September 2022 | VOL. 21, NO. 9| www.McGowan.pitt.edu
Dr. Eric Lagasse to Receive 2022 Innovation Award from the Carnegie Science Center

The Carnegie Science Center (CSC) in Pittsburgh established in 1997 the Carnegie Science Awards. These awards have honored the accomplishments of more than 600 scientists, technologists, educators, business leaders, and organizations who are making a difference in Pittsburgh and beyond. Winners are chosen by a committee of peers — both past awardees and industry leaders — who selected the most deserving innovators whose contributions have led to significant economic or societal benefit in western Pennsylvania.
On October 13, 2022, the CSC during their first-ever Geek Out Gala will present to McGowan Institute for Regenerative Medicine faculty member Eric Lagasse, PharmD, PhD (pictured), its 2022 Innovation Award. This award seeks to recognize an individual or team whose breakthrough approach to creatively solving a problem has had a positive impact on the community leading to significant business, economic, or societal benefit for the region.
Dr. Lagasse, an Associate Professor in the Department of Pathology, University of Pittsburgh, a member of Pitt’s Clinical and Translational Institute, and the Director of the Cancer Stem Cell Center at the McGowan Institute, has demonstrated that mini-livers can be created in vivo by injecting liver cells into lymph nodes. This discovery provides an alternative to liver transplantation for patients with liver failure. The discovery has reinforced that Pittsburgh is the capital for organ transplantation and organ engineering and offers the prospects to save many lives.
In Dr. Lagasse’s laboratory, research is focused primarily on stem cell biology and cell-based therapy for a variety of diseases including cancer. Dr. Lagasse’s continued research interests include cell therapy for liver diseases and organogenesis and tissue bioengineering. Dr. Lagasse is also co-founder of LyGenesis, an organ regeneration company enabling a patient’s own lymph nodes to be used as bioreactors to regrow functioning ectopic organs.
LyGenesis uses the lymph node’s evolutionary function – to serve as an efficient and rapid bioreactor for T-cells to help fight infection – to help vascularize allogenic cell therapies, a key barrier to the successful clinical application of many other cell therapies. Using donated but not transplanted organs, the therapies upend the current supply-demand disparity, enabling one donated organ to serve as the cell source to treat multiple patients. Moreover, the use of an endoscopic ultrasound to engraft our cell therapies into lymph nodes significantly decreases the costs and associated medical risks of the procedure relative to receiving a full organ transplant.
Dr. Lagasse, a world leader ectopic transplantation research, discovered that hepatocytes (liver cells) transplanted into lymph nodes would not just survive, but thrive, organize, and begin to function as miniature ectopic livers, exerting life-saving effects in otherwise fatal small and large animal models of end stage liver disease. The initial proof of concept studies in mice demonstrated the ability to develop functional, life-saving ectopic liver tissue in the lymph nodes in animals with fumarylacetoacetate hydrolase-deficiency (Fah-/-), a well-accepted model of hereditary tyrosinemia type 1. These initial findings helped to elucidate the biological principles guiding in vivo organogenesis within the lymph nodes using both autologous and allogeneic cell sources.
Congratulations, Dr. Lagasse!
Illustration: LyGenesis.
RESOURCES AT THE MCGOWAN INSTITUTE
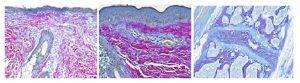
October Histology Special – Herovici Staining
The Herovici combination of aniline blue or picro methyl blue and picro acid fuchsin results in Type I mature collagen staining red while reticulum and newly formed Type III collagen stains blue.
You’ll receive 25% off your Herovici staining October when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
UPCOMING EVENTS
Save the Date! 2023 McGowan Institute Scientific Retreat

The venue for the 2023 Retreat is the University Club, and the dates are March 6 & 7, 2023.
The program committee for the 2023 McGowan Institute Scientific Retreat, under the leadership of Bryan Brown, PhD, has begun to formulate the program and is requesting your suggestions for session topics.
The committee is also seeking to learn of possible interest by individuals in organizing a session. Please submit your program suggestions here.
9th Annual International Symposium on Regenerative Rehabilitation

The McGowan Institute for Regenerative Medicine invites you to join us for the 9th Annual International Symposium on Regenerative Rehabilitation, to be held October 27-29 in Austin, TX! Now in its 9th year, this symposium is the largest medical and scientific conference specific to Regenerative Rehabilitation in the world and brings together renowned experts in the fields of regenerative medicine and rehabilitation. This year’s featured speakers include, Theresa A. Jones, PhD (University of Texas at Austin), Karunesh Ganguly, MD, PhD (University of California, San Francisco), Conor Walsh, PhD (Harvard University), William R. Wagner, PhD (University of Pittsburgh), and José del R. Millán, PhD (University of Texas at Austin).
Join us in discovering integrated methods to enhance tissue healing and regeneration through mechanotransduction and applied biophysics, as well as how these approaches relate to the application of clinically available rehabilitation approaches. Cutting-Edge Research, Trainee Opportunities, Travel Awards, Diversity Grants, Networking and More!
View the agenda and register here.
HERETIC Symposium, October 11-12, 2022, Wyndham Pittsburgh University Center

The University of Pittsburgh’s Center for Military Medicine Research (CMMR) and the Trauma and Transfusion Medicine Research Center (TTMRC), along with McGowan Institute for Regenerative Medicine (MIRM), and in collaboration with the Trauma Hemostasis and Oxygenation Research (THOR) Network, are proud to announce the inaugural HEmostatic REsuscitation and Trauma Induced Coagulopathy (HERETIC) Symposium!
This Symposium, scheduled for October 11-12, 2022, at the Wyndham Pittsburgh University Center in Pittsburgh, Pennsylvania, will disseminate and discuss the most up-to-date research and best practices in the field of hemostatic resuscitation and trauma-induced coagulopathy, and celebrate the growing partnerships and research opportunities available through private and public research programs.
The Symposium’s intent is to bring together a rich mix of researchers, providers, the Department of Defense, and private and public research program managers in order to present the current state of the science, stimulate discussion, and build connections that are only possible through this forum.
The poster abstract submission deadline is September 29, 2022. Find the form here.
The latest Symposium agenda is here.
Register for the Symposium here.
More Symposium information is here.
SCIENTIFIC ADVANCES
Growing New Organs in a Person for the First Time
Reported by Jessica Hamzelou for MIT Technology Review, a volunteer in Boston, Massachusetts, will be the first to trial a new treatment that could end up creating a second liver in their body. And that’s just the start—in the months that follow, other volunteers will test doses that could leave them with up to six livers in their bodies.
The company behind the treatment, LyGenesis, hopes to save people with devastating liver diseases who are not eligible for transplants. Their approach is to inject liver cells from a donor into the lymph nodes of sick recipients, which can give rise to entirely new miniature organs. These mini livers should help compensate for an existing diseased one. The approach works in mice, pigs, and dogs. Now we’ll find out if it works in people.
The LyGenesis treatment is the culmination of years of scientific research conducted by McGowan Institute for Regenerative Medicine faculty member Eric Lagasse, PharmD, PhD (pictured), Associate Professor in the Department of Pathology, University of Pittsburgh, and Director of the Cancer Stem Cell Center at the McGowan Institute. Dr. Lagasse is a co-founder of LyGenesis.
If it works, the treatment could be revolutionary. Donor organs are in short supply, and many of those donated can’t be used—for example, sometimes the tissue is too damaged. The new approach can make use of organs that would otherwise have been discarded, and the researchers reckon they can get treatments for around 75 people from a single donated organ.
“It’s very promising,” says Valerie Gouon-Evans, PharmD, PhD, a stem-cell biologist at the Boston University School of Medicine with a focus on liver regeneration, who is not involved in the research or with the company. “I’m really happy … that this idea is getting into the clinic.”
Transplants aren’t always an option for people who are very unwell, however. That’s why Dr. Lagasse and his colleagues at LyGenesis have taken this different approach. Dr. Lagasse has spent years researching cell-based treatments for liver disease. Around 10 years ago, he was experimenting with the idea of injecting cells from healthy livers into diseased ones in mice.
It is difficult to access the livers of small, 25-gram mice, which Dr. Lagasse was studying, so instead he and his colleagues injected the cells into the spleens of mice with liver disease. They found that the cells were able to migrate from the spleen to the liver. To find out if they could migrate from other organs, Dr. Lagasse’s team injected liver cells at various sites in the mice’s bodies.
Only a small number of mice survived. When Dr. Lagasse and his colleagues later performed autopsies on those survivors, “I was very surprised,” he recalls. “We had a mini liver present … where the lymph node would be.”
Little incubators
Lymph nodes are small, bean-shaped structures found throughout the body. They play a crucial role in our immune health, making cells that help fight infections. While Dr. Lagasse was initially surprised that liver cells could multiply and grow in lymph nodes, it makes sense, he says.
Around five years ago, Dr. Lagasse, along with entrepreneur and drug developer Michael Hufford, PhD, and transplant surgeon/scientist/entrepreneur and McGowan Institute affiliated faulty member Paulo Fontes, MD, founded LyGenesis to take the technology further. The team is also exploring the use of lymph nodes to grow new thymuses, kidneys, and pancreases.
But the company’s priority is livers. Over the last 10 years, members of the team have collected promising evidence that shows they can use their approach to grow new mini livers in mice, pigs, and dogs. The mini livers don’t grow indefinitely—the body has an internal regulator that stops liver growth at a certain point, which is why healthy livers don’t overshoot when they regenerate.
New human livers
The team at LyGenesis will now trial their treatment in 12 adults with end-stage liver disease who are not eligible for liver transplants. People with this disorder have chronic liver failure that worsens over time. Liver cells die, and healthy tissue is replaced with scar tissue. As a result, harmful substances that are normally filtered by the liver, like ammonia, build up in the blood. When the liver stops making substances that help blood to clot, people can bleed and bruise easily. People with the disease are also at risk of diabetes, infections, and liver cancer.
The clinical trial will test the safety of the treatment and look for health benefits among the volunteers. The first recipient will receive a one-milliliter dose of around 50 million cells.
The second volunteer will receive the same dose seven days later—leaving the medical team a week-long window to check for any potential problems. Once four volunteers have received the lowest dose, a further four will have a total of 150 million cells injected into three lymph nodes. A final four volunteers will later have 250 million cells injected across five lymph nodes. If all goes to plan, these final four individuals will end up with five mini livers in addition to their original organ.
The trial volunteers will be closely monitored. Researchers will assess blood samples for signs of liver function improvement, and will also track any potential improvements in energy, cognition, and general quality of life. “Each patient is studied for a full year,” says Dr. Hufford. “We hope to be wrapped up in just under two years.”
Because they are receiving cells from other people, all the volunteers will need to take immunosuppressant drugs that stop their immune systems from rejecting their new mini livers for the rest of their lives, just as people who receive transplanted donor organs do.
If the liver treatment works, LyGenesis plans to trial other cells, and potentially grow other organs. “We’ve been able to grow ectopic kidneys and an ectopic thymus… and pancreatic beta cells to help [regulate the blood sugar levels of] animals with diabetes,” he says. The company’s approach could also be useful for implanting organoids—tiny lab-grown, organ-like clumps of cells—into people, says Dr. Gouon-Evans.
“The program is just getting off the ground,” says Dr. Hufford.
Ms. Hamzelou is a senior reporter at MIT Technology Review, where she covers biomedicine and biotechnology. She was previously a health and medical science reporter at New Scientist.
Illustration: LyGenesis.
Developing a Novel Extracorporeal Hemoadsorption Device

Cell-free plasma hemoglobin (PHb) is now recognized as a common and problematic sequela of extracorporeal therapies (ECT) such as cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO) with contributions to organ injury such as acute kidney injury. The goal of new research is to develop a novel and selective extracorporeal hemadsorption device capable of removing PHb during all forms of ECT. The prototype will be evaluated for compatibility with ECT, the capability to effectively remove PHb, and the ability to ameliorate ECT-related kidney injury.
McGowan Institute for Regenerative Medicine faculty member William Federspiel, PhD (pictured), John A. Swanson Professor in the Department of Bioengineering with secondary appointments in Chemical Engineering, Critical Care Medicine and Clinical and Translational Sciences, and Director of the Medical Devices Laboratory at the McGowan Institute, is the co-principal investigator on the project entitled, “Hemoadsorption device for selective removal of cell-free plasma hemoglobin during extracorporeal therapies.” The 2-year project began on September 2, 2022, and was funded by the NIH’s National Heart, Lung, and Blood Institute.
The abstract for this project reads:
Extracorporeal therapies (ECT) including cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO) have been refined over the years, yet unfavorable outcomes such as acute kidney injury (AKI) continue to occur and are associated with mortality and prolonged intensive care unit and hospital length of stay. Increased cell-free plasma hemoglobin (PHb) from hemolysis during CPB and ECMO has been identified as playing a central role in such dysfunction and is thus an obvious clinical target. Despite this, there are no clinically available therapies for the selective removal of PHb during ECT. The focus of this proposal is to develop a novel extracorporeal hemoadsorption device to serve as an easily implementable therapy to selectively remove PHb during ECT.
The hemoadsorption device will use a bead-based matrix containing immobilized haptoglobin (Hp), a native protein with high-affinity binding for PHb, to selectively remove PHb from whole blood flow. Preliminary work has been conducted to establish the foundational feasibility of the proposed approach. This includes the development of methods to produce Hp-modified beads, confirmation of the ability of these beads to bind PHb, demonstration of the ability of a bead-based column to accommodate whole blood flow, and model-based evidence of the ability for clinically reasonable device flow rates to achieve therapeutic PHb removal. In addition, we have established a rat model of ECT-induced AKI to be used during in vivo assessment of the proposed approach.
The two proposed Aims will (i) fabricate functional, scaled-down prototypes and characterize PHb binding kinetics during benchtop studies in whole blood flow and (ii) use a rat model to evaluate the feasibility of device implementation during ECT and its ability to prevent ECT-induced renal injury. Thus, the proposed work involves elements of design and fabrication of extracorporeal blood-contacting devices, porous media fluid dynamics, blood-based mass transfer, hemocompatibility, kidney injury, hemolysis-associated pathology, and clinical implementation of ECT. The research team, led by principal investigators Nahmah Kim-Campbell, MD, MS, and William Federspiel, PhD, possesses the unique engineering and clinical expertise necessary to pursue this highly multidisciplinary research. Combined with the rich research environment and resources of the University of Pittsburgh and the McGowan Institute of Regenerative Medicine, this team is well-positioned to successfully develop this mechanistically supported approach to improve adverse outcomes in ECT.
Congratulations, Dr. Federspiel!
Grant Boosts Respiratory Support for Lung Disease Patients

More than 16 million Americans suffer from chronic obstructive pulmonary disease (COPD), with veterans being 1.5 to 3 times more likely to develop COPD versus the general population. Across the board, COPD patients experience a gradual decline in respiratory function along with acute exacerbations that lead to a transient, but dangerous, worsening of their disease state. Each year in the U.S. alone, COPD patients account for two million emergency room visits, 700,000 hospital discharges, and 156,000 deaths.
An interdisciplinary team led by McGowan Institute for Regenerative Medicine affiliated faculty member Keith Cook, PhD (pictured top), Carnegie Mellon University (CMU) biomedical engineering professor and department head, has been awarded $8.7 million dollars from the U.S. Army Congressionally Directed Medical Research Programs (CDMRP) program to create and integrate new technologies to sustain permanent at-home artificial lung support. Such advances will allow chronic lung disease patients to lead more normal lives in which they feel comfortable engaging in everyday activities, such as walking or driving.
One of Dr. Cook’s collaborators is McGowan Institute affiliated faculty member Jana Kainerstorfer, PhD (pictured bottom), CMU associate professor of biomedical engineering. Dr. Cook and his team are building a novel Pulmonary Assist System (PAS) that will enable long-term, ambulatory respiratory support for COPD patients. The PAS weighs in under three pounds and consists of a small, lightweight axial flow pump coupled with a compact, highly biocompatible gas exchanger. To date, long-term respiratory support has not been performed outside the intensive care unit (ICU); however, the group is focused on developing supporting technologies that will allow for safe, uncomplicated support for COPD patients outside of the ICU.
“Respiratory support requires a means of drug delivery and patient and artificial lung monitoring that are not possible on a regular hospital floor, and definitely not possible at home,” explained Dr. Cook. “We are developing different drug regimens, new drugs, and new monitoring systems to enhance patient care and overall quality of life.”
As part of the funded work, there are four interconnected projects. They include the development of more stable anticoagulation strategies to support the PAS outside of the ICU, new surgical methods that allow a patient freedom of movement on the artificial lung, and enhanced remote monitoring technologies.
“Monitoring of device performance is crucial for at-home artificial lung support to enable early intervention in case of device failure,” said Dr. Kainerstorfer. “We are developing the technology to monitor, for instance, blood oxygenation, a crucial parameter of device performance.”
Fruit from these efforts stand to offer hope and practical solutions for chronic lung patients, explained Dr. Cook. “The reality is that there just aren’t enough lung transplants for all the people who need them. Even if someone does receive a transplant, the chances are that within five years that lung transplant will fail. Our work in developing alternative treatment technologies that allow chronic lung patients to maintain their quality of life is a huge win.”
Popular Mechanics Features Human Sight-Restoring Technologies

One of the four human sight-restoring technologies featured in Popular Mechanics includes the work of the University of Pittsburgh School of Medicine Eye Center’s researchers who are developing goggles that have a camera and processor to translate images from the environment into pulses of light. Reporter Connie Chang zeros in on their work.
Pittsburgh scientists are pursuing vision restoration through optogenetics, in which retinal neurons are induced—via a virus that targets specific cells—to express light-sensitive proteins in their cell membranes. McGowan Institute for Regenerative Medicine affiliated faculty member José-Alain Sahel, MD (pictured), the Chair of Ophthalmology at the University of Pittsburgh School of Medicine and Director of the UPMC Eye Center, and colleagues were the first to successfully implement this technique in patients, as reported in a Nature Medicine paper published in 2021.
Their method requires a camera and processor to translate images from the environment into pulses of light that can excite the transformed neurons, which is likely more reliable and versatile than traditional prostheses. Cells in the retina continue to express the photosensitive protein after a one-time injection of the virus, while physical implants could degrade and stop working, accompanied by risks of infection. “And the goggles, since they’re outside the eyes, can evolve over time,” Dr. Sahel says. “They’re already different from the first goggles we developed, with more to come.”
What do patients see?
“The type of signal that’s produced with this [light-sensitive] protein is a new type of signal, and the patient has to learn how to make sense out of it,” Dr. Sahel says, comparing the process to learning a foreign language. Each pixel in the camera reacts to a change in light, so it’s like looking at a scintillating screen, where pixels flash as the light changes at that position. This neuromorphic camera, notes Dr. Sahel, is designed to partially mimic how the retina responds to light.
Signals arise, therefore, when the object moves or the patient shifts their head or their eyes in a type of edge detection, explains Dr. Sahel. Gradually, patients are able to discern shapes, light sources, edges, and objects in motion. Dan Dunfee, a patient participating in the clinical trial in Pittsburgh, says it’s like looking out of glasses smeared with Vaseline at a world bathed in different intensities of blue.
“I see contrast and light and the edges where the contrast and light change,” Mr. Dunfee tells Popular Mechanics. “And when I find what I think is an edge of something, I’ll scan around to find the other edges of it—and from that, I get a sense of size, shape, and how reflective an object is.”
Ms. Chang is a freelance writer in the Bay Area — covering science, parenting, and health. She’s a recovering scientist, inveterate knitter, and fan fiction enthusiast.
Art Therapy Assists in the Recovery of a Burn Patient

UPMC Mercy is Western Pennsylvania’s only hospital with a comprehensive Burn Center and Level I Regional Resource Trauma Center under one roof. In 1967, UPMC Mercy became the first burn center in Pennsylvania and the 14th in the United States. UPMC Mercy has more than 54 years of excellence in burn care.
UPMC Mercy’s Burn Center, which is directed by McGowan Institute for Regenerative Medicine affiliated faculty member Jenny Ziembicki, MD (pictured), Department of Surgery, Division of Trauma and Burns, embraces a cutting edge, multidisciplinary approach that brings together experts in plastic surgery, wound care, rehabilitation, and emotional support to assist burn patients and their families. These highly specialized services focus on the delivery of expert medical care and the development of therapeutic plans designed to reunite patients with their families, allowing them to heal at home. These include:
- State-of-the-art wound care therapies.
- The region’s first outpatient services program — open 365 days a year — which enables burn patients to return to the hospital for daily hydrotherapy, dressing changes, and physical and occupational therapies.
- The Enchanted Forest, a specially designed resource for pediatric burn patients, staffed by a certified child life specialist.
It was because of the emotional support she received through art therapy that inspired a 12-year-old girl who suffered burns during a house fire when she was 5 years old to give back to UPMC Mercy’s Burn Center. She told her story to Pittsburgh CBS News affiliate KDKA.
Meira Loring just celebrated her bat mitzvah, and one of her projects was collecting art supplies for patients at the UPMC Mercy Burn Center. The center is where she received care and healing that went beyond just medical attention. The hospital has a robust art therapy program that Ms. Loring loved when she was a patient there for two months.
“It was mostly to give back to the hospital because of all they’ve done for me and my family,” Ms. Loring said.
Her mom, Leah Ackner, said, “The child life specialists helped out tremendously, both through art therapy and just talking us through what would happen. So, it was important for us to come back.”
The family visits UPMC Mercy regularly to show their gratitude. Dr. Ziembicki talked about how important compassion and creativity can be in someone’s recovery.
“This is a great example of a former patient being able to overcome so much injury and help others in similar situations,” Dr. Ziembicki said.
As a comprehensive ABA (American Burn Association)-verified Burn Center, UPMC Mercy is nationally recognized as a leader in providing outstanding, quality care to individuals of all ages with severe burn injuries. This designation means that the compassionate, high quality of care given patients meets the rigorous standards established by the American Burn Association and American College of Surgeons. We are among only 68 ABA-verified burn centers nationwide.
Advancing Circuit Manipulation Capabilities in the Brain

Adeno-associated viruses (AAVs) are potent gene delivery vectors that are widely used in laboratory animals and in a growing number of clinical trials. However, AAVs are not naturally cell type specific; this limitation prevents the widespread use of genetically coded tools to study circuit function in genetically intractable species, like non-human primates (NHPs), and hinders the development of cell type specific and circuit targeted therapeutic strategies.
In this 4-year project funded by the NIH’s National Institute of Mental Health, researchers will use cutting edge experimental, genomic, and computational tools to create and validate a suite of next generation AAVs that drive cell type specific expression in the NHP cognitive and reward systems and establish a foundation for circuit-targeted translational applications. McGowan Institute for Regenerative Medicine affiliated faculty member Leah Byrne, PhD (pictured), Assistant Professor, Department of Ophthalmology, University of Pittsburgh, with secondary appointments in the Departments of Neurobiology and Bioengineering, is a co-principal investigator on this project which began on September 1, 2022.
The abstract for this project reads:
Adeno-associated viruses (AAVs) are potent gene delivery vectors for neuroscience studies and gene therapy applications. However, naturally occurring AAVs are not cell type specific: they must be combined with other technologies, such as transgenic animals, to achieve cell type specific gene expression. This requirement limits the use of genetically coded ‘circuit-breaking’ tools to study behavior in nonhuman primates (NHPs) – the experimental animal model with the greatest similarity to humans – and hinders development of cell type specific targeting strategies for achieving direct clinical benefits.
To expand cell type specific access in NHPs and lay the foundation for circuit specific gene therapy, we propose to create, test, and validate next generation, cell type specific AAVs. First, we will define cell type specific enhancers – distal regulatory elements that have demonstrated considerable promise as cell type specific AAV drivers. In preliminary data, we collected transcriptomic and chromatin accessibility (i.e., “multi-omic”) single cell data from the striatum, dorsolateral prefrontal cortex (dlPFC), primary motor cortex (M1), insula, and ventral midbrain of 2 rhesus macaque monkeys. We combined the rhesus monkey data set with existing human and mouse data and used convolutional neural networks (CNNs) to rank open chromatin sequences according to their potential as cell type specific enhancers. We packaged AAVs with the top candidate enhancers, injected them into NHP striatum, and observed cell type specific, enhancer driven expression in striosomes – a cell type specific striatal compartment related to reward processing.
To broadly advance this agenda and develop AAVs that drive robust, cell type-specific expression, we propose to expand our multi-omic single cell database with additional data from macaque and marmoset. We will leverage this updated, sex-balanced database, which will include data from 8 NHPs to identify cell type specific enhancers that are likely to drive robust expression in primates. In parallel, we will use our validated scAAVengr pipeline to screen AAV capsid mutants for cell-type biased infection patterns in the NHP cognitive and reward systems. We will combine the top cell type specific enhancers with the most biased AAV capsids to generate new, cell type specific AAVs for targeting neurons in the NHP cognitive and reward systems. We will validate AAV specificity using Fluorescent in situ hybridization (FISH).
This data will be combined with ultra-high resolution MRI scans to create a rhesus macaque brain atlas, and the validated vectors will be stored and distributed by The University of Pittsburgh BioForge Initiative. NHPs are critical for studying human cognition and disease, and thus there is a pressing need to define the molecular properties of NHP cell types and study their behavioral functions. This proposal will generate a unique NHP multi-omic single cell database, provide cell type specific AAVs for neuron types in cognitive and reward systems, and establish a new multimodal rhesus brain atlas. These contributions will significantly advance circuit manipulation capabilities in the primate brain and promote fundamental research in basic and preclinical science.
Congratulations, Dr. Byrne!
Laying the Groundwork for the Treatment of Temporomandibular Disorders

Jansen’s metaphyseal chondrodysplasia (JMC) is a rare autosomal disorder characterized by malfunction of the temporomandibular joint (TMJ) among other bone deformities that are associated with hypercalcemia and hypophosphatemia. Caused by constitutive activation of genetically dominant heterozygous mutants of the parathyroid hormone (PTH) type 1 (PTHR) receptor, the cell surface receptor for PTH and its related protein (PTHrP), there is no cure or efficient therapeutic drug for JMC patients.
The goal of a new research effort funded by the NIH’s National Institute of Dental & Craniofacial Research is to identify effective small (non-peptidic) molecules for directly blocking anomalous constitutive activity of PTHR JMC mutants while maintaining the action of endogenous ligands (PTH and PTHrP). The project entitled, “Identification of selective inhibitors of PTH-receptor for Jansen’s metaphyseal chondrodysplasia,” began on September 1, 2022, for 2 years. The project’s co-principal investigator is McGowan Institute for Regenerative Medicine affiliated faculty member Juan Taboas, PhD (pictured), Associate Professor with the Department of Oral and Craniofacial Sciences in the School of Dental Medicine and the Department of Biomedical Engineering in the Swanson School of Engineering at the University of Pittsburgh.
The abstract for this project reads:
The objective of this project is to test and optimize our recently identified small non-peptidic molecules (referred to as Pitt molecules) to restore normal parathyroid hormone type 1 receptor (PTHR) signaling and cellular temporomandibular joint (TMJ) condylar morphogenic processes, which are defective in cells expressing constitutive active receptor mutants causing Jansen’s metaphyseal chondrodysplasia (JMC). Based on existing collaborations between PIs (Dr. Vilardaga, Dept. of Pharmacology & Chem. Biol., and Dr. Taboas, Dept. Oral and Craniofacial Sciences, and of Bioengineering) and co-I (Wipf, Dept. of Chemistry) at U.Pitt, an interdisci- plinary approach has been developed to demonstrate the efficacy of Pitt molecules in restoring normal PTHR signaling and cellular morphogenic processes in vitro, and thereby better understand the effects of PTHR signaling on condylar morphogenesis. The project has 2 aims:
Aim 1 will identify the most effective allosteric small molecules for blocking constitutive PTHR activation using state-of-the-art optical analysis of receptor signaling in live cells expressing PTHR mutants encountered in JMC patients (PTHR-H223R, PTHR-T410P, or PTHR-I458R), and medicinal chemistry methods to optimize blocking properties of Pitt molecules.
Aim 2 will take a step further by determining the effect of small molecules on temporomandibular joint condylar morphogenesis using biochemical and histological assays on engineered microtissues composed of condylar mesenchymal progenitors from wild-type and mice expressing the JMC mutant PTHR-H223R.
The significance of this research program lies in its premise to better understand the effects of PTHR signaling on condylar morphogenesis and lay the groundwork for a future translational research program that examines the therapeutic utility of these Pitt molecules for treatment of temporomandibular disorders.
Congratulations, Dr. Taboas!
New Animal Model to Study Human T Cell-Mediated Immunity

McGowan Institute for Regenerative Medicine affiliated faculty member Ipsita Banerjee, PhD (pictured), Professor in the Department of Chemical and Petroleum Engineering at the University of Pittsburgh, is the co-author of a journal article entitled, “De novo construction of T cell compartment in humanized mice engrafted with iPSC-derived thymus organoids,” which appeared in Nature Methods, September 5, 2022. Dr. Banerjee’s research interests and collaborations include:
- pluripotent stem cells
- systems modeling of signaling pathways
- biomanufacturing of pluripotent stem cells
- pancreatic islet organoid engineering
- organ engineering
- nanotoxicity evaluation
The abstract of the Nature Methods paper follows:
Hematopoietic humanized (hu) mice are powerful tools for modeling the action of human immune system and are widely used for preclinical studies and drug discovery. However, generating a functional human T cell compartment in hu mice remains challenging, primarily due to the species-related differences between human and mouse thymus.
While engrafting human fetal thymic tissues can support robust T cell development in hu mice, tissue scarcity and ethical concerns limit their wide use. Here, we describe the tissue engineering of human thymus organoids from inducible pluripotent stem cells (iPSC-thymus) that can support the de novo generation of a diverse population of functional human T cells. T cells of iPSC-thymus-engrafted hu mice could mediate both cellular and humoral immune responses, including mounting robust proinflammatory responses on T cell receptor engagement, inhibiting allogeneic tumor graft growth and facilitating efficient Ig class switching.
Our findings indicate that hu mice engrafted with iPSC-thymus can serve as a new animal model to study human T cell-mediated immunity and accelerate the translation of findings from animal studies into the clinic.
Illustration: University of Pittsburgh Swanson School of Engineering.
Working to Restore a Healthy Microbiome

McGowan Institute for Regenerative Medicine affiliated faculty member Tagbo Niepa, PhD (pictured), Assistant Professor in the Department of Chemical & Petroleum Engineering with affiliations and secondary appointments in Civil & Environmental Engineering; Bioengineering; Mechanical Engineering & Materials Science; and the Center for Medicine and the Microbiome, is the principal investigator on the 3-year grant entitled, “Designing a High-Throughput Platform to Bioprospect the Human Microbiome and Manipulate Its Interplay with Host Environments.” The NIH’s National Institute of General Medical Sciences funded this project which began on September 8, 2022.
There is an urgent need to develop technologies for the growth and manipulation of microbial consortia to assess the beneficial effects attributed to some microorganisms and synthetic communities. Dr. Niepa team’s long-term goal is to develop a microbial bank of live-biotherapeutics of human origin comprising defined microbial communities applicable for personalized and precision medicine. In the next pursuit of this goal, this investigation will develop a microfluidics-based human microbiome coculture platform to eradicate C. difficile infection and thereby restore a healthy microbiome.
The abstract of the project reads:
The human microbiome, comprising hundreds of microbial species living in and on the body, is now recognized to play critical roles in human health and performance as well as disease prevention and management. A healthy microbiome (which has not yet been fully characterized because some key species cannot be cultured) keeps in check harmful microbes that are normally present. However, when this balance is perturbed, pathogenic microbes may overgrow, a condition called dysbiosis, and compromise both gut and immune functions.
Development of technologies for the growth and manipulation of microbial consortia are urgently needed to assess the beneficial effects attributed to probiotics and synthetic communities. Developing such ability would enable clinicians to reverse microbial imbalance by providing a personalized set of microorganisms capable of restoring gut functions associated with infectious, inflammatory, metabolic, cardiovascular, and cognitive diseases in patients. To this end, my group aims to advance a bold and unique microfluidic-based technology to isolate, culture, reconstruct, and, in the long-term, manipulate the human gastrointestinal (GI, gut) microbiome to treat diseases.
This application specifically aims to develop a nanoculture system to grow microbial isolates from the gut, including those as yet unculturable, and identify beneficial interactions or bioactive metabolites essential to design synthetic communities capable of eradicating or inhibiting the growth of pathogens such as Clostridium difficile.
The preliminary effectiveness of the ‘designed’ communities will be determined by treating Clostridium difficile Infection (CDI) in an established mouse model. Our long-term goal is to develop a microbial bank of live biotherapeutics of human origin comprising defined microbial communities applicable for personalized and precision medicine. We envision this technology to be a safe, easy-to-deliver, and efficient alternative to fecal microbiota transplant (FMT) to treat diverse dysbiotic conditions, and thus help restore a healthy gut microbiome.
Congratulations, Dr. Niepa!
The Blood Pressure Monitor of Your Dreams

Geckos can stick to just about anything, and their feet are helping University of Pittsburgh researchers revolutionize how medical professionals can monitor blood pressure.
The small reptilians’ toe pads are covered in thin hairs called setae, which is what adheres to surfaces. Feng Xiong, PhD, an associate professor of electrical and computer engineering, and his team are using gecko feet to improve the adhesion of cuff-less 24-hour ambulatory blood pressure monitoring. This research will be funded for $580,000 through the National Institute of Health (NIH) over the next three years.
Partnering with the University of Pittsburgh School of Medicine, Dr. Xiong is joined by co-investigators Ramakrishna Mukkamala, PhD, professor of bioengineering and anesthesiology and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, and Matthew Muldoon, MD, professor of medicine. Aman Mahajan, MD, PhD, professor of anesthesiology and perioperative medicine, and Sanjeev Shroff, PhD, interim dean of the Swanson School of Engineering and a faculty member of the McGowan Institute, will serve as faculty associates.
“Blood pressure is hard to track longitudinally,” Dr. Xiong explained. “Patients, for example, are likely to be more stressed when inside a doctor’s office, likely creating higher blood pressure readings than normal, which is why 24-hour monitoring is important.”
Cuff-based devices are most commonly used to track changes in blood pressure, as well as diagnose hypertension, but the devices come with their own set of limitations. The cuff periodically inflates and deflates every 15 minutes or so, even when the patient is trying to sleep. Monitoring blood pressure during sleep is crucial for medical professionals, but interrupting patients’ sleep can cause mis-readings during clinical observation.
Cuffless blood pressure monitoring devices would not only improve patient sleep but provide medical professionals with a more effective clinical approach to diagnosing hypertension.
Together, the team will design wearable tonometric sensors at arterial sites on the neck and ankle which will collect tonometric waveforms and pulse transit time (PTT) – or the time it takes for the pulse to travel between two arterial sites – simultaneously. Ultimately, the team plans to combine high-fidelity arterial tonometry and PTT principles to achieve cuffless 24-hour blood pressure monitoring.
“We will be challenging existing barriers with a number of innovations,” Dr. Xiong explained. “These include integrating ionic liquid and microstructures in the sensor to enhance both the sensitivity and dynamic range, mimicking the feet of geckos at the sensor substrate for better adhesion, and develop PTT-based calibration to extract both diastolic and systolic blood pressure.”
Dr. Muldoon said this research is needed to help patients struggling with hypertension as well as for the doctors trying to diagnose it.
“Dr. Xiong’s lab seeks to catapult research on devices to measure blood pressure in the hospital and at home every minute without noise or pain,” said Dr. Muldoon.
The goal of the research is that more accurate readings from cuffless blood pressure monitors will lead to improved hypertension diagnosis, better post-surgery hypotension surveillance, and fewer cardiovascular disease mortalities.
Illustration: Shutterstock/Papa Bravo.
Therapeutic Approaches to Help People Living with HIV

Human immunodeficiency virus (HIV)-associated central nervous system (CNS) disease occurs in more than 50% of the people living with HIV (PLWH) that use combination antiretroviral therapy (cART). A lack of appropriate brain/CNS models to study HIV neuropathogenesis limited the understanding of HIV’s effect on cognition and hampered the development of therapeutic approaches.
Using recently developed novel tri-culture human 3D-brain organoids (hBORGs) that mimic the brain of HIV infected individuals, McGowan Institute for Regenerative Medicine affiliated faculty member and co-principal investigator Shilpa Sant, PhD (pictured), Associate Professor at University of Pittsburgh School of Pharmacy in the Department of Pharmaceutical Sciences, and the team will assess how HIV exacerbates HIV-associated neurocognitive disorder (HAND) progression and the role of viral, host, and external (opioids) factors in disease development to further develop therapeutic approaches to help PLWH.
This 1-year project entitled “Modeling HIV-1 Neuropathogenesis and Neuronal Dysregulation Using 3D-Organoids Containing Multiple CNS Cell Lineages,” was funded by the National Institute of Neurological Disorders and Stroke. Work began on September 15, 2022.
The abstract of this project follows:
HIV-associated neurocognitive disorder (HAND) ranges from mild to severe deficits in cognition and occurs in more than 60% of HIV-1 subjects receiving combined antiretroviral therapy (cART). Pathological evaluation of the frontal cortex of HIV-1 positive subjects revealed neuroinflammation with presence of HIV-1 infected microglia/macrophages, astrogliosis, dendrites loss and synaptic pruning in neurons.
The degradation of dendrites that accompany these neuronal changes leads to synaptic loss, neuronal and cognitive decline, and this causes development of HAND in people living with HIV. Moreover, these effects may be exacerbated by illicit drug use abuse such as opioids, a drug commonly abused by this population, thus, there is a great and increasing need to study HAND and understand HIV-associated neuropathogenesis to develop better treatment options and improve disease management. However, these efforts are significantly hampered by the lack of a physiologically relevant brain/CNS model. To address this need, we developed cutting-edge tri-culture human 3D-brain organoids (hBORGs) that contain primary neurons, astrocytes, and microglia (HIV-1-infected and uninfected) to accurately model the brain environment, chronic virus replication, and neuroinflammatory milieu observed in HIV-1 infected individuals.
Using this 3D-BORG model, we propose to test our hypothesis that neuroinflammation caused by HIV-1 infected microglia, induces degeneration of neurons, synaptic pruning and loss of dendrites, astrocytosis and these effects worsen by the addition of opioid drug abuse. We propose the following aims to achieve the goals:
Aim 1. Delineate how infected microglia contribute to pruning and scaling of synaptodendrites, loss of synaptic functions and neuronal loss;
Aim 2. Determine how use of illicit drugs such as Morphine drives the development and/or worsening of the neuronal dysfunction and pathology; and
Aim 3. Identify how the viral and cellular factors from HIV-1 infected microglia cause the HAND-associated pathological features.
We have assembled a team of investigators who have expertise in HIV-1 Virology, 3D-Organoids and drug abuse and synaptic function, thus, are poised to define how infected microglia interact with neurons and astrocytes and further delineate the molecular mechanisms involved in dendritic damage, synaptic plasticity and neuronal survival in the presence and absence of drug abuse. This will help us develop novel therapeutics that can prevent neuronal damage and loss of synaptic function and cognitive impairment observed in PLWH.
Congratulations, Dr. Sant!
Newly Issued Patents for McGowan Institute Affiliated Faculty Members

During the month of August 2022, the following patents were issued based on research work conducted by McGowan Institute for Regenerative Medicine affiliated faculty members. Their newly issued patents include:
U.S. Patent No: 11,406,736
Vascular Extracellular Matrix Hydrogel
Julie Phillippi; Thomas Gleason; George Fercana; Stephen Badylak
U.S. Patent No: 11,406,687
Activators of CXCR3 for the Treatment of Angiopathies of the Eye
Alan Wells; Cecelia Yates-Binder; Joel Schuman
U.S. Patent No: 11,413,375
Methods for Preparation of a Terminally Sterilized Hydrogel Derived from Extracellular Matrix
Timothy Keane Jr.; Stephen Badylak; Christopher Dearth; Neill Turner
U.S. Patent No: 11,413,245
Artificial Cell Constructs for Cellular Manipulation
Steven Little
U.S. Patent No: 11,419,926
Identification of Mutations in Herpes Simplex Virus Envelope Glycoproteins That Enable or Enhance Vector Retargeting to Novel Non-HSV Receptors
Justus Cohen; Joseph Glorioso; Hioaki Uchida
U.S. Patent No: 11,427,625
Expression of NKG2D Activating Ligand Proteins for Sensitizing Cancer Cells to Attack by Cytotoxic Immune Cells
Paola Grandi; Ndukaku Amankulor; Joseph Glorioso
U.S. Patent No: 11426490
Mixture of Polymers, Lubricating Fluid and Porous Materials Comprising Said Mixture, and Surface Bearing Said Mixture
Xavier Banquy, Jimmy Faivre, Buddha Ratna Shrestha, Krzysztof Matyjaszewski, Joanna Burdynska, Florina Moldovan
Congratulations on these successful developments!
 Also, the University of Pittsburgh has once again moved up the list of the top recipients of U.S. utility patents among worldwide universities in 2021, according to the National Academy of Inventors (NAI) and the Intellectual Property Owners Association (IPO). Published annually since 2013, the report ranks the top 100 global universities named as the first assignee on utility patents granted by the U.S. Patent and Trademark Office. Pitt ranked #18 for the 2021 calendar year with 104 patents. In the previous year, the university ranked #20, and ranked #28 the year before that. The full report can be found here.
Also, the University of Pittsburgh has once again moved up the list of the top recipients of U.S. utility patents among worldwide universities in 2021, according to the National Academy of Inventors (NAI) and the Intellectual Property Owners Association (IPO). Published annually since 2013, the report ranks the top 100 global universities named as the first assignee on utility patents granted by the U.S. Patent and Trademark Office. Pitt ranked #18 for the 2021 calendar year with 104 patents. In the previous year, the university ranked #20, and ranked #28 the year before that. The full report can be found here.
Illustration: U.S. Patent and Trademark Office logo/Academy of Inventors Top 100 logo.
AWARDS AND RECOGNITION
Dr. MaCalus Hogan Named David Silver Professor and Chair of Pitt’s Department of Orthopaedic Surgery and Chair of Orthopaedic Surgery at UPMC

The University of Pittsburgh School of Medicine and UPMC have selected McGowan Institute for Regenerative Medicine affiliated faculty member MaCalus Hogan, MD, MBA (pictured), as the new David Silver Professor and Chair of Pitt’s Department of Orthopaedic Surgery and Chair of Orthopaedic Surgery at UPMC.
Dr. Hogan will begin his tenure in the position once held by Freddie Fu, MD, whose legacy as department chair from 1998 to 2021 attained a stellar international reputation for leading key scientific and clinical innovations. The department grew into one of the most ethnic- and gender-diverse orthopaedic departments in the nation, preparing dozens of physicians to become leaders in orthopaedics at universities and hospitals throughout the world. Dr. Fu passed away in September 2021.
“The appointment of an individual to replace the ‘irreplaceable’ Dr. Freddie Fu is a decision that required great consideration. After a lengthy and very deliberate selection process, Dr. Hogan was clearly the preferred choice to not only build upon Dr. Fu’s legacy, but also lead the department into new territories for the future,” said Anantha Shekhar, MD, PhD, Senior Vice Chancellor for the Health Sciences, Pitt; and John and Gertrude Petersen Dean, Pitt School of Medicine. “His commitment to building a diverse environment at Pitt/UPMC and growing the academic excellence in musculoskeletal research made him the ideal next chair.”
Dr. Hogan was recruited to Pitt and UPMC by Dr. Fu in 2013. Dr. Hogan’s focus has been serving as the Chief of Foot and Ankle Surgery at UPMC, Vice Chair of Education and Residency Program Director in the Department of Orthopaedic Surgery at UPMC. He is a Professor of Orthopaedic Surgery, with secondary appointments in the Department of Bioengineering and the Katz School of Business. Dr. Hogan was the founder and Director of the Foot and Ankle Injury Research (F.A.I.R.) group at Pitt, within the Department of Orthopaedic Surgery. He serves as a foot and ankle consultant for the athletic departments at the University of Pittsburgh, Carnegie Mellon University, Duquesne University, and Robert Morris University. He is the Assistant Team Physician for Point Park University, including the Conservatory of Performing Arts, and the Pittsburgh Ballet Theatre. Dr. Hogan also serves as the foot and ankle consultant for the Pittsburgh Steelers and Pittsburgh Penguins as part of the UPMC Sports Medicine Institute.
“I am honored to take on this leadership role and look forward to taking UPMC and Pitt orthopaedics to the next level. There is no other option for excellent care than UPMC. What we offer locally, nationally, and globally is unmatched,” said Dr. Hogan. “I am excited to get to work, amplifying and advancing this department and orthopaedic service line into the future through cutting-edge care and innovation.”
Originally from Muscle Shoals, AL, Dr. Hogan completed his undergraduate studies at Xavier University of Louisiana with a BS in biochemistry and minor in biology. He received his medical degree from Howard University College of Medicine in Washington, DC, and completed his orthopaedic surgery residency at the University of Virginia Health System in Charlottesville, VA, which included a National Institutes of Health Clinician Scientist fellowship year with a focus in musculoskeletal tissue repair and regeneration. He completed his foot and ankle fellowship at the Hospital for Special Surgery in New York, where he served as a consultant for the New York Ballet Company, American Ballet Theatre, and several collegiate and professional sports teams. In 2018, Dr. Hogan also earned an Executive Master of Business Administration in health care at the University of Pittsburgh – Katz Graduate School of Business.
Congratulations, Dr. Hogan!
Illustration: UPMC.
Dr. Bernard Costello Moves from Dental Medicine to Associate Vice Chancellor

McGowan Institute for Regenerative Medicine affiliated faculty member Bernard Costello, MD, DMD (pictured), has been named the associate vice chancellor for health science integration, which aims to advance interprofessional education among the University of Pittsburgh Schools of the Health Sciences. Dr. Costello was named interim dean of the School of Dental Medicine in February 2018 and in April of that year took on the job permanently.
When Anantha Shekhar, MD, PhD, Senior Vice Chancellor for the Health Sciences, Pitt; and John and Gertrude Petersen Dean, Pitt School of Medicine, noted that at his previous job at Indiana University, a Center for Interprofessional Education was formed in the health sciences to make sure that curriculum in medicine, nursing, public health, dental medicine, and more were “intertwined, so that there were periods when medical students and nursing students and dental school students were all working in teams on certain courses. You learn to respect other providers’ schools, as well as work with their students as early as your first and second year in medical school and in nursing school.”
In the August 2022 newsletter from the Dental School, Dr. Costello thanked his colleagues “for your incredibly hard work and commitment to advancing the mission of our School. Improving oral and craniofacial health in our region is an incredibly important cause and working with such a special group of people in pursuit of this mission has been both an honor and a pleasure.”
Congratulations, Dr. Costello!
Dr. Ellen Gawalt Becomes First Female Dean of Duquesne’s Bayer School

McGowan Institute for Regenerative Medicine affiliated faculty member Ellen Gawalt PhD (pictured), is the first female dean of the Bayer School of Natural and Environmental Sciences at Duquesne University. She had been interim dean of the school since July 2021.
Since becoming interim dean, Dr. Gawalt has overseen an increase in undergraduate enrollment as the school’s reputation continues to experience positive growth. Despite being a relatively new school, Bayer’s faculty and students are regularly recognized on a national scale. In May, two Bayer students received the prestigious Goldwater Scholarships, becoming the seventh and eighth female Bayer students to receive the honor in the last 10 years.
“Dr. Gawalt’s commitment to our students is unparalleled,” said Ken Gormley, JD, president of Duquesne University. “She is a wonderful collaborator, whether partnering with faculty on a research project or working together with students in the lab or classroom. The school’s faculty, staff and students will continue to benefit from her expertise.”
A professor at Duquesne since 2003, Dr. Gawalt is a Hillman Distinguished Professor and formerly served as Chair of the school’s Chemistry and Biochemistry Departments. She has authored or co-authored more than 100 scientific papers and presentations. Her research efforts have generated more than $3.5 million in funding, with a focus on scientific discovery and student education.
“For nearly twenty years, Dr. Gawalt has played a vital role in preparing our science students for exciting careers in industry and academia,” said Duquesne Provost David Dausey, PhD. “A prolific researcher and dedicated educator, she will be an excellent dean for our next generation of students.”
Dr. Gawalt is a mentor for the Bayer Scholars Program, which provides full-tuition scholarships and research opportunities for underrepresented groups in chemistry. Under her supervision, 18 students have graduated under the program to work in industry or attend graduate schools since 2013. The program serves as another example of Duquesne’s commitment to creating equity and opportunity in the region.
“I am honored to be named dean of the Bayer School of Natural and Environmental Sciences,” Dr. Gawalt said. “I look forward to working alongside our nationally-recognized faculty who care deeply about the education of each of our students and work to continue our tradition of excellent research in the core sciences.”
Dr. Gawalt received her bachelor’s degree in chemistry from Duke University and her master’s and doctoral degrees in chemistry from Princeton University.
Congratulations, Dr. Gawalt!
Dr. Savio Woo Receives Distinguished Honor from ASME
Savio Woo, PhD (pictured), distinguished university professor emeritus and founding director of the Musculoskeletal Research Center at the University of Pittsburgh, is being recognized as an Honorary Member of the American Society of Mechanical Engineers (ASME) for his pioneering work in biomechanics.
The Board of Governors of ASME unanimously elected Dr. Woo for his “dedication to joint biomechanics, exemplified by innovative use of robots; and for excellence in mentorship.”
“As a pioneer in bioengineering, his research has significantly contributed to the use of tissue engineering to provide positive outcomes to patients with ligament and tendon injuries,” Thomas Costabile, PE, executive director and CEO of ASME, said. “We are proud of his distinguished career, service to the engineering profession and his years of dedication to ASME as a Life-Fellow.”
McGowan Institute for Regenerative Medicine faculty member Sanjeev Shroff, PhD, Interim Dean of the Swanson School of Engineering and a longtime colleague of Dr. Woo in the Department of Bioengineering, said this honor is just further recognition of Woo’s contributions to the school and field.
“Dr. Woo is a giant in the field of musculoskeletal biomechanics and tissue engineering,” Dr. Shroff said. “The election as an ASME Honorary Member is a recognition of his outstanding scholarship, teaching, and professional service. I am honored and proud to be Dr. Woo’s colleague at the Swanson School of Engineering.”
Throughout the course of his career, Dr. Woo has received more than 70 of the highest awards from universities and engineering and medical professional societies. He is a member of the National Academy of Medicine, the National Academy of Engineering, and the Academia Sinica. Dr. Woo also received the Olympic Prize for Sports Science from the International Olympic Committee and the first Olympic Gold Medal at the 1998 Nagano Games in Japan, the only non-athlete to be so honored.
Congratulations, Dr. Woo!
Illustration: University of Pittsburgh Swanson School of Engineering.
Dr. Anna Balasz Follows Her Scientific Bliss
While having a veterinarian father couldn’t help but spark the interest of McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD (pictured), in biology and the sciences, inspiration from the natural world also played a profound role in fueling the Hungarian native’s career-shaping curiosity. The University Times writer, Shannon Wells, MS, recently spoke with Dr. Balazs, and here is what he learned about the researcher.
“When I was a kid and I looked at a tree trunk or section of a tree, I just couldn’t believe how amazingly complex and interesting it was,” she recalls. “And I was just wondering, if I got into it more and more, what does the tree trunk — the cross section of the tree trunk — look like as you got to smaller life scales. Because it just looked so beautiful and so amazing and so interesting — and again, you know, led to a tree!”
Dr. Balazs also engaged in a more mundane activity that fired her young imagination — albeit with mixed results.
“My father used to (collect) mechanical pencils. And when I was very young, I would steal them and play with them when I was getting introduced, very simply, to mechanics. And I always broke the pencils,” she recalls with a chuckle. “They were expensive mechanical pencils, and I always broke them. But those are the two things that stand out in my mind, just moments where, ‘A ha! This is what I like.’”
Dr. Balazs, distinguished professor of chemical engineering at the University of Pittsburgh’s Swanson School of Engineering and adjunct professor in the chemistry department, has enjoyed a flurry of accolades in the past two years that complement an already impressive roster of recognition. On the heels of her election to the National Academy of Sciences, Dr. Balazs was named earlier this year to the National Academy of Engineering (NAE). The first Pitt and Swanson faculty member to be accepted into both academies, she will be inducted in early October 2022 along with 111 new NAE members and 22 international members at its annual meeting in Washington, D.C.
The academy accepted Dr. Balazs’ membership “in recognition of distinguished contributions to engineering and for creative and imaginative work in predicting the behavior of soft materials that are composed of multiple cooperatively interacting components,” the NAE said.
Creative prediction
Those soft materials of interacting components — commonly known as polymers — form the basis of Dr. Balazs’ extensive research using “biomimicry,” in which elements from nature are emulated to address human- and material-world challenges. Her work in theoretical and computational modeling of polymers has drawn international acclaim, including the American Physical Society’s Polymer Physics Prize in 2016. She was the first woman to receive the award.
Flexibility and failures
McGowan Institute faculty member Steven Little, PhD, distinguished professor and chair of Pitt’s Department of Chemical Engineering, benefitted from Dr. Balazs’ engaging expertise soon after arriving at Pitt from MIT in 2006.
“Everybody knew Anna, and so it was one of the first connections I made,” he recalls. “My very first grant with Anna. I wrote a large NSF multi-PI (principal investigator) proposal, and it was funded on the first try. Part of that had to have been because I did it with Anna.”
Dr. Little describes Dr. Balazs as a “tireless researcher” and “a pinnacle of academic productivity.”
“She knows what excellence looks like — the definition of it in her field and in engineering as a whole. And she will not settle for less than that,” he says. “She is great to have in the department because everybody — especially my assistant professors that I hire — can look at what she does and know what that bar is set at, and they know what it looks like for someone to achieve that level. And that’s very valuable to have in any department.”
While enjoying her latest wave of accolades, Dr. Balazs emphasizes that peer recognition in one’s field only tells part of the trial-and-error-fueled story.
“I’ve had 9,000 failures,” she admits. “I’m very happy that my successes are celebrated, but we don’t mention the 9,000 failures. … You see people and you look at their success and you think, ‘Oh my goodness, they’ve clearly developed a means of being successful.’ Yes, but that means of being successful involves failing 9,000 times.
“And I think that’s an incredibly important message,” Dr. Balazs adds. “We certainly don’t celebrate people’s failures, but we don’t celebrate their attempts either.”
Mr. Wells, a native of Charleston, WV, joined the University Times staff early in 2022 as its full-time writer. A longtime journalist and communications specialist, he recently taught journalism courses at West Virginia State University and served as adviser for its student newspaper. He returned to Charleston in 2020 after 14 years in Portland, OR, where he served as executive news editor for three Pamplin Media Group publications and covered news, business, and entertainment reporting beats.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#237 –– Dr. Rory Cooper discusses his research in rehabiliation and medical devices for the disabled.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: José-Alain Sahel, Elise Boulanger-Scemama, Chloé Pagot, Angelo Arleo, Francesco Galluppi, Joseph N. Martel, Simona Degli Esposti, Alexandre Delaux, Jean-Baptiste de Saint Aubert, Caroline de Montleau, Emmanuel Gutman, Isabelle Audo, Jens Duebel, Serge Picaud, Deniz Dalkara, Laure Blouin, Magali Taiel & Botond Roska
Title: Partial recovery of visual function in a blind patient after optogenetic therapy
Summary: Optogenetics may enable mutation-independent, circuit-specific restoration of neuronal function in neurological diseases. Retinitis pigmentosa is a neurodegenerative eye disease where loss of photoreceptors can lead to complete blindness. In a blind patient, we combined intraocular injection of an adeno-associated viral vector encoding ChrimsonR with light stimulation via engineered goggles. The goggles detect local changes in light intensity and project corresponding light pulses onto the retina in real time to activate optogenetically transduced retinal ganglion cells. The patient perceived, located, counted and touched different objects using the vector-treated eye alone while wearing the goggles. During visual perception, multichannel electroencephalographic recordings revealed object-related activity above the visual cortex. The patient could not visually detect any objects before injection with or without the goggles or after injection without the goggles. This is the first reported case of partial functional recovery in a neurodegenerative disease after optogenetic therapy.
Source: Nat Med 27, 1223–1229 (2021).
GRANT OF THE MONTH
PI: William Federspiel, PhD
Title: Hemoadsorption device for selective removal of cell-free plasma hemoglobin during extracorporeal therapies
Description: Extracorporeal therapies (ECT) including cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO) have been refined over the years, yet unfavorable outcomes such as acute kidney injury (AKI) continue to occur and are associated with mortality and prolonged intensive care unit and hospital length of stay. Increased cell-free plasma hemoglobin (PHb) from hemolysis during CPB and ECMO has been identified as playing a central role in such dysfunction and is thus an obvious clinical target. Despite this, there are no clinically available therapies for the selective removal of PHb during ECT. The focus of this proposal is to develop a novel extracorporeal hemoadsorption device to serve as an easily implementable therapy to selectively remove PHb during ECT.
The hemoadsorption device will use a bead-based matrix containing immobilized haptoglobin (Hp), a native protein with high-affinity binding for PHb, to selectively remove PHb from whole blood flow. Preliminary work has been conducted to establish the foundational feasibility of the proposed approach. This includes the development of methods to produce Hp-modified beads, confirmation of the ability of these beads to bind PHb, demonstration of the ability of a bead-based column to accommodate whole blood flow, and model-based evidence of the ability for clinically reasonable device flow rates to achieve therapeutic PHb removal.
In addition, we have established a rat model of ECT-induced AKI to be used during in vivo assessment of the proposed approach. The two proposed Aims will (i) fabricate functional, scaled-down prototypes and characterize PHb binding kinetics during benchtop studies in whole blood flow and (ii) use a rat model to evaluate the feasibility of device implementation during ECT and its ability to prevent ECT-induced renal injury. Thus, the proposed work involves elements of design and fabrication of extracorporeal blood-contacting devices, porous media fluid dynamics, blood-based mass transfer, hemocompatibility, kidney injury, hemolysis-associated pathology, and clinical implementation of ECT. The research team, led by principal investigators Nahmah Kim-Campbell, MD, MS, and William Federspiel, PhD, possesses the unique engineering and clinical expertise necessary to pursue this highly multidisciplinary research. Combined with the rich research environment and resources of the University of Pittsburgh and the McGowan Institute of Regenerative Medicine, this team is well-positioned to successfully develop this mechanistically supported approach to improve adverse outcomes in ECT.
Source: National Heart, Lung, and Blood Institute
Term: September 2, 2022 – August 31, 2024
Amount: $217,496
