September 2021 | VOL. 20, NO. 9| www.McGowan.pitt.edu
Dr. Fabrisia Ambrosio Named a 2021 Women of Influence Award Winner

Presented by the Pittsburgh Business Times, the 2021 Women of Influence Awards honor the region’s most influential businesswomen at for-profit companies, nonprofits, and government entities. This year, McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, MPT, is one of the 25 women being honored as Women of Influence. The awardees are women who were effective in their communities, blazed a trail for others, and are leaving an indelible mark on business in Greater Pittsburgh. This year’s presentation event will take place on October 6, 2021.
Dr. Ambrosio is an associate professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh, and Director of Rehabilitation for UPMC International. She holds secondary appointments in the Departments of Physical Therapy, Orthopaedic Surgery, Bioengineering, Environmental Health & Occupational Safety, as well as Microbiology & Molecular Genetics at the University of Pittsburgh, and she serves on the Executive Committee of the McGowan Institute for Regenerative Medicine.
Dr. Ambrosio was the first to identify the potential benefits of “marrying” regenerative medicine and rehabilitation, and it is now a rapidly growing approach to patient care. Her research has the long-term goal of developing Regenerative Rehabilitation approaches to improve skeletal muscle healing and functional recovery of tissue impacted by trauma, disease, or surgery. Her
Of laboratory investigates the underlying mechanisms by which mechanical and electrical signals can be used to enhance donor and/or host stem cell functionality in mouse and human models. Her research has received numerous awards, including, most recently, “Best Paper of the Year” awards both in 2017 and 2018.
One example of Dr. Ambrosio’s pioneering research is a recently issued study report that offers significant potential enhancement in the quality of life while we age. The study reveals which molecules in the muscle are most significantly altered at different life stages, and shows that a molecule called Klotho, when administered to mice in old, but not very old age, was able to improve muscle strength.
Age-related loss of skeletal muscle mass and function – called sarcopenia – is associated with loss of mobility and increased risk of falls. Dr. Ambrosio and her team looked at whether giving the mice Klotho could reverse age-related declines in muscle quality and function. They found that Klotho administration led to improvements in the old mice: force production was improved by 17% when supporting whole body weight and was 60% greater compared to mice without treatment. But this was only seen in the old mice, and not in the oldest-old animals. Further investigation showed that Klotho affected genes associated with the hallmarks of aging in all age groups, but that the oldest-old mice showed a dysregulated gene response.
Her data suggests that treatment with Klotho may be more effective in slowing the progression of sarcopenia at an earlier time point, rather than rescuing advanced age-related disease, by which time the gene responses seem to be more random.
Through her founding of the new field of Regenerative Rehabilitation, which is non-profit centered as it is a consortium of non-profit institutions, she has had a significant impact on non-profits. It is noteworthy that she has established Pittsburgh as the epicenter of this new field.
Finally, as a female researcher of Latina descent, Dr. Ambrosio is particularly interested in mentoring women and under-represented minorities (URMs). Her commitment to this objective is highlighted by her leadership, programmatic efforts, and research endeavors.
A major focus of Dr. Ambrosio’s programmatic efforts has been to encourage the participation of URMs in science and technology training programs. For example, in 2015, Dr. Ambrosio served as co-course director of the NIH-funded Rehabilitative and Regenerative Medicine for Minority Health & Health Disparities (ReMedy). ReMedy was a T15 advanced training course designed to recruit URMs and provide hands-on introduction to research strategies and state-of-the-art methods in bioengineering as well as cellular, molecular, and genetic approaches for advancing the frontiers in Regenerative Rehabilitation. Working in close partnership with historically black universities, this course included >80% URM trainees.
More recently, Dr. Ambrosio has used her leadership role as Director for the Alliance for Regenerative Rehabilitation Research & Training (AR3T) Center to promote inclusion in the domain of Regenerative Rehabilitation. Specifically, Dr. Ambrosio has implemented a diversity travel award mechanism that executes targeted efforts to recruit URMs to AR3T events and programs, such as the Annual Symposium for Regenerative Rehabilitation and sabbatical opportunities.
As a result of these efforts, AR3T has enjoyed participation of an average of 15% URMs across the Center’s programs. Dr. Ambrosio also recognizes that efforts to promote inclusion must be a priority for all trainees and investigators, regardless of one’s own race or ethnicity. With the goal of facilitating a community-wide dialogue to identify steps that will promote the participation of URMs, in 2019, Dr. Ambrosio organized and led a “diversity breakfast” at the 8th Annual Symposium for Regenerative Rehabilitation. This breakfast, which included approximately 50 participants, was designed to serve as a forum for the exchange of ideas and experiences as they relate to diversity. The enthusiasm for the breakfast was such that it has now become an annual component of the Symposium series, for which Dr. Ambrosio serves as Course Director.
Honorees for the Women of Influence award were selected from a public pool of nominations and determined by a panel of judges made up of program alumnae. Nominees were women from every industry and profession — those with a strong record of performance in business or at nonprofits who had influence in their communities and are leaving their mark on the western Pennsylvania business community.
Congratulations, Dr. Ambrosio!
In Memoriam: Dr. Freddie Fu
 Members of the McGowan Institute for Regenerative Medicine mourn the passing of Freddie Fu, MD. Dr. Fu was internationally recognized as a pioneer in the field of orthopedic medicine and acclaimed worldwide for his innovative research and teaching, leading to many clinical advancements in sports medicine and orthpaedic care, particularly in treating knee injuries. Throughout his life and career, Dr. Fu worked passionately to always set the bar higher for his local, national, and international medical/surgical colleagues, thousands of medical students, surgical residents, and fellows who came to Pittsburgh to learn from the best. Read Dr. Fu’s full obituary from UPMC here. Condolences are extended to Dr. Fu’s wife, children, and family.
Members of the McGowan Institute for Regenerative Medicine mourn the passing of Freddie Fu, MD. Dr. Fu was internationally recognized as a pioneer in the field of orthopedic medicine and acclaimed worldwide for his innovative research and teaching, leading to many clinical advancements in sports medicine and orthpaedic care, particularly in treating knee injuries. Throughout his life and career, Dr. Fu worked passionately to always set the bar higher for his local, national, and international medical/surgical colleagues, thousands of medical students, surgical residents, and fellows who came to Pittsburgh to learn from the best. Read Dr. Fu’s full obituary from UPMC here. Condolences are extended to Dr. Fu’s wife, children, and family.
RESOURCES AT THE MCGOWAN INSTITUTE
October Histology Special – Safranin O Staining
The Histology lab offers decalcification, processing and embedding, cutting and staining of those difficult bone samples. Our staff has years of experience with bone samples that include special staining and IHC on large and small bone samples that can be difficult to work with. We have even developed a technique to ensure your sample will not lift from the slide during aggressive antigen retrieval protocols.
Below are just some of the special stains we offer at the McGowan Histology Core:
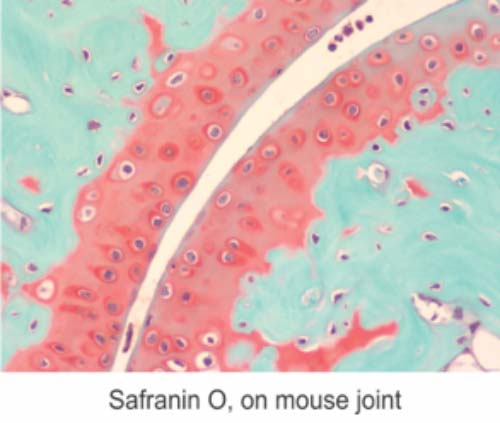
You’ll receive 25% off bone samples in October when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
Are COVID Variants Treated Differently, or Is COVID Just COVID?
That’s the question Kelly Sasso from Pittsburgh’s WTAE received from a man in Latrobe, PA. Ms. Sasso received the answer from McGowan Institute for Regenerative Medicine faculty member Alan Wells, MD, the Thomas J. Gill III Professor of Pathology and the Executive Vice-Chairman of the Department of Pathology at the University of Pittsburgh. He is also the medical director at UPMC’s Clinical Laboratories.
Ms. Sasso received from Dr. Wells a tour of the largest academic clinic lab in the US which is located at UPMC.
Dr. Wells explained, “For any individual that is sick, COVID is COVID.” Current COVID tests do not identify the variant of the virus. Treatment is the same based on how sick a patient is and what that patient’s risks are.
Dr. Wells further explained that 90% of current COVID cases in the Pittsburgh area are the delta variant. Further testing is done on random COVID test samples from Dr. Wells’ lab at the Microbial Genomic Epidemiology Lab, also at UPMC. Here is where the virus’ variant is determined.
Watch the entire interview here.
Illustration: WTAE.
UPMC and Pitt Provide First Results of Largest Comparative Randomized Trial of Monoclonal Antibody Treatment for COVID-19

Monoclonal antibodies—an outpatient treatment that must be given soon after COVID-19 diagnosis—significantly decrease hospitalization and death from the disease. Real-world data from UPMC patients now show that two antibody combination treatments – bamlanivimab-etesevimab and casirivimab-imdevimab – were safe and appeared to be equally effective.
These interim results come from UPMC’s innovative OPTIMISE-C19 study, a randomized, adaptive trial designed to simultaneously expand access to monoclonal antibodies and compare the effectiveness of different treatments for outpatients with COVID-19. The next phase of the trial, which already is in progress, will evaluate how well the currently authorized treatments work against coronavirus variants, including Delta, to prevent hospitalization and death.
UPMC and University of Pittsburgh School of Medicine physician-scientists published the findings in medRxiv, a preprint journal, and announced the results ahead of peer-reviewed publication. McGowan Institute for Regenerative Medicine affiliated faculty members Derek Angus, MD, MPH, UPMC’s chief innovation officer, distinguished professor and chair of Pitt’s Department of Critical Care Medicine, and Alan Wells, MD, DMS, the Thomas J. Gill III professor of pathology and the executive vice-chairman of the Department of Pathology at the University of Pittsburgh, are co-authors on this paper.
“Before we launched OPTIMISE-C19, only a small percentage of eligible patients were receiving monoclonal antibody treatment,” said lead author Erin McCreary, PharmD, UPMC infectious diseases pharmacist and Pitt clinical assistant professor of medicine. “Now, we’re able to offer monoclonal antibodies in the context of a clinical trial at every single one of our available treatment sites—resulting in a 7.5-fold increase in the number of eligible patients receiving this treatment. That level of outreach and access is virtually unprecedented, allowing us to build a foundation to roll-out future treatments quickly and safely within our learning health system.”
Monoclonal antibodies are potent versions of the natural defense that our bodies build to fight off an infection. By giving monoclonal antibodies to newly infected people, they can immediately start neutralizing and eliminating the virus, preventing it from infecting cells and causing damage. Previous research by UPMC and others found that monoclonal antibodies significantly reduce the risk of hospitalization and death when given soon after infection.
“The whole world is in a race to tame the virus that causes COVID-19,” said study co-author Dr. Angus. “If we get COVID-19, monoclonal antibodies are currently our best bet to keep ourselves and our loved ones alive and out of the hospital. In our quest for a cure, we’ve had the good fortune to have multiple options available, leaving doctors with the question: Which one is best for my patient? Right now, the answer is that the best option is the one you can give your patient fastest.”
In February 2021, UPMC partnered with the White House COVID-19 Response Team to expand clinical use of monoclonal antibodies and evaluate their comparative effectiveness.
The OPTIMISE-C19 trial randomizes the allocation of the monoclonal antibody treatments that were granted emergency use authorization (EUA) by the U.S. Food and Drug Administration (FDA) to patients who qualify for them. All patients in the trial receive monoclonal antibody treatment, are followed for 28 days and their outcomes compared.
UPMC randomized either Eli Lilly or Regeneron’s combination monoclonal antibody treatment to 1,935 patients from March 10 to June 25, 2021. The results were pulled for analysis after the federal government stopped distributing Lilly’s monoclonal antibody treatment due to lower laboratory effectiveness against the Gamma and Beta variants of the virus.
The first results of OPTIMISE-C19 show that before Delta became the predominant strain in the U.S., both Lilly and Regeneron’s combination monoclonal antibody treatments performed well in keeping patients with COVID-19 alive and out of the hospital in a geographic region of Alpha variant predominance. Safety also was similar, with very few serious complications due to the treatment among UPMC patients.
While data were pulled for analysis, GSK and Vir Biotechnology’s monoclonal antibody, sotrovimab, was granted an EUA and incorporated into UPMC’s OPTIMISE-C19 trial for comparison against Regeneron’s treatment.
“That’s the beauty of an adaptive learning health system trial,” said senior author and principal investigator David Huang, MD, M.P.H., an intensivist at UPMC and professor of critical care medicine, emergency medicine, and clinical and translational science at Pitt. “As new treatments are authorized, we can immediately begin offering them to patients and collect randomized data to inform future treatment protocols. We can then compare outcomes as the virus evolves and new variants emerge.”
Delta is now the predominant strain of COVID-19 in the communities UPMC serves. UPMC offers monoclonal antibodies to people who have tested positive for COVID-19, have had symptoms for 10 days or less and meet other FDA eligibility criteria. Certain people who have been exposed to COVID-19 also can receive monoclonal antibodies as a preventive treatment, to keep them from contracting the virus. More information can be found at www.upmc.com/antibodytreatment or by calling 866-804-5251.
Additional authors on this research are J. Ryan Bariola, MD, Tami Minnier, MS, Richard J. Wadas, MD, Judith A. Shovel, BSN, Debbie Albin, BS, Oscar C. Marroquin, MD, Kevin E. Kip, PhD, Kevin Collins, MBA, Mark Schmidhofer, MD, Mary Kay Wisniewski, MA, David A. Nace, MD, Colleen Sullivan, MHA, Meredith Axe, BS, Russell Meyer, MBA, Alexandra Weissman, MD, William Garrard, PhD, Stephen Koscumb, BS, Octavia M. Peck-Palmer, PhD, Robert D. Bart, MD, Anne Yang, MD, Tina Khadem, PharmD, Kelsey Linstrum, MS, Stephanie K. Montgomery, MS, Daniel Ricketts, MT, Jason Kennedy, MS, Caroline J. Pidro, BS, Ghady Haidar, MD, Graham M. Snyder, MD, Bryan J. McVerry, MD, Christopher W. Seymour, MD, and Paula L. Kip, PhD, all of UPMC, Pitt or both; and Lindsay Berry, PhD, Scott Berry, PhD, Amy Crawford, PhD, and Anna McGlothin, PhD, all of Berry Consultants. Donald M. Yealy, MD, UPMC’s chief medical officer and professor and chair of Pitt’s Department of Emergency Medicine, provided data and safety monitoring for the trial.
The U.S. government provided the monoclonal antibody treatments reported in this manuscript.
Controlling the Output Signals of the Basal Ganglia

McGowan Institute for Regenerative Medicine affiliated faculty member Jonathan Rubin, PhD, Professor and Chairman in the Department of Mathematics and adjunct professor with the Department of Computational and Systems Biology at the University of Pittsburgh, is a co-principal investigator on a three-year Collaborative Research in Computational Neuroscience Grant, funded by the NIH National Institute of Neurological Disorders and Stroke. Dr. Rubin and co-principal investigator Aryn Gittis, PhD, from Biological Sciences at Carnegie Mellon University, will work together on the project entitled “Diverse effects of GABAergic inputs on a basal ganglia output center.”
Drs. Rubin and Gittis will study control of the output signals of the basal ganglia, which is a brain area involved in action selection and reward-based learning. The project will combine recordings from neurons with data analysis and mathematical modeling to understand the origins of surprisingly diverse firing patterns of these neurons as well as changes that occur during dopamine depletion associated with conditions like Parkinson’s disease (PD). The work could have implications for the development of new therapies for PD.
The project abstract follows:
The basal ganglia are a collection of subcortical nuclei studied for their contributions to movement, action selection, habit formation, and reward learning as well as their dysfunction in movement disorders. While basal ganglia processing of cortical inputs and the emergence of the direct and indirect pathway communication channels within the striatum have been the subject of extensive investigation, the integration of these channels at the level of basal ganglia output nuclei including the substantia nigra pars reticulata (SNr) has been relatively understudied. This imbalance is problematic for our understanding of basal ganglia function, because basal ganglia impacts on other areas of the nervous system, and hence on behavior, are funneled through basal ganglia output nuclei and depend on how they process the signals they receive. This project will build on and test ideas recently proposed based on computational and experimental work from the PIs’ groups to investigate SNr activity in ways that redress this knowledge gap. Specifically, this work will advance our knowledge about: how SNr neuron responses to GABAergic inputs from a major source, the external segment of the globus pallidus (GPe) in the indirect pathway depend on SNr neuron characteristics, the locomotor state of the subject, and dopamine levels; how they are expected to impact dynamics at the level of the SNr network and its outputs; the extent to which chloride dynamics and its effect on the GABA reversal potential give rise to these behaviors; and how these factors contribute to the delta band oscillations that emerge in SNr specifically under dopamine depletion. These advances will be achieved via an interdisciplinary approach of model development, simulations, and analysis done in synergy with experiments in slice and in vivo in mice involving optogenetics, neural recording, pharmacological manipulations, and behavior across control and dopamine-depleted conditions. RELEVANCE: Basal ganglia dysfunction contributes to a range of disorders including Parkinson’s disease, which is characterized by significant dopamine depletion. The proposed research will provide new insights about how dopamine depletion alters communication among key neural populations within the basal ganglia, as well as output signaling from the basal ganglia, which can impact motor behavior. These findings will supply information that is of direct utility in the search for therapeutic targets and the development of efficient, effective brain stimulation paradigms to reduce the motor complications of Parkinson’s disease.
Congratulations, Dr. Rubin!
Pitt TEDx 2021: William Wagner, PhD

William Wagner, PhD, Director of the McGowan Institute for Regenerative Medicine and Distinguished Professor of Surgery, Bioengineering and Chemical Engineering at the University of Pittsburgh, recently participated in the University of Pittsburgh’s TEDx 2021 (click for full program—Dr. Wagner is the last presenter). Dr. Wagner highlighted the history of the McGowan Institute and also several current research efforts underway during his presentation.
The McGowan Institute was named after William McGowan, the head of MCI, a competing local and long distant phone service for AT&T. It was through the Starzl Transplantation Institute that Mr. McGowan became familiar with the University of Pittsburgh Medical Center when in 1986 he needed a heart transplant. While convalescing, Mr. McGowan engaged his restless, entrepreneurial mind with all he could learn about medical research. Ever the visionary, Mr. McGowan believed that artificial organs could alleviate the shortage of donor organs and perhaps provide permanent alternatives to transplantation. In 1990, William and Sue Gin McGowan donated $1 million to fund a like-minded center. The McGowan Center for Artificial Organ Development was established in 1992, shortly after Mr. McGowan’s death.
As science has evolved, so has Mr. McGowan’s namesake institute. The twin revolutions in genetic and tissue engineering began to shape the idea that, ultimately, regrowing the patient’s own tissues to replace those lost to disease is an achievable — and preferable — goal. When the mission of the McGowan Center expanded to include “biohybrid” organs (those that combine artificial and natural components), tissue engineering, and cellular therapies, it was renamed the McGowan Institute for Regenerative Medicine. The Institute is the single base of operations for leading scientists and clinicians at UPMC and the University of Pittsburgh working to develop new regenerative therapies.
Today, the synergy between clinicians and engineers and later the support of technology companies is what drives the research successes at the McGowan Institute. In addition, the tight integration of clinicians and engineers with students is what moves the research forward through the next generations of scientists to come.
Dr. Wagner described several McGowan Institute technologies developed and/or in the pipeline for development:
- HeartMate II Continuous Flow Left Ventricular Assist Device (LVAD): The HeartMate II is intended for a broad range of advanced heart failure patients and is the only continuous-flow LVAD approved by the FDA for both Bridge to Transplantation and Destination Therapy. The device is a miniature rotary pump with axial flow bearings and is intended for patients with end-stage heart failure. A key feature of the design is a sophisticated control system developed by researchers at the McGowan Institute for Regenerative Medicine that senses when to increase or decrease the rate of blood flow. Other approved and experimental devices require manual adjustments. The control system developed by the researchers involves a patented algorithm that permits the device system to respond to the needs of the patient based on the level of activity, generating up to10 liters of blood flow per minute, a rate that would be required to climb stairs, for example. The controller was the brainchild of McGowan Institute for Regenerative Medicine affiliated faculty member James Antaki, PhD, then an associate professor in Biomedical Engineering at Carnegie Mellon University and adjunct professor in the University of Pittsburgh’s Departments of Surgery and Bioengineering. McGowan Institute for Regenerative Medicine faculty member Robert Kormos, MD, formerly a professor with tenure specializing in cardiothoracic surgery at the University of Pittsburgh, director of the University of Pittsburgh Medical Center (UPMC) Artificial Heart Program, and co-director of the UPMC Heart Transplantation Program, was the clinician involved in this work.
- Artificial lung development through ALung Technologies, Inc., a respiratory dialysis device: During the COVID-19 pandemic, the FDA approved ALung’s device for emergency use. More than 100 patients used the Hemolung® Respiratory Assist System in lieu of being placed on a ventilator. This work is lead by William Federspiel, PhD, John A. Swanson Professor in the Department of Bioengineering with secondary appointments in Chemical Engineering, Critical Care Medicine, and the Clinical Translation Institute. He is also the Director of the Medical Devices Laboratory at the McGowan Institute.
- Pediatric device technology development: The U.S. Food and Drug Administration announced that it has awarded five grants totaling up to $6 million per year over the next five years to Pediatric Device Consortia (PDC) across the country that will provide advice and support services to innovators of children’s medical devices. Dr. Wagner is a co-principal investigator on this effort.
- Livers grown in lymph nodes: For patients with liver failure, LyGenesis has been approved for a Phase 1 clinical trial. LyGenesis’ technology uses lymph nodes as bioreactors to regrow functioning organs within a patient’s own body. LyGenesis was built on nearly a decade of groundbreaking academic research by Eric Lagasse, PharmD, PhD, Associate Professor in the Department of Pathology at the University of Pittsburgh and LyGenesis’ Chief Scientific Officer.
- Revolutionizing Metallic Biomaterials: An interdisciplinary team of investigators from North Carolina A&T State University (NCAT), the University of Pittsburgh (Pitt), and the University of Cincinnati (UC), has been formed based on its members individual track records of success in science, engineering, and technology. The intellectual merit of the current proposal is the intertwining of carefully planned, cutting edge research, education, and economic activities on a global level among partner institutions. The major goal is to revolutionize metallic biomaterials and smart coatings with built-in, responsive biosensory capabilities which can adapt to biological changes to create novel bio-functional Engineered Systems: Craniofacial and Orthopedic Applications, Cardiovascular and Thoracic Devices, and Responsive Biosensors and Neural Applications. Dr. Wagner is the Deputy Director of this effort.
Watch here… (click for full program—Dr. Wagner is the last presenter; starts at 1:14:30 )
Dr. Q&A: Plastic vs Cosmetic Surgery with Dr. Peter Rubin

McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, FACS, Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh, was recently interviewed by Olga Villaverde, the host of Dr. Q&A of AllHealthTV.com. Dr. Rubin answered many medical questions from Ms. Villaverde and viewers.
Dr. Rubin had the opportunity to also talk about the research he and other McGowan Institute faculty members are conducting on volumetric muscle loss in patients who have been injured in the Armed Services, motor vehicle accidents, industrial accidents, or through other traumas. The story of one wounded warrior that Dr. Rubin supported can be viewed here.
Dr. Rubin helped his audience to understand the risks and outcomes of both plastic and cosmetic surgery. He stressed to everyone the importance of ensuring that whatever medical/surgical procedure you may be interested in, to do research on your practitioner and where the procedure will be conducted. The American Board of Plastic Surgery has a list of safe, qualified, and competent doctors who have participated in the required educational and clinical training.
Dr. Rubin’s full conversation with Ms. Villaverde can be watched here.
Predicting Complications Postpartum

A relevant cardiac health trend is the United States’ growing maternal mortality rate — the worst among industrialized countries. The majority of maternal deaths occur postpartum and result from hypertensive disorders like preeclampsia and gestational hypertension.
Alisse Hauspurg, MD, assistant professor of obstetrics, gynecology, and reproductive sciences at Pitt’s School of Medicine, is the lead investigator on the NIH R21 Trailblazer Award entitled, “Development of a smartphone-based device to detect fluid overload among postpartum women with hypertensive disorders of pregnancy.” Ramakrishna Mukkamala, PhD, professor of bioengineering at Pitt’s Swanson School of Engineering and professor of anesthesiology and perioperative medicine at Pitt’s School of Medicine is a co-investigator on this award that uses arterial waveforms to predict fluid buildup in patients with preeclampsia. McGowan Institute for Regenerative Medicine faculty member Sanjeev Shroff, PhD, distinguished professor and Gerald E. McGinnis Chair of bioengineering, and Aman Mahajan, MD, PhD, Peter and Eva Safar Professor and chair of anesthesiology and perioperative medicine and professor of bioengineering, will also collaborate on the project.
“A subset of women with high blood pressure in pregnancy develop fluid overload and heart failure symptoms postpartum, which can lead to an increased risk of hospital readmission and significant complications after delivery,” explained Dr. Hauspurg.
“Home blood pressure monitors improve manageability of hypertension,” Dr. Mukkamala said, “but they do not reduce the incidence of postpartum hospital readmission related to fluid overload.”
The team will develop a smartphone-based device for convenient monitoring of postpartum fluid overload. Such a device could indicate which patients should receive more aggressive treatment prior to discharge and may potentially save the life of a new mother.
The project abstract follows:
Hypertensive disorders of pregnancy contribute to a significant proportion of maternal morbidity and mortality and are the most common reason for hospital readmission postpartum. While treatment of hypertension is the cornerstone of postpartum management, emerging evidence suggests that implementation of robust remote blood pressure monitoring and treatment programs alone do not reduce maternal morbidity or hospital readmissions related to postpartum fluid overload and heart failure symptoms. The ability to predict which women will develop these complications and pursue more aggressive treatment with diuresis prior to hospital discharge or in the first week postpartum is limited. The overall goal of this line of research is to develop and implement a convenient congestion prediction device for home use in conjunction with home blood pressure monitoring in a postpartum population following a hypertensive disorder of pregnancy. In this application, the objective is to develop and test a smartphone-based device to predict fluid overload in patients with preeclampsia through three specific aims: 1) to build multiple smartphone-based devices to assess volume status via a congestion prediction index in postpartum women with preeclampsia, 2) to collect patient training data with the devices, optimize their accuracy in assessing volume status, and identify and create the final real-time device and 3) to validate the final device prospectively in response to known fluid changes among a new cohort of postpartum women with preeclampsia. The research proposed in this application is innovative in its novel adaptation of evidence-based approaches to fluid assessment in heart failure patients to an understudied postpartum population with significant morbidity, its use of a smartphone form factor and the design and the proposed testing of multiple devices to optimize effectiveness and convenience. This proposal is significant as it will provide an opportunity to develop and assess the feasibility of smartphone-based assessment of fluid status among postpartum women with preeclampsia. Effective interventions to improve postpartum hypertension care coupled with innovative remote strategies have broad implications for improving maternal morbidity and reducing disparities in care. Our findings will provide a valuable framework to inform a future randomized trial of remote assessment and management of fluid status in the postpartum period. Successful completion of this line of research would represent a transformation in postpartum management of women with preeclampsia and have a direct impact to improve hypertension and cardiovascular-related maternal morbidity and mortality.
Dr. John Pacella to Serve as Co-Investigator on NHLBI Grant

McGowan Institute for Regenerative Medicine affiliated faculty member John Pacella, MD, Associate Professor in the School of Medicine at the University of Pittsburgh, an adjunct Associate Professor of Biomedical Engineering at Carnegie Mellon University, and an interventional cardiologist within the UPMC Heart and Vascular Institute, and Xiaoning Jiang, PhD, Dean F. Duncan Distinguished Professor of Mechanical and Aerospace Engineering at the North Carolina State University, Adjunct Professor of Biomedical Engineering at the University of North Carolina at Chapel Hill and North Carolina State University, and Adjunct Professor of Neurology at Duke University, are co-investigators on a novel R33 award as part of the recently launched NHLBI Catalyze Program which aims at “facilitating the transition of basic science discoveries into viable diagnostic and therapeutic candidates cleared for human testing.”
Microvascular Therapeutics (MVT) has received the funds for the project entitled, “Ultrasound-mediated thrombolysis for MVO and PAO treatment,” from the National Heart, Lung, and Blood Institute (NHLBI), a part of the National Institutes of Health, for its program to treat cardiovascular disease, which kills more Americans than any other disease. As a leader in fluorocarbon technology, MVT is currently developing Phase Shift Microbubbles (PSMB) for several therapeutic applications.
Evan Unger, MD, founder and Interim CEO of MVT said, “This extends our ongoing work in therapeutic PSMB in important ways for patients suffering one of the most difficult to treat diseases. We are optimistic that this will lead to better options for patients.” Dr. Unger has been pioneering the use of fluorocarbons and ultrasound in diagnostics and therapeutics for more than 30 years and is the inventor of Definity®, the leading ultrasound contrast agent.
The team of MVT hope to translate the discoveries to the clinic in collaboration with SonoVascular. “We are very excited to work with Dr. Unger and the team at MVT on the further development of this exciting technology. Dr. Unger has a proven track record of bringing life-saving technologies to the clinic and fluorocarbon phase shift microbubbles have the potential to make a significant impact in patient care,” said Daniel Estay, CEO of SonoVascular. “SonoVascular, in collaboration with Dr. Xiaoning Jiang’s lab at North Carolina State University, has developed an ultrasound catheter technology which is able to treat blood clots and we will be using the PSMB from MVT. We anticipate to jointly develop the technology for treatment of deep vein thrombosis, arterial occlusions, and pulmonary emboli. These are life-threatening diseases that afflict more than one million Americans each year. This new technology combining therapeutic ultrasound with MVT’s PSMB technology has potential to more rapidly and safely treat vascular disease.
This new grant from the NHLBI will “allow us to improve on the formulation of our products and test them in combination with the technology of SonoVascular to treat blood clots. This work funded by the NHLBI represents one step closer towards clinical testing,” says the Principal Investigator of the award, Emmanuelle Meuillet, PhD, Chief Scientific Officer of MVT.
Dr. Kenton Gregory to Support Development of AI-Based Applications for Mass Casualty Events

An earthquake in a major city, a bomb in a subway, a bus crash in rural town, COVID-19 pandemic hot zones — any event that overwhelms the capacity of available health care facilities is considered a mass casualty event. Lung damage and other internal injuries are particularly challenging to diagnose in multiple casualties during these events.
Handheld ultrasound devices, small enough to fit in a coat pocket, are already being used by first responders, emergency medical services medics and health professionals. The development of new artificial intelligence (AI)-based applications has the potential to improve obtaining, reading and interpreting images quickly and accurately, saving lives during a mass casualty event. Such advances could help guide rapid diagnosis, triage, and early treatment, whether they occur in urban or remote areas, especially in those with limited medical resources.
Oregon Health & Science University (OHSU) and Philips North America are collaborating to develop such applications as part of a Biomedical Advanced Research and Development Authority, or BARDA program. Philips will lead the development of the AI-based ultrasound program, including development of advanced software for its Lumify handheld ultrasound solution that could expand its user base to non-physician emergency medical service providers.
OHSU will conduct multi-center clinical studies to acquire ultrasound images of participants with smoke-inhalation lung injuries from fires, COVID-19 lung disease, and traumatic abdominal and extremity injuries. OHSU imaging and AI experts will interpret images and electronically explain the images for machine learning and algorithm development.
“In this project, a computer will be taught to read ultrasound images — and have the result in less than a minute — that should be as accurate as a physician expert,” said Kenton Gregory, MD, founding director of the OHSU Center for Regenerative Medicine, professor of biomedical engineering in the OHSU School of Medicine, and an affiliated faculty member of the McGowan Institute of Regenerative Medicine.
Five Years, Four Program Work Streams
The OHSU and Philips collaboration focuses on the development of machine learning algorithms for the Lumify handheld ultrasound solution to detect COVID-19 lung injury, smoke inhalation lung injury, trauma-based abdominal bleeding, and severe extremity injury.
Dr. Gregory is the principal investigator charged with managing all clinical and AI imaging work for the program. OHSU will subcontract with the Department of Defense and civilian sites around the country to acquire images, which will then come to OHSU for machine-learning processing.
“By using AI-enabled ultrasound in the field, we are helping to empower first responders with a technology that can give them better insight into traumatic injuries in the field at the first point of contact,” said Joseph Frassica, MD, head of research for Philips North America. “With this type of solution, they can make better, faster assessments of patients, improve the accuracy of field triage and potentially improve outcomes.”
The system has broad application not only for mass casualty events, but also for routine health care delivery in the U.S., especially as a cornerstone diagnostic tool for rural primary care medicine, and globally in locations where chest X-ray and computerized tomography (CT) devices and expert physicians for image interpretation are not readily available.
Partnership with OmniLife to Support the Starzl Network for Excellence in Pediatric Transplantation

The Starzl Network for Excellence in Pediatric Transplantation led by UPMC Children’s Hospital of Pittsburgh has partnered with OmniLife to drive innovation around transplant graft selection and acceptance, specifically with the goal of reducing pediatric waitlist mortality.
OmniLife has been leading efforts to improve communication between all partners involved in the organ transplantation process with its novel technology since 2016. Through multiple clinical partnerships and funding awarded from the NIH, the company delivers clinical decision support in the palm of a surgeon’s hand to aid in organ selection for transplant. A clinical feasibility trial involving two centers within the Starzl Network is underway through the end of 2021.
“OmniLife is looking at a problem in the transplant realm that does not currently have a solution,” said Asad Zaman, MHA, Director, UPMC Children’s. “Working on communication during organ acceptance can help a tremendous amount of people. Even improving the process by 10 percent could mean improving hundreds or thousands of lives in addition to what they’re already doing.”
“It is wonderful to work with companies that are aligned with our mission of eliminating child waitlist deaths. Working with OmniLife will help us to push to the next step in accomplishing this goal,” says George Mazariegos, MD, the Chief of Transplantation and Surgery at UPMC Children’s and an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
“We are very excited to be working with UPMC Children’s Hospital and supporting the Starzl Network” said Eric Pahl, PhD, one of the founders of OmniLife. “We pride ourselves on providing industry-leading innovative solutions to the transplantation community, and our partnership with UPMC Children’s, the first pediatric transplant program in the USA and the wider network of top centers in America is a real honor.”
The Starzl Network for Excellence in Pediatric Transplantation is a community of the ten best programs in America. The Starzl Network honors the life and legacy of Thomas E. Starzl, MD, PhD, recognized as the father of transplantation, and carries on his work of discovery and lifesaving innovation. Given its focus on innovation, this partnership grew naturally from the work the two organizations have been collaborating on over the past few years.
Currently the Starzl Network is comprised of Seattle Children’s Hospital, University of California, San Francisco Benioff Children’s Hospitals, Ann & Robert H. Lurie Children’s Hospital of Chicago, Sick Kids (Canada), Columbia University Medical Center, Mount Sinai, UPMC Children’s Hospital of Pittsburgh, University of Virginia, Children’s Healthcare of Atlanta, and Advent Health.
Illustration: The Starzl Network for Excellence in Pediatric Transplantation and OmniLife.
Walking – It’s in Your Genes!

It’s good to walk every day. This form of exercise is a very effective way to enjoy our vitality. McGowan Institute for Regenerative Medicine affiliated faculty member Urszula Zdanowicz, MD, a surgeon/specialist in orthopedics and sports medicine at the Carolina Medical Center in Poland, explains why it is worth walking, how it affects our health, and whether it is necessary to prepare for it.
“There is a famous Schopenhauer’s saying: ‘Life is about movement and movement is its essence,’ and with this one maxim I could end my expert statement, because it is the quintessence of the matter,” laughs Dr. Zdanowicz. “The human body was made to be in constant movement. Walking is therefore the nature of man as a biological being,” she adds.
In the past, it was physical fitness, movement, and good condition that guaranteed survival. Technological progress, the development of civilization, modern inventions that make life easier have made us much less mobile. And it’s not just our fault. Nowadays, unlike in historical times, in order to survive most of the time you have to sit. It is required by the style of our work, commuting, the need to travel quickly, to name a few examples.
“There are several ways to change habits, despite a sedentary lifestyle, and move more during the day,” says Dr. Zdanowicz. “Let us choose to walk wherever possible. If we commute to work by car, park a little further away to reach the office on foot. Instead of an elevator, let’s choose stairs. If we use public transport, let’s get off two stops earlier. In the afternoon, instead of watching TV on a couch, let’s go for a walk to the park or the nearby forest.”
Why is it worth walking?
“Not only is it worth it, it is crucial! Let us not treat walks as an option, but as a permanent element in our daily schedule. Walking, exercise, this is healthy not only for our locomotor system, but also for the whole organism in many aspects, i.e., internal medicine or cardiology,” clarifies Dr. Zdanowicz. “The problem of insufficient daily activity is so serious that even local and state authorities in some countries are involved in activating the society to walk or simply move. During my stay in the United States, I came across a social campaign aimed at encouraging people to walk in shopping malls. This shows that no matter where or how, it is important to walk a certain number of kilometers every day,” explains Dr. Zdanowicz.
For our body, a dose of easy, but systematic movement in the form of walking is definitely better than an intense sport practiced once in a while.
“My patients are often managers, directors, or basically people in high professional positions—very ambitious and used to achieving their goals. When they start practicing some sport, such as running, they fully commit to it. When they start training in the gym, they start at the highest level. Competition in this area has nothing to do with health, on the contrary. It leads to injuries, excessive fatigue, or an increase in stress levels. Excessive and dynamic use of the body in combination with daily chores, work, and not getting enough sleep can lead to exhaustion.”
“When patients ask how to increase their mobility, I very often say to take frequent walks,” adds the expert. “The longer the walk, the more kilometers we go, the better impact it has on our health and fitness as well as general well-being. Such a determinant for everyday walks can be the goal of 10,000 steps, i.e., about 6 – 8 kilometers a day. I encourage patients to use popular pedometers to check how many steps we take every day. When we work in a sitting position for 8 hours and commute to work by car, we perform only 20 – 30 percent of the set goal a day. Motivation in the form of setting and measuring our goal by the number of steps per day will be the first stage to build a strong habit of movement,” says Dr. Zdanowicz.
“In addition, the great thing about walking is that it is simply pleasant, perfectly relaxing, and we can practice it at various levels of intensity: from lazy and calm, to energetic and fast – everyone can choose something for themselves. Moreover, walking is very safe, the risk of injury is low, and the activity itself is practically cost-free.”
Should we wear special shoes?
“We need shoes that fit our feet,” says the expert. “Regardless of the brand, price, or modern technologies used, if we feel comfortable in a given shoe, it means that it is just for us. It is worth investing in shoes, but the point is not to buy the most expensive ones. Just look for the ones that are best suited to our feet. Remember, that when choosing footwear, it is not only the size that matters, but additional parameters such as the width of the foot, its shape, or the difference in length between one foot and the other. Footwear that is poorly fitted will cause discomfort and reduce our willingness to walk,” explains Dr. Zdanowicz.
The problem of uncomfortable shoes can sometimes be solved by choosing the correct insole. “Our feet are designed for walking barefoot on sand, grass, or moss where the short muscles of the feet work best. If our shoes do not provide comfort, consider wearing shaped insoles that will help increase the convenience of use,” she adds.
Walking doesn’t have to be boring!
There are plenty of ideas to spice up our walk. We can take children or pets on expeditions, choose interesting routes full of greenery and a pleasant atmosphere, practice Nordic walking which engages many muscles of our body, and, let’s face it, gives us a lot of fun. Starting with gradual, slow walks, as we improve our condition, we can increase the pace and try longer and longer distances or more vigorous marches. They will have a beneficial effect not only on our musculoskeletal system and general well-being, but will also burn a large dose of calories, which will help us shape the correct figure.
Through TED-Ed, Dr. Elizabeth Wayne Explains mRNA Technology and COVID Vaccines

Elizabeth Wayne, PhD, is a McGowan Institute for Regenerative Medicine affiliated faculty member, a Biomedical Engineer at Carnegie Mellon University, a TED Fellow, and advocate for women in higher education. Dr. Wayne’s areas of expertise include biomedical engineering, cancer, gene delivery, nanomedicine, and STEM education.
Dr. Wayne and Kaitlyn Sadtler, PhD, a postdoctoral fellow at MIT who received her PhD from the Johns Hopkins University School of Medicine, dig into the science of mRNA technology in a TED-Ed original lesson entitled, “How the COVID-19 Vaccines Were Created So Quickly.”
In the 20th century, most vaccines took over a decade to research, test, and produce. But the vaccines for COVID-19 were cleared for emergency use in less than 11 months. The secret behind this speed is a medical technology that’s been developing for decades: the mRNA vaccine. So how do these revolutionary vaccines work? Find out by watching Drs. Wayne and Sadtler’s TED-Ed lesson here.
This TED program features the words and ideas of educators brought to life by professional animators. Their lesson was directed by Igor Ćorić, Artrake Studio, narrated by Bethany Cutmore-Scott, with music by Nikola Radivojevic.
Dr. Kathryn Whitehead Participates in TED/Monteray

McGowan Institute for Regenerative Medicine affiliated faculty member Kathryn Whitehead, PhD, Associate Professor and Dean’s Career Fellow in the Departments of Chemical Engineering and Biomedical Engineering at Carnegie Mellon University, recently participated in TED/Monteray. Her lab develops RNA and protein drug delivery systems and has a long-term goal of predicting the behavior of delivery materials in humans.
What if you were holding life-saving medicine … but had no way to administer it? During her presentation, “The Tiny Balls of Fat that Could Revolutionize Medicine,” you will zoom down to the nano level with engineer Dr. Whitehead as she:
- gives a breakdown of the little fatty balls (called lipid nanoparticles) perfectly designed to ferry cutting-edge medicines into your body’s cells
- explains how her work is already powering mRNA-based COVID-19 vaccines
- discusses forging the path for future therapies that could treat Ebola, HIV, and even cancer
Watch Dr. Whitehead’s presentation here.
Illustration: TEDMonteray.
AWARDS AND RECOGNITION
Dr. Rory Cooper Receives Science and Society Award

Sigma Xi, the Scientific Research Honor Society, has honored McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, with the John P. McGovern Science and Society Award. Recipients of this award represent individuals who have supported research, the communication of science, and the impact of science on society.
Presentation of the award will take place at the virtual Sigma Xi Annual Meeting and Student Research Conference on Nov. 4-7. Dr. Cooper will present the talk, “Participatory Action Design and Engineering: Forging a New Freedom!” that will suggest pathways to expand the talent pool of scientists and engineers. Dr. Cooper will also speak about the work that he and his colleagues are doing to create technologies and systems for older adults and people with disabilities.
Dr. Cooper is the founding director and VA senior research career scientist of the Human Engineering Research Laboratories at Pitt. He is also an elected fellow of the National Academy of Inventors and has authored or co-authored more than 300 peer-reviewed journal publications.
Congratulations, Dr. Cooper!
Dr. Thomas Rando Named Director of UCLA Broad Stem Cell Research Center

McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Rando, MD, PhD, a renowned neurologist and stem cell biologist, has been named Director of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at the University of California, Los Angeles (UCLA). His appointment is effective October 1.
Dr. Rando, who was chosen after an international search, is currently a Professor of Neurology and Neurological Sciences at the medical school at Stanford University, where he also serves as Director of the Glenn Center for the Biology of Aging and Deputy Director of the Stanford Center on Longevity. In addition, he is Chief of Neurology at the Veterans Affairs Palo Alto Health Care System.
“As a trailblazing clinical investigator and inspired scientific leader, Tom is well positioned to lead the Broad Stem Cell Research Center to even greater successes,” said UCLA Chancellor Gene Block. “His accomplishments as a director of numerous multidisciplinary and collaborative scientific programs, both at Stanford and the VA, are a testament to his abilities as a champion for innovative research, a mentor and an administrator.”
Dr. Rando will succeed S. Thomas Carmichael, MD, PhD, who has served as Interim Director of the Center since 2020, and Owen Witte, MD, who served as Founding Director from 2005 to 2020.
With a membership of nearly 250 researchers and clinicians representing more than 55 UCLA departments, schools and institutes, the Broad Stem Cell Research Center is committed to revolutionizing the treatment of disease through personalized cellular therapies and regenerative medicine. The center is well regarded for its dynamic and highly collaborative research program, its success in supporting cutting-edge research and translating discoveries into life-saving therapies, and its dedication to training the next generation of stem cell investigators.
As Director, Dr. Rando will seek to build on the Center’s strengths and promote a culture of scientific excellence, academic integrity and interdisciplinary collaboration. He will also work to bring about new partnerships with the larger scientific community — including other institutions and private companies — to address the most urgent challenges of medicine.
“I see the opportunity to lead a world-class center like this one as a chance to contribute to the field of regenerative medicine to a degree that far surpasses what I could accomplish as an individual investigator,” Dr. Rando said. “It is an honor to join the Center’s remarkable community of scientists, clinicians and trainees to advance our shared mission of revolutionizing the treatment of disease.”
Dr. Rando received a bachelor’s degree in biochemistry, a doctorate in cell and developmental biology and a medical degree from Harvard University. He completed his internship at Massachusetts General Hospital, his residency in neurology at UC San Francisco and a research fellowship in molecular pharmacology at Stanford.
Dr. Rando began his career researching the development and progression of muscular dystrophies. This led to an interest in regenerative medicine, particularly somatic cell function, which has been the central focus of his laboratory for the past 20 years. He has published extensively and founded biotechnology startups in his major areas of research, which include stem cell aging and epigenetic rejuvenation, tissue engineering, muscle stem cell biology and muscular dystrophies.
Dr. Rando has also served as director of the Palo Alto VA’s Geriatric Research, Education and Clinical Center and its Rehabilitation Research and Development Center of Excellence. At Stanford, he has championed diversity, equity and inclusion through outreach programs that include the Postdoctoral Recruitment Initiative in Science and Medicine, the Hispanic Center of Excellence and the Stanford Medical Youth Science Program.
Dr. Rando is an elected member of the National Academy of Medicine, the American Academy of Arts and Sciences, and the American Neurological Association. Among other honors, he has received a Director’s Pioneer Award and a Transformative Research Award from the National Institutes of Health, a Paul Beeson Physician Faculty Scholar Award in Aging Research, a Breakthroughs in Gerontology Award from the American Federation for Aging Research and a Senior Scholar Award from the Ellison Medical Foundation.
Congratulations, Dr. Rando!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#225 –– Dr. Partha Roy discusses his research in directed cell migration and actin-binding proteins (ABPs).
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Erin K. McCreary, J. Ryan Bariola, Tami Minnier, Richard J. Wadas, Judith A. Shovel, Debbie Albin, Oscar C. Marroquin, Kevin E. Kip, Kevin Collins, Mark Schmidhofer, Mary Kay Wisniewski, David A Nace, Colleen Sullivan, Meredith Axe, Russell Meyer, Alexandra Weissman, William Garrard, Octavia M. Peck-Palmer, Alan Wells, Robert D. Bart, Anne Yang, Lindsay Berry, Scott Berry, Amy Crawford, Anna McGlothin, Tina Khadem, Kelsey Linstrum, Stephanie K. Montgomery, Daniel Ricketts, Jason N. Kennedy, Caroline J. Pidro, Ghady Haidar, Graham M. Snyder, Bryan J. McVerry, Derek C. Angus, Paula L. Kip, Christopher W. Seymour, David T. Huang
Title: A Learning Health System Randomized Trial of Monoclonal Antibodies for Covid-19
Summary: Background: Neutralizing monoclonal antibodies (mAb) targeting SARS-CoV-2 decrease hospitalization and death in patients with mild to moderate Covid-19. Yet, their clinical use is limited, and comparative effectiveness is unknown.
Methods: We present the first results of an ongoing, learning health system adaptive platform trial to expand mAb treatment to all eligible patients and evaluate the comparative effectiveness of available mAbs. The trial launched March 10, 2021. Results are reported as of June 25, 2021 due to the U.S. federal decision to pause distribution of bamlanivimab-etesevimab; patient follow-up concluded on July 23, 2021. Patients referred for mAb who met Emergency Use Authorization criteria were provided a random mAb allocation of bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab with a therapeutic interchange policy. The primary outcome was hospital-free days (days alive and free of hospital) within 28 days, where patients who died were assigned -1 day. The primary analysis was a Bayesian cumulative logistic model of all patients treated at an infusion center or emergency department, adjusting for treatment location, age, sex, and time. Inferiority was defined as a 99% posterior probability of an odds ratio < 1. Equivalence was defined as a 95% posterior probability that the odds ratio is within a given bound.
Results Prior to trial launch, 3.1% (502) of 16,345 patients who were potentially eligible by an automated electronic health record (EHR) screen received mAb. During the trial period, 23.2% (1,201) of 5,173 EHR-screen eligible patients were treated, a 7.5-fold increase. After including additional referred patients from outside the health system, a total of 1,935 study patients received mAb therapy (128 bamlanivimab, 885 bamlanivimab-etesevimab, 922 casirivimab-imdevimab). Mean age ranged from 55 to 57 years, half were female (range, 53% to 54%), and 17% were Black (range, 12% to 19%). Median hospital–free days were 28 (IQR, 28 to 28) for each mAb group. Hospitalization varied between groups (bamlanivimab, 12.5%; bamlanivimab-etesevimab, 14.7%, casirivimab-imdevimab, 14.3%). Relative to casirivimab-imdevimab, the median adjusted odds ratios were 0.58 (95% credible interval (CI), 0.30 to 1.16) and 0.94 (95% CI, 0.72 to 1.24) for the bamlanivimab and bamlanivimab-etesevimab groups, respectively. These odds ratios yielded 91% and 94% probabilities of inferiority of bamlanivimab versus bamlanivimab-etesevimab and casirivimab-imdevimab respectively, and an 86% probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab, at the prespecified odds ratio bound of 0.25. Twenty-one infusion-related adverse events occurred in 0% (0/128), 1.4% (12/885), and 1.0% (9/922) of patients treated with bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab, respectively.
Conclusion: In non-hospitalized patients with mild to moderate Covid-19, bamlanivimab, compared to bamlanivimab-etesevimab and casirivimab-imdevimab, resulted in 91% and 94% probabilities of inferiority with regards to odds of improvement in hospital-free days within 28 days. There was an 86% probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab at an odds ratio bound of 0.25. However, the trial was unblinded early due to federal distribution decisions, and no mAb met prespecified criteria for statistical inferiority or equivalence.
Source: JAMA Intern Med. 2021 Jan 1;181(1):41-51.
GRANT OF THE MONTH
PI: Alisse Hauspurg
Co-I: Ramakrishna Mukkamala, Sanjeev Shroff, and Aman Mahajan
Title: Development of a smartphone-based device to detect fluid overload among postpartum women with hypertensive disorders of pregnancy
Description: Hypertensive disorders of pregnancy contribute to a significant proportion of maternal morbidity and mortality and are the most common reason for hospital readmission postpartum. While treatment of hypertension is the cornerstone of postpartum management, emerging evidence suggests that implementation of robust remote blood pressure monitoring and treatment programs alone do not reduce maternal morbidity or hospital readmissions related to postpartum fluid overload and heart failure symptoms. The ability to predict which women will develop these complications and pursue more aggressive treatment with diuresis prior to hospital discharge or in the first week postpartum is limited. The overall goal of this line of research is to develop and implement a convenient congestion prediction device for home use in conjunction with home blood pressure monitoring in a postpartum population following a hypertensive disorder of pregnancy. In this application, the objective is to develop and test a smartphone-based device to predict fluid overload in patients with preeclampsia through three specific aims: 1) to build multiple smartphone-based devices to assess volume status via a congestion prediction index in postpartum women with preeclampsia, 2) to collect patient training data with the devices, optimize their accuracy in assessing volume status, and identify and create the final real-time device and 3) to validate the final device prospectively in response to known fluid changes among a new cohort of postpartum women with preeclampsia. The research proposed in this application is innovative in its novel adaptation of evidence-based approaches to fluid assessment in heart failure patients to an understudied postpartum population with significant morbidity, its use of a smartphone form factor and the design and the proposed testing of multiple devices to optimize effectiveness and convenience. This proposal is significant as it will provide an opportunity to develop and assess the feasibility of smartphone- based assessment of fluid status among postpartum women with preeclampsia. Effective interventions to improve postpartum hypertension care coupled with innovative remote strategies have broad implications for improving maternal morbidity and reducing disparities in care. Our findings will provide a valuable framework to inform a future randomized trial of remote assessment and management of fluid status in the postpartum period. Successful completion of this line of research would represent a transformation in postpartum management of women with preeclampsia and have a direct impact to improve hypertension and cardiovascular-related maternal morbidity and mortality.
Source: National Institute of Biomedical Imaging and Bioengineering
Term: July 1, 2021 – March 31, 2024
Amount: $160,214 (one year)
