September 2023 | VOL. 22, NO. 9 | www.McGowan.pitt.edu
Stories by Cristina D’Imperio
McGowan Researchers Examine Wound Healing in Diabetics
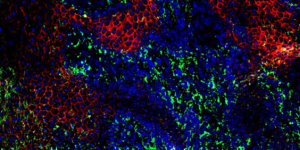
Image: Skin cells at the edge of a diabetic wound. Red and green colors depict two proteins involved in the release of exosomes from these cells (CREDIT: Subhadip Ghatak).
McGowan affiliated faculty Mohamed S. El Masry, PhD; Subhadip Ghatak, PhD; and Sashwati Roy, PhD, alongside McGowan Associate Researcher Anu Sharma, PhD; Research Assistant Piya Das Ghatak; and Director of the McGowan Institute Chandan K. Sen, PhD, recently authored a study published in Nano Today.
The study, titled “Nanoscopic and functional characterization of keratinocyte-originating exosomes in the wound fluid of non-diabetic and diabetic chronic wound patients,” provides insight into why wounds often heal poorly in patients with diabetes.
“In patients with diabetes, wound healing is impaired because of excess inflammation,” said Dr. Sen. When exosomes, small particles that carry information between cells, do not function properly in patients with diabetes, inflammation can increase and impair wound healing.
Researchers collected bandages containing wound fluid from 22 diabetic and 15 non-diabetic patients in order to isolate and examine exosomes.
They found that if signals contained within the exosomes were correct, then inflammation in the wound would ultimately be resolved. However, they found that diabetic exosomes, or “diaexosomes,” compromised wound healing and led to the production of pro-inflammatory compounds.
“Diaexosomes drive deviation from the healing cascade, so that resolution of inflammation is compromised,” said Dr. Sen. “And this isn’t just limited to wounds. Because exosomes are responsible for many functions in the body, diaexosomes could play a role in other diabetic complications.”
“Left untreated,” Dr. Sen continued, “these non-healing, or chronic, wounds can lead to limb amputations. More than 100,000 diabetes-related amputations occur in the U.S. each year, but by understanding more about wound healing and developing new therapies, our goal is to bring down this number.”
Read more from UPMC here.
RESOURCES AT THE MCGOWAN INSTITUTE
October Histology Special – Bone Samples
The Histology lab offers decalcification, processing and embedding, cutting and staining of those difficult bone samples. Our staff has years of experience with bone samples that include special staining and IHC on large and small bone samples that can be difficult to work with. We have even developed a technique to ensure your sample will not lift from the slide during aggressive antigen retrieval protocols. Below are just some of the special stains we offer at the McGowan Histology Core:
You’ll receive 25% off bone samples* in October when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: Please contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
* 25% off applies to routine sample preparation (processing, embedding, cutting, and staining). It does not apply to specialized services such as immunohistochemistry workups, and IHC staining.
SCIENTIFIC ADVANCES
The Story Behind Restoring Sight

At 25 years old, José-Alain Sahel (pictured) was a medical resident at the University of Strasbourg in France tasked with the job of informing parents when their children were found to have permanent vision loss.
Dr. Sahel, now Distinguished Professor of the Department of Ophthalmology at the University of Pittsburgh School of Medicine, director of the UPMC Eye Center, and the Eye and Ear Foundation Endowed Chair of Ophthalmology, as well as a McGowan affiliated faculty member, spoke with the Richard King Mellon Foundation about his experiences.
“I tried to be nice,” he said, “but you cannot be nice when you say that. People hate you. They just hate you. It had a real impact on me.” At times, parents would yell, patients would cry, and Dr. Sahel would feel frustrated he was unable to help them.
These early frustrations as a resident, however, spurred Dr. Sahel to dedicate himself to research.
He completed his residency at the University of Strasbourg and became a research fellow at the Massachusetts Eye and Ear Infirmary, a visiting scholar in the Department of Molecular and Cellular Biology at Harvard University, and a visiting lecturer at Harvard Medical School. He eventually founded the Vision Institute in Paris and served as its director from 2008 until 2020.
Dr. Sahel is now globally recognized for his expertise in vision restoration techniques. He has developed several interventions, including stem cell implantation, gene therapy, innovative pharmacologic approaches, and retinal prostheses for numerous vision impairments that currently are untreatable.
In the last decade, he has led pioneering efforts in optogenetic vision restoration, a technique in which cells in the retina are genetically modified to express light sensitive proteins. Now, Dr. Sahel has overseen more than 80 clinical trials.
One recent trial combined gene therapy with light-enhancing glasses as a visual prothesis. The trials demonstrated that the patients, who suffer from blindness, were able to perceive light stimuli. One of the patients in the trial had been blind for decades.
Dr. Sahel called the people who volunteer for clinical trials “the real heroes,” the pioneers who are willing to help researchers push “the biomedical frontier.”
Fueled by early frustrations, Dr. Sahel himself has pushed the boundaries of the biomedical frontier over the past four decades. He now finds his work gratifying, stating that the most rewarding moments are working with patients and their families. He wants to give patients suffering from blindness hope. “The main thing,” he states, “is there is a path forward. It’s not desperate. It’s not the end.”
Read more from the Richard King Mellon Foundation here, by logging in to LinkedIn.
Photo courtesy of George Lange.
Treating Trauma in Children: Dr. Spinella Co-Principal Investigator in $34M Clinical Trial

“Pediatric trauma research is decades behind research in adults,” maintains Christine Leeper, MD, Assistant Professor of Surgery and Critical Care Medicine at the University of Pittsburgh.
Trauma is the leading cause of death in children in the U.S., with most deaths occurring within 24 hours of injury. In comparison to adult trauma patients, children are more likely to die in the emergency room than survive long enough to be either admitted into the hospital or transferred to the operating room.
“Not only is trauma the leading cause of death in kids,” states Philip Spinella, MD (pictured), “their mortality rate with life-threatening bleeding is almost twice that of adults. And we think that’s because of delay in recognition and treatment, not that children are less resilient.”
Dr. Spinella, who is McGowan affiliated faculty and Co-Director of the Trauma and Transfusion Medicine Research Center, alongside Dr. Leeper and Angelo D’Alessandro, PhD, Professor of Biochemistry and Molecular Genetics at the University of Colorado, is launching a clinical trial to test, simultaneously, multiple interventions for life-threatening bleeding in children.
The trial, Massive Transfusion in Children-II (MATIC-II) will be the first randomized “platform” trial of its kind to assess traumatically injured children. Platform trials test multiple therapies at once, whereas more typical trials assess only one intervention. This will enable researchers to assess the safety and effectiveness of treatments based on data collected during the trial, further allowing the researchers to add or remove treatments in order to provide the best outcomes for patients.
The MATIC-II platform trial will study blood transfusions in pediatric patients. When blood is donated, it is usually separated into red blood cells, platelets, and plasma. Many patients who experience substantial bleeding require all three. Recent research indicates that “whole” blood, rather than blood that has been separated, may improve survival and reduce the amount of overall blood needed by patients.
MATIC-II will study the safety and effectiveness of several different types of whole blood transfusions in 1,000 traumatically injured children across 20 U.S. pediatric trauma centers. The study is funded by the Biomedical Advanced Research and Development Authority, part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health & Human Service. It currently has a budget of $34 million for the first five years, with the potential for funding to increase to more than $81 million.
Looking to the near future with optimism, Dr. Spinella upholds, “This trial will be transformative when it comes to trauma care for children.”
Read more from the Pittsburgh Business Times here.
Find the story on UPMC here.
Dr. Kim Awarded Grant from the Brain Aneurysm Foundation

Seungil Kim, PhD (pictured), McGowan affiliated faculty and Research Assistant Professor at the University of Pittsburgh, is the recipient of a research grant from the Brain Aneurysm Foundation (BAF).
The BAF is the leading advocacy organization supporting education, research, and policy in the treatment of brain aneurysms. Dr. Kim is one of 17 leading academic researchers chosen from across the U.S. and Canada to receive funding.
The work of this year’s awardees includes efforts to develop biomarkers to better predict and develop potential therapeutics for aneurysms, understand the impact of genetics on rupture outcomes, and create new device technology, drug delivery, and robotic approaches to evolve treatment.
“I am thrilled with the quality and relevance of the programs being led by this year’s grant recipients,” states Christopher Ogilvy, MD, Director of Endovascular and Operative Neurovascular Surgery at the Brain Aneurysm Institute at Beth Israel Deaconess Medical Center, states. “In each case we believe their work will directly support the field and build upon our understanding of why and how aneurysms form, who they are more likely to impact, and how to continuously improve treatment.”
Approximately 1 in 50 people in the U.S. experience a brain aneurysm, with more than 30,000 suffering from a rupture annually. Many are misdiagnosed in the emergency room, and federal funding for brain aneurysm research amounts only to around $2 per afflicted person.
Christine Buckley, the BAF’s executive director, maintains, “These early grants are key to fostering an ecosystem that brings ideas from academia to the clinic with the goal of transforming patient care and saving lives.”
Dr. Kim’s grant was awarded at the 17th Annual research Grant Symposium on September 21, 2023, in South Carolina.
Read the press release from the Brain Aneurysm Foundation here.
BOOM: Pioneering Digital Bladder Collaboration Awarded $3.2 million, Dr. Robertson Co-Principal Investigator

Image: Pictured left to right: Professor Naoki Yoshimura and Professor Anne Robertson from the University of Pittsburgh with Dr Paul Watton from the University of Sheffield.
Anne Robertson, PhD (pictured center), McGowan affiliated faculty, is collaborating with researchers from the University of Pittsburgh and the University of Sheffield to develop the digital twin of a bladder.
The project, titled “A Digital Twin for Designing Bladder Treatment informed by Bladder Outlet Obstruction Mechanobiology (BOOM),” has been awarded a $3.2 million R01 grant from the National Institutes of Health (NIH) to help design patient-specific treatments for those with bladder outlet obstruction (BOO).
BOO can negatively impact men as they age and is characterized by a blockage that stops or slows the flow of urine from the bladder. Symptoms include abdominal pain, the sensation of a continuously full bladder, and trouble urinating. Men between the ages of 50 and 60 have an 80% chance of experiencing some degree of BOO.
Over 200 million people globally are affected by symptoms related to BOO. However, as Dr. Robertson states, “The connection between changes to the BOO bladder wall structure and bladder functionality are not understood. Our digital twin of the bladder, informed by extensive data, will enable us to better understand this connection.”
Currently, surgical procedures to treat BOO are only 70% effective. A digital representation of the bladder, however, would not only enable a greater understanding of BOO but also how it can be more effectively treated, both surgically and pharmacologically. The project seeks to make use of state-of-the-art studies of BOO in rat models and corresponding computational data as a basis for the development of the new digital technology.
“By creating this computational model of a real physiological system,” says Dr. Robertson, “we can gain data in real time and try different interventions to prime our model so it can be used on a patient-specific basis in the future.” Adjusting the digital twin in real time with personalized data would enable clinicians to better predict the success of a treatment on individual patients.
In short, Dr. Robertson maintains, “The idea would be that a medical doctor could put an individual’s own measurements and clinical information into the model and then provide a patient-tailored treatment.”
Other researchers on this project include:
- Paul Watton, co-principal investigator, University of Sheffield
- Naoki Yoshimura, University of Pittsburgh
- Kang Kim, University of Pittsburgh
- Yasutaka Tobe, University of Pittsburgh
- Simon Watkins, University of Pittsburgh
- Spandan Maiti, University of Pittsburgh
- Alan Watson, University of Pittsburgh
- Kanako Matsuoka, University of Pittsburgh
- Tadanobu Kamijo, University of Pittsburgh
- Richard Clayton, University of Sheffield
Read more from Pitt’s Swanson School of Engineering here.
Living-Donor Transplant Program: Dr. Mazariegos Collaborates to Save Toddler’s Life
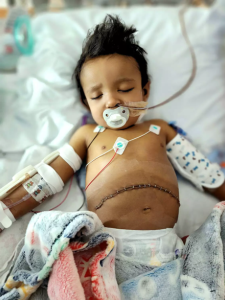
Logan Salva at UPMC Children’s Hospital of Pittsburgh.
Nearly two years ago, Logan Salva of Ocala, Florida, was born with Alagille Syndrome, a rare genetic mutation that caused bile to build up in his liver. Alagille Syndrome appears in one out of 70,000 babies and can ultimately cause the liver to fail, necessitating a transplant.
As Logan’s symptoms persisted, it became apparent that he needed a liver transplant. However, his parents Raska Marletto-Salva and Skyler J. Salva discovered that Logan could possibly spend months or years on a transplant waitlist. Based on his condition, they feared he would be unable to survive the wait.
According to the United Network for Organ Sharing (UNOS), there are approximately 400 children waiting for a liver transplant. An average of 6% of these children die while on the waitlist.
The Salvas then turned to living-donor transplantation and a pediatric liver transplant program at UPMC Children’s Hospital of Pittsburgh.
George Mazariegos, MD, McGowan affiliated faculty, pediatric liver and intestine transplant surgeon, and the Director of the Hillman Center for Pediatric Transplantation at the Children’s Hospital of Pittsburgh of UPMC is part of a collaboration with Advent Health for Children, which works with UPMC Children’s Hospital to expand access to lifesaving pediatric liver transplants to central and northern Florida.
Through Advent Health for Children, Logan’s family in central Florida was connected with UPMC Children’s Hospital and Makenzie Beach, a nurse from Erie, Pennsylvania, who volunteered to be a living donor.
“The great thing about […] living donation,” said Dr. Mazariegos in an interview with WTAE, “is that we are able to take a perfectly healthy segment of the liver in a transplant situation and be able to optimize [the] chance for a really great recovery.”
Logan’s family traveled to Pittsburgh for his surgery, and on June 8, 2023, surgeons successfully transplanted a portion of Beach’s liver into Logan. Only about 5% of people who undergo a liver transplant receive the organ from a living donor.
Three months later and both Beach and Logan are doing well.
“It’s incredible the change that we saw in him physically, just in his appearance and complexion and everything,” Marletto-Salva, Logan’s mother, said. “It happened within days, with his complexion, and that was just the beginning of it, and then he just started to fill out and look like a normal little kid again.”
Beach has already returned to work. “It’s much more manageable than people realize,” she said. “My liver has probably already grown back to its full size again, and I was able to save a life for a couple weeks of some discomfort. It seems like a small price.”
In an interview with WPXI, Dr. Mazariegos stated, “In most cases, when a child undergoes a transplant, their disease does not come back, and they are able to achieve a normal childhood, normal growth, and a normal transition into adulthood that wouldn’t have been possible without the transplant.”
Thanks to the altruistic donation by Beach, the efforts of Advent Health, and the research, studies, and surgeries performed by Dr. Mazariegos and others at UPMC, Logan now has the chance to live a healthy life.
“I feel like I was part of a miracle,” said Beach.
Read the original reporting by WTAE News here.
Dr. Angus Co-Authors Study that Seeks to Improve Equitable Access to Scarce Medication

Derek Angus, MD, MPH, FRCP (pictured), McGowan affiliated faculty, co-authored a study published this September in JAMA Health Forum.
The study, titled “Weighted Lottery to Equitably Allocate Scarce Supply of COVID-19 Monoclonal Antibody,” sought to determine whether a weighted lottery could promote the “equitable allocation of scarce resources to disadvantaged populations” in a large health system.
The study implemented a weighted lottery in a 35-institution health system that spanned parts of Pennsylvania, New York, and Maryland. A total of 10,834 individuals were eligible for the lottery: 16.6% were from disadvantaged neighborhoods, 7.1% were Black, and 50.5% were women.
Ultimately, the study found that with a weighted lottery system in a large health system does, in fact, help to allocate resources to disadvantaged populations. However, it also found that “Black individuals who are allocated [the resources] may be less likely to accept allocation and receive it.”
Lead author Erin McCreary, PharmD, Clinical Assistant Professor in the University of Pittsburgh’s School of Medicine’s Division of Infectious Diseases, and Director of Infectious Diseases Improvement and Clinical Research Innovation at UPMC, stated, “Equitable allocation of a resource is not the same as equitable receipt of that resource.”
“The weighted lottery and UPMC’s efforts to remove barriers to care worked to get [the resource] to many patients who otherwise likely would have been unable to receive it,” said senior author Douglas White, MD, professor and UPMC Endowed Chair for Ethics in Critical Care Medicine. “But we also learned that we must continue to enhance efforts to remove barriers to care if we are ever to close the considerable gap in racial equity during times of scarcity.”
Read more from UPMC here.
Left Stimulated: Dr. Weber’s Work to Restore Hand and Arm Function after Stroke

Image from left to right: Drs. Weber, Pirondini, and Capogrosso
More than a decade ago, college student Heather Rendulic suffered a series of five brain bleeds over an 11-month period. An active runner and horseback rider, Rendulic had to relearn how to walk. The left half of her body had become paralyzed.
Globally, one quarter of adults over the age of 25 will experience a stroke. Approximately 75% of all people who suffer a stroke will also experience loss of motor control in an arm and hand. Six months after a stroke, or the “chronic stage,” there are no effective federally approved treatments for paralysis in the U.S.
A team of Pittsburgh-based researchers are using electric stimulators to change that.
In 2021, Douglas Weber, PhD, McGowan affiliated faculty; Elvira Pirondini, PhD, Assistant Professor of Neural Engineering and Physical Medicine and Rehabilitation; and Marco Capogrosso, PhD, Assistant Professor in the Department of Neurological Surgery at the University of Pittsburgh, began a collaboration at Pitt’s Neural Engineering Labs.
Drs. Weber, Pirondini, and Capogrosso worked alongside a team of researchers to begin the first clinical trial to use spinal implants to treat arm and hand paralysis in stroke patients. One of the patients they enrolled was Heather Rendulic.
Shortly after the trial began, surgeons threaded two small electrodes through a small incision in Rendulic’s upper back. The electrodes were positioned in her neck, in the epidural space over the spinal cord, in order to engage intact neural circuits. Prior to the surgery, Rendulic’s left arm was all but paralyzed.
After the surgery, Rendulic was able to fully open and close her fists, raise her arms above her head, and complete everyday tasks that had previously been impossible.
The technology implanted in Rendulic has also been used in patients experiencing high-grade, persistent pain, as well as patients who are paralyzed from the waist down. However, restoring the dexterity of a hand and the full range of motion to an arm comes with a unique set of challenges.
“We discovered that electrical stimulation of specific spinal cord regions enables patients to move their arm in ways that they are not able to do without the stimulation,” says Dr. Capogrosso. The stimulation occurs only when patients are trying to use their arm or hand.
“The sensory nerves from the arm and hand send signals to motor neurons in the spinal cord that control the muscles of the limb,” Dr. Weber explains in an interview with PittMed. “By stimulating these sensory nerves, we can amplify the activity of muscles that have been weakened by stroke. Importantly, the patient retains full control of their movements.”
Surprisingly, researchers also learned from the trial that the implants could prompt longer-lasting mobility improvements. “We found that after a few weeks of use,” states Dr. Capogrosso, “some of these improvements endure when the stimulation is switched off, indicating exciting avenues for the future of stroke therapies.”
Rendulic, too, is optimistic. “That very first day, none of us knew for sure if it was going to work, or how it would feel if it did,” she says. “But that very first day in the lab, I opened and closed my hand for the first time in the nine years since my stroke. My husband and my mom were there, and when I opened that hand, there were just tears of joy.”
Today, as she continues to work with Drs. Weber, Pirondini, and Capogrosso, Rendulic notes, “The future of this is so exciting. […] I wholeheartedly believe this technology can change millions of lives.”
Read more at the New York Times, CNN, NPR, and the BBC.
Dr. Sen Pens Article for the Pittsburgh Post Gazette

Chandan K. Sen, PhD (pictured), Director of the McGowan Institute, wrote an article for the Pittsburgh Post-Gazette this week, titled “Expert Insights: One small step in diabetic wound care, one giant leap for amputation prevention.” It appeared online on September 22, 2023.
The article discusses the dangers of diabetic foot wounds, including the reality that some patients may require amputation. Of the patients who undergo amputations due to vascular disease, almost half will die within five years.
In Pennsylvania alone, Dr. Sen maintains, 1.5 million people suffer from diabetes, ranking the state among the top 10 with the worst outcomes in leg amputation. In order to better address the many issues stemming from diabetes and foot wounds, the McGowan Institute and the University of Pittsburgh have become home to the national Diabetic Foot Consortium (DFC).
The DFC is funded by the National Institutes of Health Institute of Diabetes and Digestive Kidney Diseases (NIDDK); there are 21 wound clinics state-wide.
Read the full article at the Pittsburgh Post-Gazette, here.
Dr. Singh Proposal Recommended for Department of Defense Funding

Kanhaiya Singh, PhD (pictured), McGowan faculty, has had a proposal recommended for funding by the Department of Defense (DoD) office of the Congressionally Directed Medical Research Programs (CDMRP).
Dr. Singh’s award is anticipated to be funded with FY23 funds, which will extend until September 30, 2029.
His proposal is titled “Endothelial-Targeted Metallothionein Gene Editing to Enable VEGF Therapy in Rescuing Diabetic Pressure Ulcer.”
Dr. Singh’s laboratory focuses on epigenetics of diabetic vasculopathy and wound healing.
His laboratory is investigating epigenetic mechanisms such as DNA methylation as a proxy for abnormal gene–environmental interactions in diabetic ischemic wounds. The lab’s current line of study aims to develop a simple and point-of-care approach to achieve targeted epigenetic editing of adult diabetic ischemic tissue in vivo.
His laboratory is also investigating the molecular mechanism in diabetic vasculopathy and limb ischemia. Researchers are applying single-cell RNA sequencing technology to study the endothelial cells of the human diabetic skin in order to identify novel ligands and receptors that can improve outcomes of the vascular endothelial growth factor (VEGF) therapy. To that end, his team is employing tissue nanotransfection technology as a delivery platform to administer combination gene therapy for diabetic ischemic limb rescue.
AWARDS AND RECOGNITION
Diversifying the Silver Screen: Dr. Cooper Featured in ReelAbilities Film Festival

From September 7-13, Film Pittsburgh hosted its 11th Annual ReelAbilities Film Festival at the Pittsburgh Playhouse.
One of this year’s films featured McGowan affiliated faculty, Rory Cooper, PhD (pictured).
On CBS News Pittsburgh’s Pittsburgh Today Live, Dr. Cooper spoke about his impetus to participate in Bumps in the Road, a film that follows him and “a team of wheelchair-using engineers” at the University of Pittsburgh.
“The film briefly chronicles the work that we do, and there’s a little bit about my life and my wife’s life,” said Dr. Cooper in the interview. “And the work we do is to create technologies to help people fully participate in society and to help them achieve their dreams.”
Dr. Cooper has co-authored more than 375 peer-reviewed publications and has over 30 awarded or pending patents. His work as Director of the Human Engineering Research Laboratories (HERL) focuses on wheelchair technology that expands mobility and reduces second-hand injuries for millions of people with disabilities.
“We also work with people with disabilities to help them become scientists and engineers, to get engaged in research, and to become healthcare professionals themselves,” Dr. Cooper told CBS.

Dr. Cooper is an advocate for diversity and in 2020 spoke to the U.S. Congress about the lack of diversity among American inventors.
Executive Director of Film Pittsburgh, Kathryn Spitz Cohan, appeared on CBS alongside Dr. Cooper and maintained the importance of representation. “It is so important to see yourself on the screen,” she told CBS. “One in 4 people in the U.S. live with a disability. In the last hundred years, less than 5% of movies and TV shows have disability content.”
In an interview with the Pittsburgh Post-Gazette, Dr. Cooper said, “It is important to tell stories of lived experiences and contributions of people with disabilities as scientists, engineers, and inventors to encourage more people with disabilities to pursue these paths. It helps the public to understand the multitude of contributions made to society by people with disabilities and that we are not one-dimensional.”
Bumps in the Road was screened at the Playhouse on September 8.
Read more from the Pittsburgh Post-Gazette.
Watch Dr. Cooper’s interview on CBS News Pittsburgh.
PUBLICATION OF THE MONTH
Author: Poornachander R. Guda, Anu Sharma, Adam J. Anthony, Mohamed S. El Masry, Andrew D. Couse, Piya Das Ghatak , Amitava Das, Lava Timsina, Jonathan C. Trinidad, Sashwati Roy, David E. Clemmer, Chandan K. Sen, Subhadip Ghatak
Title: Nanoscopic and functional characterization of keratinocyte-originating exosomes in the wound fluid of non-diabetic and diabetic chronic wound patients
Summary: Exosomes, a class of extracellular vesicles of endocytic origin, play a critical role in paracrine signaling for successful cell-cell crosstalk in vivo. However, limitations in our current understanding of these circulating nanoparticles hinder efficient isolation, characterization, and downstream functional analysis of cell-specific exosomes. In this work, we sought to develop a method to isolate and characterize keratinocyte-originated exosomes (hExoκ) from human chronic wound fluid. Furthermore, we studied the significance of hExoκ in diabetic wounds. LC-MS-MS detection of KRT14 in hExoκ and subsequent validation by Vesiclepedia and Exocarta databases identified surface KRT14 as a reliable marker of hExoκ. dSTORM nanoimaging identified KRT14+ extracellular vesicles (EVκ) in human chronic wound fluid, 23% of which were of exosomal origin. An immunomagnetic two-step separation method using KRT14 and tetraspanin antibodies successfully isolated hExoκ from the heterogeneous pool of EV in chronic wound fluid of 15 non-diabetic and 22 diabetic patients. Isolated hExoκ (Ø 75–150 nm) were characterized per EV-track guidelines. dSTORM images, analyzed using online CODI platform followed by independent validation using Nanometrix, revealed hExoκ Ø as 80–145 nm. The abundance of hExoκ was low in diabetic wound fluids and negatively correlated with patient HbA1c levels. The hExoκ isolated from diabetic wound fluid showed a low abundance of small bp RNA (<200 bp). Raman spectroscopy underscored differences in surface lipids between non-diabetic and diabetic hExoκ. Uptake of hExoκ by monocyte-derived macrophages (MDM) was low for diabetics versus non-diabetics. Unlike hExoκ from non-diabetics, the addition of diabetic hExoκ to MDM polarized with LPS and INFγ resulted in sustained expression of iNOS and pro-inflammatory chemokines known to recruit macrophages (mϕ).This work provides maiden insight into the structure, composition, and function of hExoκ from chronic wound fluid thus providing a foundation for the study of exosomal malfunction under conditions of diabetic complications such as wound chronicity.
Source: Nano Today. Volume 52, October 2023, 101954
GRANT OF THE MONTH
Co-PIs: Philip C. Spinella, Christine M. Leeper, Stephen Wisniewski, and Abdus S. Wahed
Title: Massive Transfusion in Children-II (MATIC-II)
Description: Trauma is the leading cause of death in children, with firearm-related injury and motor vehicle crashes as the top two sources of injury. In the U.S., an estimated 2,000 pediatric deaths caused by bleeding-related trauma are preventable with timely and effective care every year. During a mass casualty incident, such as a radiological or nuclear incident, children are likely to suffer from hemopoietic radiation injury as well as blunt, penetrating, and blast injuries that will cause significant blood loss that necessitate blood transfusions. Although children’s coagulation and physiologic response to bleeding differs from adults, pediatric trauma resuscitation practice guidelines are derived from adult data due to a lack of high-quality research in children. Survival outcomes are also worse in children with life-threatening traumatic injury compared to adults. This highlights the need to determine optimal transfusion practices in pediatric populations.
The trial will examine the safety and effectiveness of transfusion with whole blood versus component therapy (CT), as well as tranexamic acid (TXA), a drug that helps prevent bleeding, vs. placebo. The current standard of care for blood transfusions is to transfuse red blood cells, frozen plasma, and platelet concentrates separately in a balanced proportion to address specific deficiencies and clotting abnormalities. Whole blood is unseparated and contains all the component products in one bag.
In children with life-threatening hemorrhage from trauma, whole blood transfusion compared to CT may improve patient outcomes by decreasing the total blood transfused, improving oxygen delivery and hemostasis (stopping the flow of blood from an injury), and increasing survival. This clinical trial will also examine TXA, which may reduce the volume of blood needed for transfusion and improve survival in injured children. No prospective multicenter trials compare whole blood to CT or TXA to placebo in pediatric trauma patients, therefore this study may address a critical gap in care.
This effort aligns with BARDA’s overarching strategy, outlined in the 2022-2026 Strategic Plan, to protect all members of our communities against an evolving threat landscape. This includes supporting development of MCMs to address critical gaps in the treatment of children.
Source: Biomedical Advanced Research and Development Authority
Amount: $34 million
