January 2023 | VOL. 22, NO. 1| www.McGowan.pitt.edu
Newly Issued Patents for McGowan Institute Affiliated Faculty Members

During the months of November and December 2022, the following patents were issued based on research work conducted by McGowan Institute for Regenerative Medicine affiliated faculty members. Their newly issued patents include:
Patent No. 11,484,515
Thermoresponsive Hydrogel Containing Polymer Microparticles for Controlled Drug Delivery to the Ear
Morgan Fedorchak; Joel Schuman; Steven Little
Patent No. 11,504,286
Pneumatic Powered Mobility Devices
Hongwu Wang; Rory Cooper; Brandon Daveler; Benjamin Gebrosky; Garrett Grindle; Jonathan Pearlman; Urs Schneider; David Minzenmay
Patent No. 11,517,234
Electrode Systems, Devices and Methods
Xinyan Tracy Cui; Mingui Sun; Nicolas Alba
Patent No. 11,517,629
Methods of Treating Cells Containing Fusion Genes by Genomic Targeting
Yangping Yu; Jianhua Luo; Zhanghui Chen; George Michalopoulos; Joel Nelson; Chien-Cheng Tseng
Patent No. 11,529,318
Devices and Methods for Local Delivery of Tacrolimus or Derivatives Thereof
Kia Washington; William Wagner; Michael Steketee; Yolandi Van der Merwe; Xinzhu Gu
Patent No. 11,530,388
Methods of Engineering Human Induced Pluripotent Stem Cells
Alejandro Soto-Gutierrez; Tomoji Mashimo; Alexandra Collin Del Hortet; Jorge Lepe; Kazuki Takeishi; Yang Wang; Kan Handa; Eduardo Alvarez; Banimir Popovic
Patent No. 11,529,122
Methods and Apparatuses for Measuring Tissued Stiffness Changes Using Ultrasound Elasticity Imaging
Kang Kim; Jingping Xu; Jonathan Rubin
Congratulations on these successful developments!
Illustration: U.S. Patent and Trademark Office logo.
RESOURCES AT THE MCGOWAN INSTITUTE
February Histology Special – Cardiac Samples
The Histology Core wants to be your Valentine this month with big saving that will warm your heart.
Bring your cardiac tissue to the McGowan Histology Core and receive 25% off your entire order in the month of February when you mention this ad.
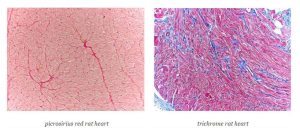
You’ll receive 25% off cardiac samples* in February when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
UPCOMING EVENTS
Save the Date! 2023 McGowan Institute Scientific Retreat

The venue for the 2023 Retreat is the University Club, and the dates are March 6 & 7, 2023.
The program committee for the 2023 McGowan Institute Scientific Retreat, under the leadership of Bryan Brown, PhD, has begun to formulate the program and is requesting your suggestions for session topics.
SCIENTIFIC ADVANCES
Additional Type 1 Diabetes Technology from the Lab of Dr. George Gittes Licensed to Genprex, Inc.

Genprex, Inc., a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, announced it has entered into an exclusive license agreement with the University of Pittsburgh, granting Genprex a worldwide, exclusive license to certain patent applications and related technology and a worldwide, non-exclusive license to use certain related know-how, all related to modulating autoimmunity in Type 1 diabetes by using gene therapy. The preclinical technology transforms macrophages enabling them to reduce autoimmune activity in Type 1 diabetes and could be complementary to the Genprex’s existing diabetes technology.
“Gaining exclusive access to technology that modulates the immune system by transforming macrophages could prove to be significant to our broader research partnership with the laboratory of George Gittes, MD (pictured), Professor of Surgery and Pediatrics and Chief of the Division of Pediatric Surgery at the University of Pittsburgh School of Medicine,” said Mark Berger, MD, Chief Medical Officer of Genprex. “We are making significant strides in our program with Dr. Gittes’s innovative approach to treating diabetes by the transformation of alpha cells into beta-like cells and are excited to add to our arsenal this additional technology also out of Dr. Gittes’s lab, in collaboration with the laboratory of Xangwei Xiao, MD, PhD, Assistant Professor of Surgery, also in the Division of Pediatric Surgery at the University of Pittsburgh’s School of Medicine. Not only could this new approach be used to reduce autoimmune activity in Type 1 diabetes by modulating the immune system but potentially it could also work in conjunction with the technology we have licensed previously.”
Dr. Gittes is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine and a Scientific Advisory Board Member of Genprex.
“With diabetes reaching epidemic proportions around the world, the work Dr. Gittes is pursuing in diabetes is absolutely critical. In the U.S. alone, there are more than 37 million people with diabetes (approximately 1.9 million of whom have Type 1 diabetes) and another approximately 96 million Americans who are pre-diabetic or have abnormally elevated blood sugar levels. The opportunity to change the course of this disease with gene therapy is extremely compelling and increasing our exclusive access to intellectual property could prove to be pivotal to our pathway forward,” said Rodney Varner, President and Chief Executive Officer of Genprex.
Genprex signed an exclusive license agreement with the University of Pittsburgh in 2020. The gene therapy approach under the original license is comprised of a novel infusion process that uses an endoscope and an adeno-associated virus (AAV) vector to deliver Pdx1 and MafA genes directly to the pancreas. In models of Type 1 diabetes, these genes express proteins that transform alpha cells in the pancreas into functional beta-like cells, which can produce insulin but are distinct enough from beta cells to evade the body’s immune system. In Type 2 diabetes, where autoimmunity is not at play, it is believed that exhausted beta cells will be rejuvenated and replenished.
This gene therapy approach was developed by Dr. Gittes. His preclinical research in this area has been published in peer-reviewed scientific publications, and he is the recipient of several research grants, including a $2.59 million grant awarded by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases. Earlier studies in diabetic mouse models showed that the gene therapy restored normal blood glucose levels for an extended period of time, typically around four months. It is believed that the duration of restored blood glucose levels in mice could translate to decades in humans. Preliminary data from a more recent study in a non-human primate model of Type 1 diabetes also have been promising. Data from this study are expected to be presented at a scientific meeting during the first quarter of 2023.
Searching for Chemical Clues to Uncurable Depression

Ana Gorelova, Manager, Science Writing, UPMC, reports that when Lisa Pan, MD, a psychiatrist specializing in treating and preventing suicidal behavior in adolescents, came across the case of a 19-year-old with treatment-refractory depression, she was stunned. The young man, who attempted to end his life multiple times, did not respond to any medications, and the medical team was desperate to find a therapy to help him. Out of desperation to find clues to the root cause of his condition, Dr. Pan decided to order a test analyzing the composition of the teen’s cerebrospinal fluid – CSF for short – a liquid that washes over the spinal cord and brain.
After receiving an analogue of BH4 to correct the deficiency, the patient’s depression symptoms largely disappeared.
That finding pushed Dr. Pan and her team of collaborators across the University of Pittsburgh’s departments of Psychiatry and Human Genetics—including McGowan Institute for Regenerative Medicine affiliated faculty member David Finegold, MD (pictured), Professor of Human Genetics at the University of Pittsburgh—to launch a bigger study, looking at levels of intermediate products of the normal metabolism, including BH4 and folic acid—two key molecules involved in maintaining the brain’s normal function. They wanted to check whether patients with treatment-refractory depression who had the identified disorders could benefit from receiving BH4 and folic acid supplements as an oral medication.
The study, researchers say, was driven by urgent necessity: Working with families pushed to their breaking point from worrying about their loved ones underscored the need to find quick and effective solutions.
“As my colleague Katherine Wisner said, ‘When you are working on the cutting edge, you are going to bleed,’” said Dr. Pan, who also founded New Hope Molecular, a company in Pittsburgh that offers metabolic testing for treatment-refractory depression. “Doing this work is exhausting, but it’s important to do what we can to avoid losing individuals who contemplate ending their lives.”
After a successful pilot study, which was published in 2016 in the American Journal of Psychiatry, Dr. Pan and her team have now revealed results of a bigger trial in 141 people with treatment-refractory depression, 67 of whom had an underlying metabolic abnormality.
The paper, published in Psychological Medicine, reveals that of 11 participants with low CSF BH4 levels, seven showed improvements in their depression after treatment with BH4 supplementation. Of the 20 participants with low CFS folate, 16 responded to replacement with folate metabolites.
Unfortunately, collecting a spinal fluid sample is an invasive and costly process. While researchers hope that their work will raise awareness in the psychiatric community of the value of ordering spinal fluid tests to detect metabolic abnormalities in patients with treatment-refractory depression, this option is usually available only in large academic hospitals.
To bring the therapy to more people, the team, led by David Peters, PhD, associate professor of obstetrics, gynecology and reproductive sciences at Pitt, is now developing a diagnostic test that can be used to detect these metabolism abnormalities in the blood.
“Currently, collecting the spinal fluid sample is the only way to detect low BH4 and folate levels in the brain,” said Dr. Peters. “By performing various genomic analyses, we are searching for biomarker correlates between levels of BH4 and folate in the spinal fluid and the blood. We are hoping to develop an easy-to-use test that can be available in clinics across the country, and that can potentially save the lives of hundreds of patients, giving hope to families who have nearly lost hope to help their loved ones.”
New Treatment Strategies for Improved Pain Relief

Although neurostimulation therapies, such as dorsal root ganglion stimulation and spinal cord stimulation, have shown promising results in the treatment of chronic pain, the mechanisms underlying their efficacy are largely unknown. The research team, which includes co-principal investigator Douglas Weber, PhD (pictured), Professor of Mechanical Engineering and Neuroscience at Carnegie Mellon University and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, is proposing parallel and complementary experiments using innovative behavioral, electrophysiological, imaging, and computational modeling techniques to address this lack of knowledge. The results of these studies will provide novel insights on the mechanisms fundamental to the effectiveness of these neurostimulation therapies and potentially offer new treatment strategies for improved pain relief.
The National Institute of Neurological Disorders and Stroke funded this 3-year project entitled “Defining Mechanisms of Pain Relief Associated with Dorsal Root Ganglion and Spinal Cord Stimulation,” which began on September 17, 2022.
The abstract of this project follows:
Chronic pain is a debilitating condition for which there is a pressing need for safe, effective treatments as is highlighted by the ongoing opioid crisis. One potential solution to this problem is the use of neuromodulatory devices such as spinal cord stimulators and dorsal root ganglion stimulators that are currently approved by the FDA for the treatment of pain. While these devices have consistently generated promising results, they are only used in a fraction of chronic pain patients as an intervention of last resort. One of the reasons that these devices are not used more broadly is because their mechanism of action is largely unknown. Here we propose a series of parallel and complementary experiments to investigate the mechanisms of pain relief for spinal cord (SCS) and dorsal root ganglion (DRGS) stimulation, where conventional stimulation parameters will be used for DRGS, and conventional, burst and kilohertz (kHz) stimulation will be used for SCS. We will focus on the two prevailing models used to explain the therapeutic efficacy of these devices: 1) Gate-control, wherein stimulation of large diameter afferent fibers activates inhibitory interneurons that block nociceptive signals from reaching the brain; and 2) T-junction filtering, wherein stimulation of sensory neurons leads to conduction failure at the T-junction in nociceptive fibers in the DRG, blocking nociceptive inputs from reaching the spinal cord. Experiments designed to test these models are described under four Specific Aims in mice and rats with and without chronic pain induced with chronic compression of the DRG, and in a human DRG nerve preparation. In Aim 1 we will determine which neurons (primary afferent and/or dorsal horn) are activated by SCS and DRGS with 2P-calcium imaging in mice, and single unit recording in rats and human tissue. We will use the data generated at an unprecedented level of detail to refine computational models of the cells and circuits engaged by these devices. In Aim 2 we will confirm the efficacy of DRGS and SCS neuromodulation on simple reflexive responses and complex nociceptive behaviors in rats and mice. The timing and relative efficacy of the site and parameters of neuromodulation will inform computational models and mechanistic studies. In Aim 3 we will directly test the gate-control model using a novel strategy of 2P Ca2+ imaging in mice enabling identification of subpopulations of inhibitory, excitatory, and projection neurons in the same preparation. This will be combined with single unit and MEA recordings from rats and mice, all done in an iterative fashion with computational modeling. In Aim 4 we will test the T-junction filtering model with direct measurements of transmission efficiency in rat and human tissue. Pharmacological studies and computational modeling will investigate specific mechanisms responsible for this filtering. The results from these conceptually and technically innovative studies will provide a major advance in our understanding of the mechanisms underlying the effectiveness of these neuromodulatory devices and potentially offer new treatment strategies for improved pain relief.
Congratulations, Dr. Weber!
Better Than a Hole in the Head

Just as blood pressure informs heart health, intracranial pressure (ICP) helps indicate brain health. ICP sensing is the burgeoning focus of the biomedical optics lab at Carnegie Mellon University (CMU) lead by Jana Kainerstorfer, PhD (pictured), an affiliated faculty member of the McGowan Institute for Regenerative Medicine. Her team is working to modernize ICP sensing approaches, which historically have been invasive and risky. Their noninvasive alternatives will ease risk of infection, pain, and medical expenses, as well as present new monitoring capabilities for patients with an array of brain injuries and conditions, from stroke to hydrocephalus.
Investigating pressure levels in the brain is a laborious task for health professionals and hasn’t progressed much since the 1960s. Current practice involves drilling a hole into a patient’s skull and placing a probe inside for continuous monitoring of ICP levels. It comes with the risk of infection and damaging the brain itself, and while valuable data to have, ICP measurement is reserved only for the most critical of situations.
“At the core of it, what we’ve done is build a sensor alternative that doesn’t require drilling a hole into the patient’s head,” said Dr. Kainerstorfer, an associate professor of biomedical engineering at CMU. “We recently published two papers that explore the use of optical sensors on the forehead for noninvasive ICP monitoring, using near-infrared spectroscopy and diffuse correlation spectroscopy. Both approaches represent huge strides in improving the patient experience and providing better tools to monitor pressure levels in the brain, which can be a key variable in both diagnosis and treatment decisions.”
The first body of work, in collaboration with Matthew Smith, PhD, an associate professor of biomedical engineering at CMU, was recently published in Neurophotonics, and demonstrates that noninvasive near-infrared spectroscopy can be used for ICP monitoring. The process involves small, customized sensors that are placed on the head—think a Fitbit for the brain—to measure light that has interacted with the brain. Every time the heart beats, it produces a pulse in the brain, and the sensors measure hemodynamic blood volume/blood flow changes. Then, a machine learning algorithm is applied to determine ICP.
“Our aim is to create a portable and user-friendly tool to measure pressure changes in the brain,” explained Dr. Kainerstorfer. “We don’t believe that fancy hardware is needed to measure blood flow or hemoglobin concentration, and right now, we don’t have a good handle on how much brain pressure even fluctuates. There are other non-invasive methods that are out there, but what sets us apart is that we’re using a sensor that can be made wearable and offers continuous monitoring.”
Dr. Kainerstorfer’s group also partnered with UPMC Children’s Hospital of Pittsburgh, in collaboration with Michael McDowell, MD, to conduct a clinical study utilizing more specialized sensors and hardware to measure ICP, via noninvasive diffuse correlation spectroscopy. Fifteen pediatric patients from UPMC’s pediatric intensive care unit (ICU) participated, and their results were published in the Journal of Neurosurgery.
As part of the study, a probe with optic fibers was placed on the forehead of the pediatric patients who already had invasive ICP monitors to measure blood flow changes and quantify ICP and to compare the non-invasively acquired data to the invasively acquired data. The results of the noninvasive model were compared with those of invasive monitoring and found to be very similar.
“In this study, the patients had an invasive sensor placed in their brain, and it served as our ground truth,” elaborated Dr. Kainerstorfer. “We worked alongside outstanding collaborators and were encouraged by the results we saw, which indicated that a noninvasive approach could yield accurate ICP measurement. It still requires very specialized hardware and expertise to interpret the data, but it’s much better than drilling a hole in the head.”
Noninvasive intracranial pressure sensing stands to benefit a wide range of people, from critical care and emergency medicine physicians to parents of children with conditions like hydrocephalus. Hydrocephalus is a condition caused by too much brain fluid in the head, which causes pressure on the brain. To solve for this, surgeons implant a shunt to drain the fluid; however, a reliable tool to non-invasively measure shunt function does not exist.
“Imagine a six-year-old who was born with hydrocephalus travels to Walt Disney World Resort with her family,” related Dr. Kainerstorfer. “If during their trip, she starts to exhibit symptoms of shunt failure, like headaches or drowsiness, the family currently has no way to quickly measure ICP, to see if it has increased. The only solution is to run to the ER and get CT/MRI testing and incur expensive medical bills. Many families live in a constant state of fear regarding shunt failure and being too far from a hospital capable of fixing the problem. Further, even if such a hospital is available, it often does not have access to the past medical records necessary to compare to, making diagnosis of shunt failure more difficult. Our work suggests there is a better way—a user-friendly portable sensor that can accurately measure ICP.”
Next steps with the work include plans for a larger, multi-center clinical study of adult and pediatric patients.
“ICP sensing was a side project for our lab initially,” said Dr. Kainerstorfer. “I come from an understanding of what influences blood flow, and how those changes influence neurons. My interest in this topic was piqued from an engineering challenge perspective, but also a personal empathy for patients who live with this uncertainty and don’t have any noninvasive way of evaluating brain pressure changes. Trying to make someone’s life better by an engineering approach is why I’m in this field.”
Projecting a Path for Clinical Translation of 3D-Bioprinted Human Tissues

Three-dimensional bioprinting is an emerging technology that has the potential to build human tissue, on demand, to treat a wide range of human diseases. However, bridging the gap from research at the benchtop to clinical translation requires a host of resources, time, and energy. A new Science Translational Medicine perspective authored by researchers in Carnegie Mellon University’s (CMU’s) Regenerative Biomaterials and Therapeutics Group examines core challenges to overcome in the field of 3D bioprinting and essential milestones to translate to the clinic. Adam Feinberg, PhD (pictured), CMU professor of biomedical engineering and materials science and engineering and co-author of the paper, is an affiliated faculty member of the McGowan Institute of Regenerative Medicine.
“This viewpoint takes into consideration technological advancements in the field to date, as well as our opinion of the work that’s still needed in order to 3D-bioprint patient-specific tissues for repair, regeneration and replacement,” explained Jacqueline Bliley, PhD, a post-doctoral fellow and first author of the piece. “In the bioprinting space, our lab is best known for introducing FRESH technology, a technique that allows for the 3D printing of soft materials, including cells and extracellular matrix, into complex tissue architectures. This technology enables the fabrication of vascular scaffolds, heart valves, and even contractile heart muscle tissues.”
In terms of key challenges and requirements for translating 3D-bioprinted tissues and organs for human benefit, the group suggests that a series of advances are needed within the engineering, medical, and biological sciences. These include:
- Faster 3D bioprinters that create larger and more complex tissues
- Optimized bioinks and biomaterials
- The ability to expand large numbers of cells and differentiate them to target cell types
- Vascularization and perfusion of volumetric tissue
- Immune tolerance to ensure long-term viability in patients
- Non-destructive validation of tissue structure
- Validation of the tissue and organ function that will be required for successful clinical translation.
“In 2022, the first 3D-bioprinted human tissue was implanted in patients, a major milestone,” said Dr. Feinberg, head of the Regenerative Biomaterials and Therapeutics Group. “So now it is no longer a question of if we can bioengineer new tissues and organs, but rather when we can translate that to the clinic and achieve FDA approval so everyone can benefit.”
A forward-looking timeline projecting dates for major milestones in the clinical translation of 3D-bioprinted constructs is presented in the piece. At the current pace of research, and barring changes in the regulatory landscape and current state of 3D bioprinting, 2042 is estimated as the timeframe for 3D-bioprinted solid organs to be approved as a clinical alternative for transplant, which would address the massive shortage in donor organs we face today.
While the field of 3D bioprinting continues to rapidly evolve, considerable challenges remain. Carnegie Mellon University is committed to advancing the narrative by taking a leading role in advanced research initiatives, including the Bioengineered Organs Initiative, a multi-disciplinary effort to create a new generation of long-term replacement organs, and key partnerships, including a multi-year research agreement with Mayo Clinic focused on transforming transplants.
3D Printing Ice

Big scientific breakthroughs often require inventions at the smallest scale. Advances in tissue engineering that can replace hearts and lungs will require the fabrication of artificial tissues that allow for the flow of blood through passages that are no thicker than a strand of hair. Similarly, miniature softbotic (soft-robot) devices that physically interact with humans safely and comfortably will demand the manufacture of components with complex networks of small liquid and airflow channels.
Advances in 3D printing are making it possible to produce such tiny structures. But for those applications that require very small, smooth, internal channels in specific complex geometries, challenges remain. 3D printing of these geometries using traditional processes requires the use of support structures that are difficult to remove after printing. Printing these models using layer-based methods at a high resolution takes a long time and compromises geometric accuracy.
Researchers at Carnegie Mellon University (CMU) have developed a high-speed, reproducible fabrication method that turns the 3D printing process “inside out.” They developed an approach to 3D print ice structures that can be used to create sacrificial templates that later form the conduits and other open features inside fabricated parts.
And they are creating these 3D micro-scale resolution structures out of ice. Yes, ice.
Akash Garg, a PhD student in mechanical engineering, and Saigopalakrishna Yerneni, PhD, a postdoctoral associate in chemical engineering, developed the process and conducted studies under the direction of Burak Ozdoganlar, PhD, Philip LeDuc, PhD (pictured top), and Phil Campbell, PhD (pictured bottom), professors in mechanical and biomedical engineering. Drs. LeDuc and Campbell are McGowan Institute for Regenerative Medicine affiliated faculty members.
“Using our 3D ice process, we can fabricate microscale ice templates with smooth walls and branched structures with smooth transitions. These can subsequently be used to fabricate microscale parts with well-defined internal voids,” said Mr. Garg.
As the most abundant substance on the earth’s surface and the primary building block of any living organism, water is exceptionally well-suited for use in bioengineering applications. The simple and rapid phase transition of water to ice provides exciting opportunities for using water as an environmentally friendly structural material.
“It doesn’t get any more biocompatible than water,” said Mr. Garg.
The team uses the printed ice structures as sacrificial templates for “reverse molding” or inside-out 3D printing. The ice structures are submerged into the liquid or gel form of a chilled structural material, such as resin. After the material sets or is cured, the water is removed. For this purpose, the ice can be melted to evacuate the water. Alternatively, the ice can be sublimated by converting it into water vapor without turning it into liquid water. This ability to easily sublimate the ice allows for easy and “gentle” removal after casting and solidifying the surrounding structural material.
A high-resolution 3D printing system is used to deposit water droplets onto a -35 ◦C custom-built temperature-controlled platform that rapidly transforms the water into ice. By modulating the ejection frequency of the water droplets and synchronizing it with movements of the stage, the new process enables printing branched geometries with smooth surfaces and continuous variations in diameter with smooth transitions. The researchers demonstrate this by printing multiple complex ice geometries, such as a tree, a helix around a pole, and even a one-and-a-half-millimeter tall octopus figurine. The rapid phase change of the water and the strength of the ice enabled freeform 3D printing of ice structures without requiring time-consuming layer-by-layer printing or support structures.
Experimental studies were performed to determine the printing path, motion-stage speed, and droplet frequencies to reproducibly fabricate smooth ice structures with straight, inclined, branching, and hierarchical geometries.
“Controlling so many parameters was challenging,” explained Mr. Garg. “We gradually built up in complexity.”
“This is an amazing accomplishment that will bring exciting advances,” commented Dr. Ozdoganlar. “We believe this approach has enormous potential to revolutionize tissue engineering and other fields, where miniature structures with complex channels are demanded, such as for microfluidics and soft-robotics.”
Faculty researchers at CMU frequently work together on interdisciplinary teams to solve such engineering and biological challenges.
“One of the wonderful parts of Carnegie Mellon is bringing together people from many different disciplines to develop new approaches and solve problems in unique new ways, which is exactly what occurred here to develop these exciting findings,” said Dr. LeDuc.
The researchers acknowledged the great contribution of the late Lee Weiss, PhD, who originally constructed the high-resolution 3D printing system. Dr. Weiss was a professor in the College of Engineering and School of Computer Science, as well as a founding member of Carnegie Mellon’s Robotics Institute. He was also a McGowan Institute affiliated faculty member.
While adoption of the 3D ice process for engineering applications such as creating pneumatic channels for soft robotics could be available in as little as a year, its clinical use for tissue engineering will take more time.
Designing Stimulation Paradigms to Elicit Astrocyte Activity Relevant to Sensory Restoration

Intracortical microstimulation (ICMS) is a versatile tool to study the fundamental physiology of the nervous system and restore sensation in neuroprosthetics; however, the basic neurobiological mechanisms, with relation to which cell types are activated, in what spatiotemporal activation, and how they contribute to therapeutic efficacy remain poorly understood. There has been an increased appreciation of astrocytes’ contribution to regulating proper coordination of neural activity during sensory processing, but it still remains unclear how astrocytes respond to ICMS on a network level and how their activation modulates neuronal activity. Using a combination of two-photon microscopy, mesoscale imaging, and optogenetics or genetic manipulation of astrocyte calcium activity, researchers aim to determine the dynamic relationship between astrocyte and neuronal calcium activity during ICMS and understand how astrocytes respond to and may contribute to the therapeutic efficacy of ICMS paradigms.
The NIH/National Institute of Neurological Disorders and Stroke funded a 3-year project which began on September 20, 2022. The project is entitled, “Astrocyte-Neuron Network Activity During In Vivo Brain Stimulation.” The project principal investigator is Kevin Stieger (pictured top), a graduate student in the laboratory of McGowan Institute for Regenerative Medicine affiliated faculty member Takashi Kozai, PhD (pictured bottom), Assistant Professor in the Department of Bioengineering at the University of Pittsburgh.
The project abstract follows:
Intracortical microstimulation (ICMS) is a primary component of fundamental neuroscience research and holds great potential for sensory restoration or sensory feedback in neuroprosthetics. Despite the wide use of electrical stimulation in neuroprosthetics and interventions such as spinal cord stimulation and deep brain stimulation (DBS), the fundamental physiological and mechanistic properties defining therapeutic efficacy remain poorly understood. For example, it is still unclear which cell types are activated, what role they play in therapeutic outcomes, and how to tune stimulation parameters to selectively elicit relevant activity in those cell types for sensory restoration. Of particular interest is the contribution of non-neuronal cells such as astrocytes. Astrocytes are emerging as important cells in the modulation of neuronal activity during sensory processing in which they respond to neurotransmitters with calcium elevations leading to the release of neuroactive substances to regulate synaptic function (i.e., gliotransmission). Importantly, the time scale in which astrocytes modulate neuronal and synaptic activity is consistent with ICMS-induced neural activity. It is because of these recently appreciated roles regarding astrocyte-neuron communication that astrocytes have been suggested as a vital component to brain stimulation. However, it is unclear how astrocytes respond to clinically relevant stimulation parameters on a network level and how that activation subsequently modulates neuronal activity in an awake animal. To address this gap in knowledge, this proposal will first quantify the calcium activity in cortical astrocytes and across a larger network induced by ICMS with different frequencies and temporal patterns known to have differential therapeutic efficacy using both two-photon microscopy and mesoscale imaging. Next, this proposal will quantify how ICMS-induced neuronal activity is modulated when astrocyte calcium activity is selectively increased (optogenetics) or decreased (viral transduction of a plasma membrane calcium pump) to determine how astrocytes regulate neuronal network activity during ICMS. Because astrocyte calcium activity regulates how these important cells modulate neuronal activity, the cumulative results of this proposal will provide valuable insight for fundamental neuroscience research and the design of stimulation paradigms to elicit astrocyte activity relevant to sensory restoration.
Congratulations, Mr. Stieger and Dr. Kozai!
Illustration: LinkedIn (Stieger)/McGowan Institute for Regenerative Medicine (Kozai).
AWARDS AND RECOGNITION
The American Board of Oral and Maxillofacial Surgery Names New President

The American Board of Oral and Maxillofacial Surgery (ABOMS) named McGowan Institute for Regenerative Medicine affiliated faculty member Bernard Costello DMD, MD, FACS (pictured), as its new president during its September 2022 Annual Meeting in New Orleans. Dr. Costello is an accomplished oral and maxillofacial surgeon, professor, and leader in the field; he will serve a one-year term.
“For me, the chance to work with the Board of Directors of the American Board of Oral and Maxillofacial Surgery as president is a career pinnacle. Our organization does important work stringently vetting oral and maxillofacial surgeons and, ultimately, protecting the public,” said Dr. Costello.
ABOMS conducts a robust certification process in which oral and maxillofacial surgeons submit application materials and undergo rigorous examinations. Once board-certified, Diplomates are required to annually submit evidence of certification maintenance.
This will not be Dr. Costello’s first role on the ABOMS Board of Directors; he served as Vice President from 2021-2022 under Past President Vincent J. Perciaccante, DDS, FACS. Of the board’s work, Dr. Costello says, “We work together with those in our specialty to cultivate excellence and continuously take our process to the next level. It is an honor and privilege to do this work.”
In the past, Dr. Costello has served as President of the American Academy of Craniomaxillofacial Surgeons and the American Cleft Palate-Craniofacial Association. A pediatric craniofacial specialist, Dr. Costello is also highly accomplished in the academic and hospital settings. He is the Associate Vice Chancellor for Health Science Integration and works with the senior administration in the Health Sciences and UPMC. He has been a full-time faculty member at Pitt since 2001 and is the former dean of the School of Dental Medicine. Dr. Costello is Professor of Oral and Maxillofacial Surgery, and Chief of Pediatric Maxillofacial Surgery at UPMC Children’s Hospital where he the longest serving surgeon on the cleft-craniofacial team. He has pioneered research in the use of regenerative medicine for craniofacial deformities and authored guidelines for responsible pain management that focuses on a non-opioid approach, advocating for measures to fight the opioid addiction crisis.
Congratulations, Dr. Costello!
Dr. Michael Boninger Named Co-Chief Sustainability Officer, UPMC
UPMC intends to reduce greenhouse gas emissions by 50% within the next eight years across its footprint – one component of the recently signed Health Care Sector Climate Pledge, an initiative led by the White House and the United States Department of Health & Human Services. The pledge demonstrates UPMC’s continued commitment to lowering emissions and building more climate resilient infrastructure. Also, by committing to the White House initiative, it is UPMC’s goal to achieve net zero emissions by 2050.
Meeting these environmental milestones will be met under the guidance of John Krolicki, vice president, Facilities and Support Services, UPMC Presbyterian Shadyside and UPMC Children’s Hospital, and Michael Boninger, MD (pictured), president, UPMC Innovative Homecare Solutions, and affiliated faculty member of the McGowan Institute for Regenerative Medicine. Mr. Krolicki and Dr. Boninger have assumed the roles of co-chief sustainability officers and will lead a team to create a comprehensive strategy to achieve UPMC’s environmental goals.
“UPMC will lead by example to develop approaches to health care that rapidly reduce our contributions to greenhouse gas emissions,” said Dr. Boninger. “We are making these pledges on behalf of the health and well-being of people today and for future generations.”
The creation of these new roles is the latest step UPMC has taken to directly address climate change and its threats to human health. In 2014, UPMC joined the Green Building Alliance 2030 challenge and has reduced its carbon footprint by over 10% in the Pittsburgh area, despite significant growth.
In addition, all UPMC campuses have eliminated plastic foam packaging from cafeterias, and more than 40 tons of appliances and equipment have been recycled over the last five years. UPMC campuses have been honored by the Arbor Day Foundation for tree planting and education, and efforts are underway in areas like utilizing geothermal technology and constructing green buildings.
“As we work to continue to reduce our environmental impact, UPMC looks forward to implementing additional cutting-edge solutions that will secure our place as a health care leader in sustainability,” Mr. Krolicki said. “We are eager to join the cause in the communities we serve to make a difference for our environment for the long term, which in the end helps the patients we serve.”
Pitt Department of Bioengineering Grad Students Receive NIH F30 and F31 Predoctoral Fellowships
The following graduate students received NIH F30 and F31 Predoctoral Fellowships and are advised by McGowan Institute for Regenerative Medicine affiliated faculty members.
Multicellular Scaffold for Condyle Surface Regeneration
Summary: Sara Trbojevic (pictured top left) will use her F31 to research the temporomandibular joint, or TMJ, which is often overlooked in degenerative joint disorders like osteoarthritis. The TMJ is located in the jaw and is important for eating, talking facial expression, but it’s complex, since it’s made up of two joints that are mechanically interdependent, and its cartilage has a unique, thick, superficial fibrous layer. The project will focus on the development of an acellular therapy for the regeneration of the fibrocartilage and bone regions of the TMJ condyle surface.
Advisor: Alejandro Almarza, PhD (pictured bottom left), associate professor in oral biology in the School of Dental Medicine with a secondary appointment in the Department of Bioengineering
Astrocyte-Neuron Network Activity During In Vivo Brain Stimulation
Summary: Intracortical microstimulation (ICMS) is a primary component of fundamental neuroscience research and holds great potential for sensory restoration or sensory feedback in neuroprostheses. Despite the wide use of electrical stimulation with neuroprostheses and interventions such as spinal cord stimulation and deep brain stimulation (DBS), the fundamental physiological and mechanistic properties defining therapeutic efficacy remain poorly understood. Astrocytes are emerging as important cells in the modulation of neuronal activity and synaptic function on a time scale consistent with ICMS-induced neural activity. However, it is unclear how astrocytes respond to clinically relevant stimulation parameters on a network level, and how that activation subsequently modulates neuronal activity. In this project Kevin Steiger (pictured top center) will use a combination of two-photon microscopy, mesoscale imaging, and optogenetics or genetic manipulation (adeno-associated viral techniques) of astrocyte activity, to determine the dynamic relationship between astrocyte and neuronal calcium activity during ICMS, and understand how astrocytes respond to and may contribute to therapeutic efficacy of ICMS paradigms. Ultimately, the cumulative results of this project will provide valuable insight for fundamental neuroscience research and the design of stimulation paradigms to elicit astrocyte activity relevant to sensory restoration.
Advisor: T.K. Kozai, PhD (pictured bottom center), assistant professor of bioengineering
Development of a Novel Bioinspired Pelvic Organ Prolapse Repair Graft
Summary: Structural and mechanical deficiencies of the vaginal wall and supporting tissues are defining characteristics of pelvic organ prolapse, a common gynecologic condition. The aim of this study is to develop a mechanical and structural profile of vaginal tissue and use these parameters to inform design of a novel synthetic biomimetic graft for prolapse repair. Brittany Egnot (pictured top right) and their advisors will perform experiments to phenotype the mechanical and structural properties of vaginal extracellular matrix, synthesize an elastomer-hydrogel graft that resembles healthy vaginal tissue, and examine cell attachment and differentiation on these grafts.
Advisors: Pamela Moalli, MD, PhD (pictured bottom right), professor in the Department of Obstetrics, Gynecology & Reproductive Sciences, and Steven Abramowitch, PhD, W.K Whiteford Professor of Bioengineering
Congratulations, all!
Illustration: University of Pittsburgh Swanson School of Engineering (students)/McGowan Institute for Regenerative Medicine (advisors)
Weber Lab Member Named 2022 DARPA Riser
The U.S. Defense Advanced Research Projects Agency (DARPA) has named Carnegie Mellon University’s Max Murphy, PhD (pictured top), as part of the 2022 class of DARPA Risers. Each year the agency selects a cohort of outstanding early-career researchers to be recognized for their work and participate in a symposium.
Dr. Murphy, a postdoctoral associate and biomedical engineer, works with McGowan Institute for Regenerative Medicine affiliated faculty member Douglas Weber, PhD (pictured bottom), the Akhtar and Bhutta Professor in Mechanical Engineering and the Neuroscience Institute (NI), and Darcy Griffin, PhD, a NI special faculty researcher. The NeuroMechatronics Lab brings together neuroscientists and engineers to develop new strategies and devices to treat neurological disorders. Dr. Murphy will present on brain-computer interface work.
“I’m excited to showcase some of the technical successes we’ve had and some ideas for how a future initiative might make use of these results,” Dr. Murphy said. “It’s a wonderful opportunity to make connections that could allow me to pursue these collaborative kinds of projects.”
Dr. Weber, who nominated Dr. Murphy, said the 2022 class of DARPA Risers has potential for exciting and unexpected collaborations.
“DARPA plays a vital role in driving innovation in many seemingly disparate areas of science and technology, including neuroscience,” he said. “Max’s work on high-precision noninvasive neurostimulation technology may enable new treatments for a multitude of brain disorders and is a great example of DARPA’s ability to inspire and challenge the research community to tackle important, but difficult problems.”
DARPA scientists have a history of working effectively across disciplines, yielding a long list of breakthrough technologies including the internet, autonomous vehicles, and mRNA vaccines.
DARPA Risers are up-and-coming standouts in their fields, whose research is related to national security and demonstrates the potential to lead to technological surprise — the heart of DARPA’s mission. The Risers program provides individuals in the early stages of their research career a unique opportunity to be recognized for their notable work and present their ideas directly to DARPA.
For DARPA Forward, DARPA program managers and faculty at universities near each event identified a small group of early career scientists and engineers to join the 2022 DARPA Risers cohort. An invitation-only DARPA Risers program will take place directly prior to each Forward event.
Illustration: Murphy (CMU)/Weber (McGowan Institute for Regenerative Medicine)
PUBLICATION OF THE MONTH
Author: Anne E Faust, Lorenzo Soletti, Nicole A Cwalina, Andrew D Miller, Matthew D Wood, Mark A Mahan, Jonathan Cheetham, Bryan N Brown
Title: Development of an acellular nerve cap xenograft for neuroma prevention
Summary: Neuroma formation following limb amputation is a prevalent and debilitating condition that can deeply affect quality of life and productivity. Several approaches exist to prevent or treat neuromas; however, no approach is either consistently reliable or surgically facile, with high rates of neuroma occurrence and/or recurrence. The present study describes the development and testing of a xenogeneic nerve cap graft made from decellularized porcine nerve. The grafts were tested in vitro for cellular removal, cytotoxicity, mechanical properties, and morphological characteristics. The grafts were then tested in rat sciatic nerve gap reconstruction and nerve amputation models for 8 weeks. Gross morphology, electrophysiology, and histopathology assessments were performed to determine the ability of the grafts to limit pathologic nerve regrowth. In vitro testing showed well decellularized and demyelinated nerve cap graft structures without any cytotoxicity from residual reagents. The grafts had a proximal socket for the proximal nerve stump and longitudinally oriented internal pores. Mechanical and surgical handling properties suggested suitability for implantation as a nerve graft. Following 8 weeks in vivo, the grafts were well integrated with the proximal and distal nerve segments without evidence of fibrotic adhesions to the surrounding tissues or bulbous outgrowth of the nerve. Electrophysiology revealed absence of nerve conduction within the remodeled nerve cap grafts and significant downstream muscle atrophy. Histologic evaluation showed well organized but limited axonal regrowth within the grafts without fibrous overgrowth or neuromatous hypercellularity. These results provide proof of concept for a novel xenograft-based approach to neuroma prevention.
Source: J Biomed Mater Res A. 2022 Nov;110(11):1738-1748. Epub 2022 Aug 17.
GRANT OF THE MONTH
PI: Alejandro Almarza
Co-PI: Juan Taboas
Title: Polymer Scaffolds for Mandibular Condyle Cartilage Regeneration
Description: The most severe cases of TMJ disorders consist of mandibular condyle degeneration. Unfortunately, no regenerative options exists and current treatments do not restore full function. The articulating tissue of the condyle is a fibrocartilage that consists of an intricate interface between fibrous, cartilaginous, and boney tissue that is essential for normal function and that is lost in severe TMJ disorders. The objective of this study is to regenerate fibrocartilage-bone interface of the mandibular condyle in skeletally mature goats using a comprehensive tissue engineering approach. A condylar defect will be treated with novel multilayer scaffold implant designed to promote site-specific tissue regeneration. We have strong pilot in-vivo data showing that our scaffolds components regenerate fibrous and cartilage tissue in our novel goat model, and bone in a segmental defect model. We will implant the scaffolds in a mediolateral grove-shaped condylar defect. We hypothesize that a multilayer scaffold will allow for site- specific fibrous-cartilage-bone regeneration of the mandibular condyle cartilage when compared to a homogenous sponge scaffold and untreated control defects. First, we will study the properties of a multilayer scaffold design in-vitro. We will characterize the permeability and release of TGFβs from the scaffold. Second, we will assess the functional healing of condylar defects treated with the multilayer scaffolds. We will assess mechanical properties, regenerate tissue composition, and condylar architecture formation using terminal assays at 1, 3 and 6 months post-surgery. Third, we will study the regeneration potential of three cell subpopulations found on the condyle. Successful completion of this proposal is the critical step to provide a regenerative therapy to treat TMJ mandibular cartilage degeneration, and a basis for successful osteochondral tissue regeneration in other sites.
Source: National Institute of Dental and Craniofacial Research
Term: December 1, 2022 – November 30, 2023
Amount: $596,892



