’s disease, intestinal inflammation, and necrotizing enterocolitis.
McGowan Institute for Regenerative Medicine affiliated faculty member David Hackam, MD, PhD, has been inducted into the Association of American Physicians (AAP). Dr. Hackam is the Watson Family Professor of Surgery, professor of surgery, cell biology and physiology, and associate dean for medical student research, University of Pittsburgh. He also serves as co-director of the Fetal Diagnosis and Treatment Center of the Children’s Hospital of Pittsburgh of UPMC. His research focuses on understanding the gastrointestinal mechanisms of conditions such as Crohn’s disease, intestinal inflammation, and necrotizing enterocolitis.
The AAP is a nonprofit, professional organization founded in 1885 for “the advancement of scientific and practical medicine.” Election to the AAP is an honor extended to individuals with outstanding credentials in biomedical science and/or translational biomedical research, and is limited to 60 persons per year. Today, the AAP serves as an association of the country’s most accomplished physician-scientists, as a forum to create and disseminate knowledge, and as a source of inspiring role models for upcoming generations of physicians and medical scientists. The AAP recognizes researchers who have made significant contributions to medical science over the course of their careers, unlike the Association of Clinical Investigation (ASCI), which is affiliated with the AAP, and recognizes high levels of achievement by members relatively early in their careers.
Dr. Freddie Fu Named a Top 2014 North American Sports Knee Surgeon
 The staff at Orthopedics This Week recently conducted a survey of thought leaders in the sports knee realm. The results named McGowan Institute for Regenerative Medicine faculty member Freddie Fu, MD, Distinguished Service Professor and the David Silver Professor of Orthopaedic Surgery and Chairman of the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine and University of Pittsburgh Medical Center, one of the 28 top North American sports knee surgeons.
The staff at Orthopedics This Week recently conducted a survey of thought leaders in the sports knee realm. The results named McGowan Institute for Regenerative Medicine faculty member Freddie Fu, MD, Distinguished Service Professor and the David Silver Professor of Orthopaedic Surgery and Chairman of the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine and University of Pittsburgh Medical Center, one of the 28 top North American sports knee surgeons.
From the survey it was noted, “Dr. Fu is a true pioneer in sports medicine and leading us into the next generation in the field. He has tremendous energy that is infectious; he is persistent and has built a superb research team at Pittsburgh. Over the last decade he has devoted his research endeavors towards the anatomy and biomechanics of the ACL. He is a very thoughtful innovator who takes a critical look at his results.”
Dr. Fu is known worldwide for his pioneering surgical techniques to treat sports-related injuries to the knee and shoulder and his extensive scientific and clinical research in the biomechanics of such injuries. He performs surgery at University of Pittsburgh Medical Center and sees patients at the Center for Sports Medicine. Because of his reputation, Dr. Fu attracts both athletic and non-athletic patients from all over the globe and has been featured in several publications.
Dr. Anthony Delitto Honored by the American Physical Therapy Association
 McGowan Institute for Regenerative Medicine affiliated faculty member Anthony Delitto, PhD, University of Pittsburgh professor of physical therapy, associate dean for research, and vice president for education and research for the Centers for Rehabilitation Science, was recently honored with the American Physical Therapy Association’s Helen J. Hislop Award for Outstanding Contribution to Professional Literature.
McGowan Institute for Regenerative Medicine affiliated faculty member Anthony Delitto, PhD, University of Pittsburgh professor of physical therapy, associate dean for research, and vice president for education and research for the Centers for Rehabilitation Science, was recently honored with the American Physical Therapy Association’s Helen J. Hislop Award for Outstanding Contribution to Professional Literature.
Dr. Delitto, whose clinical work specializes in the treatment of low back pain, has been honored for scientific writing as well as research in physical therapy and has won multiple awards for excellence in clinical research.
“The field of physical therapy has changed drastically since 1979. The training is so much more in-depth, and we’re now an access point where people can get first contact with health care. I think physical therapists as well as other health care professionals, including occupational therapists, pharmacists, and nurse practitioners, can play a major role in helping more people access health care as we face physician shortages.”
Regenerative Medicine Podcast Update
 The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#137 –– Dr. Robert Bowser is the Chairman of Neurobiology and a Professor of Neurology and Neurobiology at the Barrow Neurological Institute and St. Joseph’s Hospital and Medical Center in Phoenix, Arizona. Dr. Bowser discusses his research in the biomarkers that indicate early disease presence or predisposition and protein abnormalities caused by ALS.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
Picture of the Month
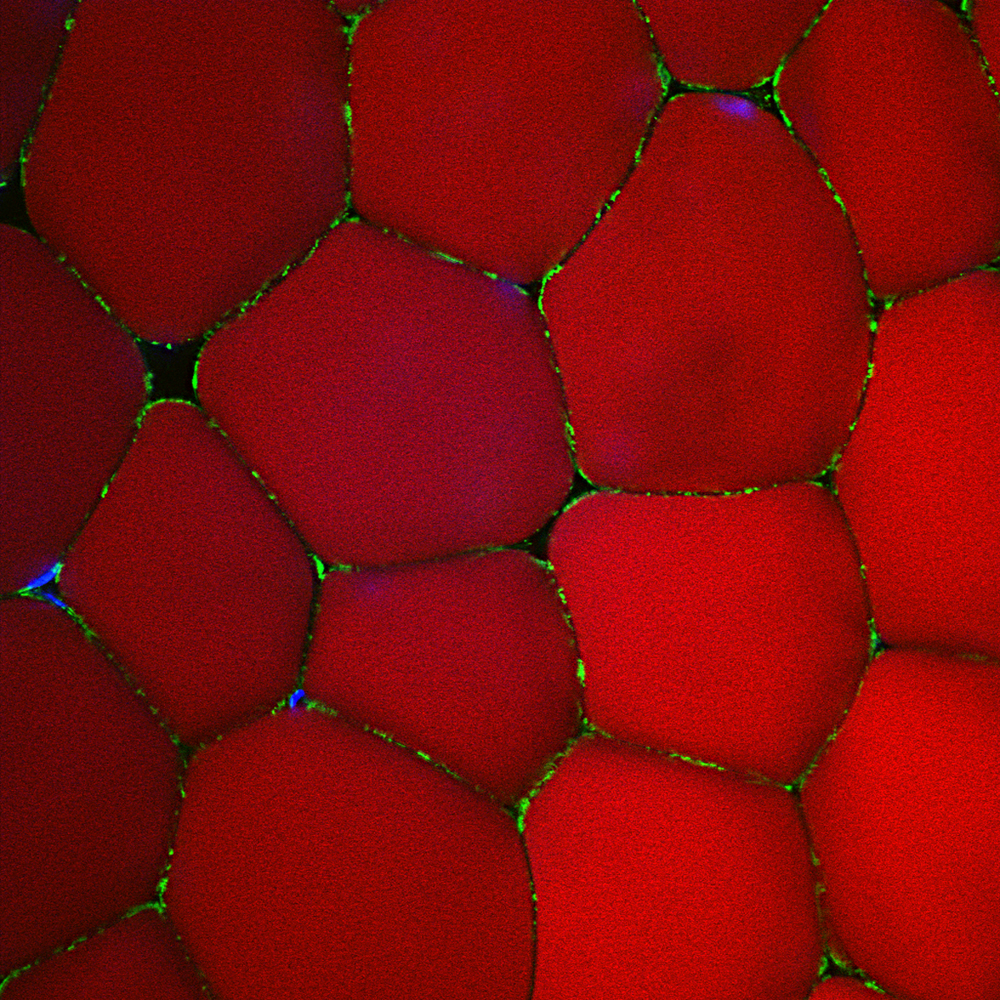
Human white adipose tissue stained for a mitochondrial marker (green), lipid marker (red) and nuclei (blue). Confocal reconstruction.
Photo by Donna Stolz, Center for Biologic Imaging.
