February 2021 | VOL. 20, NO. 2| www.McGowan.pitt.edu
2021 Class of NAI Senior Members Announced

The National Academy of Inventors (NAI) has named 63 of the world’s best emerging academic inventors to its 2021 class of Senior Members. Three of those honorees are McGowan Institute for Regenerative Medicine affiliated faculty members (pictured top to bottom):
- Bryan Brown, PhD, Associate Professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh
- Michael Lotze, MD, Professor of Surgery and Bioengineering, University of Pittsburgh; Vice Chair of Research, Department of Surgery, University of Pittsburgh School of Medicine
- Kacey Marra, PhD, Professor, Departments of Plastic Surgery and Bioengineering, Vice Chair of Research, Department of Plastic Surgery, University of Pittsburgh
NAI Senior Members are active faculty, scientists, and administrators from NAI Member Institutions who have demonstrated remarkable innovation producing technologies that have brought, or aspire to bring, real impact on the welfare of society. They also have growing success in patents, licensing, and commercialization.
Drs. Brown, Lotze, and Marra are invited to attend the NAI 10th Annual Meeting on October 31 – November 3, 2021, where they will be recognized as newly elected NAI Senior Members. The event will take place at the JW Marriott Water Street in Tampa, Florida.
The NAI’s mission is to recognize and encourage inventors with patents issued from the U.S. Patent and Trademark Office, enhance the visibility of academic technology and innovation, encourage the disclosure of intellectual property, educate, and mentor innovative students, and translate the inventions of its members to benefit society. The Senior Member Program allows the NAI to expand its reach and further its goals in recognizing the contributions of inventors around the globe.
Congratulations, Drs. Brown, Lotze, and Marra!
Illustration: McGowan Institute (Brown, Marra)/Nurix (Lotze)
RESOURCES AT THE MCGOWAN INSTITUTE
March Histology Special
The Histology Core offers specialized IHC services to fit any research needs. Our staff is dedicated to quickly and competently working up antibody protocols. We supply all ancillary reagents and will work with investigators to decide the most likely successful antibodies for every experiment need.
Any species, any marker, any challenge… we at the McGowan Histology Core Laboratory are confidant that we can help. In the month of March, all new customers submitting immunohistochemistry projects will receive 25% off the workup fee ($175 regularly) when they mention the newsletter.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SAVE THE DATE
 20th Annual McGowan Institute Virtual Scientific Retreat
20th Annual McGowan Institute Virtual Scientific Retreat
Registration Now Open for the 2021 McGowan Institute Scientific Retreat
The 20th Annual McGowan Institute for Regenerative Medicine Virtual Scientific Retreat will take place on March 9-11, 2021.
The poster session will begin on the evening of March 9th.
Under the leadership of McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, associate professor, Department of Bioengineering the program committee is planning an exciting group of speakers and topics.
Mark the dates on your calendars. Additional 2021 Retreat details and news will be available here and emailed to registrants.
NOTE: all who complete their retreat registration by March 3rd will be entered into a drawing for a $50 gift certificate!
 2021 MHSRS Call for Abstracts
2021 MHSRS Call for Abstracts
The 2021 Military Health System Research Symposium (MHSRS) Call for Abstracts opens 17 Feb 2021 and closes 31 Mar 2021. We WILL NOT accept abstract submissions after 31 Mar, so please plan accordingly.
The MHSRS is the Department of Defense’s premier scientific meeting. It provides a collaborative environment for military medical care providers with deployment experience, DoD scientists, academia, and industry to meet and exchange information on the unique medical needs of the Warfighter.
The theme of the 2021 MHSRS is “Channeling the Challenge – The Agility of Military Medical Research”. The meeting will have four presentation focus areas: Warfighter Medical Readiness, Expeditionary Medicine, Warfighter Performance, and Return to Duty. In addition, there will be breakout sessions dedicated to DoD-sponsored SARS CoV-2 (COVID-19) related research.
Go to the MHSRS website to review the titles and descriptions of the 2021 scientific breakout sessions and to submit an abstract.
The MHSRS website is a password enabled site.
- Army MEDCOM and DHA CAC Users: Signing in with a CAC is the most secure/desirable method since it uses two-factor authentication. Go to this URL (Click ‘Sign In with CAC’). If you have trouble accessing with your CAC, please register for a member account.
- All Others must have a username and password to access the site and to submit an abstract. If you have forgotten your previously issued username and password, email: detrick.medcom-usamrmc.list.usamrmc-web-team@mail.mil for assistance. DO NOT re-register. If you have never registered on the MHSRS website (new abstract submitter), go to this URL, to register for a new member account and receive username and password.
As of this time, we are projecting the 2021 MHSRS to be an in-person meeting. Meeting Approval is pending. The date and location are pending. This information will be posted on the MHSRS website and communicated via a mass e-mail to those registered on the MHSRS website when it is available.
Manuscripts prepared from MHSRS presentations are published as Supplements to the Journal of Trauma and Acute Care Surgery and Military Medicine. The links to the latest editions of these supplements can be found on the MHSRS homepage.
Please forward this 2021 MHSRS Call for Abstracts to any interested party. Thank you for your support!
Sincerely,
SCIENTIFIC ADVANCES
Study Examines Social Determinants of Disparities in Kidney Transplantation

Among US adults with kidney failure, race and social determinants of health were associated with patients’ likelihood of receiving a kidney transplant. The findings come from an analysis that is in the Clinical Journal of the American Society of Nephrology (CJASN). McGowan Institute for Regenerative Medicine affiliated faculty member Mary Amanda Dew, PhD, Professor of Psychiatry, Psychology, Epidemiology, Nursing, and Biostatistics at the University of Pittsburgh and also a Professor at the Clinical and Translational Science Institute, is a co-author of the study.
Blacks are more likely than whites to develop kidney failure, but they’re less likely to undergo kidney transplantation, the optimal treatment for kidney failure. Blacks also have disproportionately lower rates of kidney transplants from living donors, which offer superior patient and transplant survival rates compared with deceased-donor kidney transplants.
To assess whether social determinants of health–such as demographics, cultural factors, psychosocial characteristics, and transplant knowledge–may play a role in these disparities, Larissa Myaskovsky, PhD (University of New Mexico Health Sciences Center) and her colleagues prospectively followed 1,056 patients referred for kidney transplantation from 2010 to 2012 (with follow up through 2018) at the University of Pittsburgh Medical Center. Patients completed an interview soon after their initial kidney transplant evaluation and were followed until their kidney transplants was performed.
The team found that even after accounting for social determinants of health, Blacks had a lower likelihood of receiving a kidney transplant overall, and specifically a living-donor transplant but not deceased-donor transplant.
Black race, older age, lower income, public insurance, more comorbidities, being transplanted before 2014 kidney allocation policy changes, greater religiosity, less social support, less transplant knowledge, and fewer learning activities each were associated with a lower probability of receiving a kidney transplant.
“Our data suggest a critical need for transplant centers to identify and intervene on social determinants for at-risk populations,” said Dr. Myaskovsky. “Based on our findings, developing interventions that target patients with low transplant knowledge, religious objection to living-donor transplant, or poor social support may enhance equal access to kidney transplantation because transplant teams can use these risk factors to target patients who may need more support to ensure they receive a transplant.”
An accompanying Patient Voice editorial provides the perspective of a Black American woman who was suddenly diagnosed with kidney failure 7 years ago at the age of 49 years. She is a kidney transplant recipient and also the Director of Outreach and Government Relations for the National Kidney Foundation of Illinois.
Experts Study ACL Injury Features with 3D Statistical Shape Modeling

McGowan Institute for Regenerative Medicine affiliated faculty member Richard Debski, PhD, William Kepler Whiteford Faculty Fellow, Co-Director, Orthopaedic Robotics Laboratory, Professor, University of Pittsburgh Departments of Bioengineering and Orthopaedic Surgery, is a co-author of a study to investigate tibiofemoral bony morphology features associated with ACL injury and sex utilizing three-dimensional statistical shape modeling. Other co-authors of this work include:
- Sene Polamalu, BS, Bioengineering PhD Student Researcher (third year), Orthopaedic Robotics Laboratory, University of Pittsburgh
- Volker Musahl, MD, Blue Cross of Western Pennsylvania Professor, Chief UPMC Sports Medicine Medical Director, Professor, University of Pittsburgh Departments of Orthopaedic Surgery, Bioengineering, and Clinical Translational Science Institute
In the study, statistical shape modeling was employed to assess three-dimensional (3D) bony morphology between:
- distal femurs and proximal tibiae of anterior cruciate ligament (acl) injured knees
- the contralateral uninjured knees of acl injured subjects
- knees with no history of injury
Surface models were created by segmenting bone from bilateral computed-tomography scans of:
- 20 subjects of their ACL injured knees and non-injured contralateral knees
- 20 knees of control subjects with no history of a knee injury
Correspondence particles were placed on each surface, and a principal component analysis determined modes of variation in the positions of the correspondence particles describing anatomical variation. ANOVAs assessed the statistical differences of 3D bony morphological features with main effects of injury state and sex.
ACL injured knees were determined to have a more lateral femoral mechanical axis and a greater angle between the long axis and condylar axis of the femur. A smaller anterior-posterior dimension of the lateral tibial plateau was also associated with ACL injured knees.
Results of this study demonstrate that there are more bony morphological features predisposing individuals for ACL injury than previously established. These bony morphological parameters may cause greater internal and valgus torques increasing stresses in the ACL. No differences were determined between the ACL injured knees and their uninjured contralateral knees demonstrating that knees of ACL injured individuals are at similar risk for injury.
Further understanding of the effect of bony morphology on the risk for ACL injury could improve individualized ACL injury treatment and prevention.
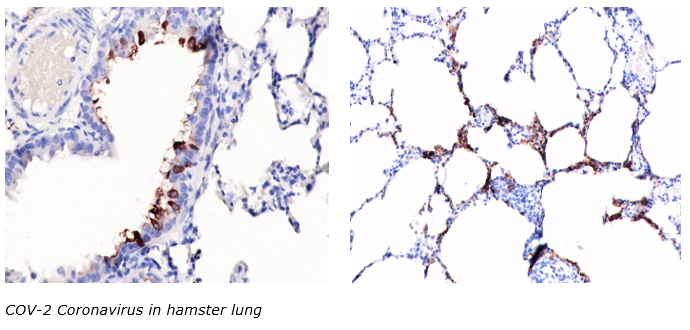
Microscopic Look at Aneurysm Repair
Hitting a pothole on the road in just the wrong way might create a bulge on the tire, a weakened spot that will almost certainly lead to an eventual flat tire. But what if that tire could immediately begin reknitting its rubber, reinforcing the bulge, and preventing it from bursting?
That’s exactly what blood vessels can do after an aneurysm forms, according to new research led by the University of Pittsburgh’s Swanson School of Engineering and in partnership with the Mayo Clinic. Aneurysms are abnormal bulges in artery walls that can form in brain arteries. Ruptured brain aneurysms are fatal in almost 50% of cases.
The research, recently published in Experimental Mechanics, is the first to show that there are two phases of wall restructuring after an aneurysm forms, the first beginning right away to reinforce the weakened points.
“Imagine stretching a rubber tube in a single direction so that it only needs to be reinforced for loads in that direction. However, in an aneurysm, the forces change to be more like those in a spherical balloon, with forces pulling in multiple directions, making it more vulnerable to bursting,” explained McGowan Institute for Regenerative Medicine affiliated faculty member Anne Robertson, PhD, Professor of Mechanical Engineering and Materials Science at Pitt, whose lab led the research. “Our study found that blood vessels are capable of adapting after an aneurysm forms. They can restructure their collagen fibers in multiple directions instead of just one, making it better able to handle the new loads without rupturing.”
Researchers have known that blood vessels have the ability to change and restructure over time, but this study represents the first observation of a new, primary phase of restructuring that begins immediately.
The researchers used a rabbit model developed by David Kallmes of the Mayo Clinic to observe this restructuring in the brain tissue over time. To see this process up close, the researchers partnered with McGowan Institute affiliated faculty member Simon Watkins, PhD, Founder and Director of the Center for Biologic Imaging at the University of Pittsburgh, a member of the Pittsburgh Cancer Institute, and a Distinguished Professor and Vice Chairman within the Department of Cell Biology, taking advantage of the center’s state-of-the-art multiphoton microscopes to image the architecture of the fibers inside the aneurysm wall.
“We found that the first phase of restructuring involves laying down an entirely new layer of collagen fibers in two directions to better handle the new load, while the second phase involves remodeling existing layers, so their fibers lie in two directions,” explained Chao Sang, PhD, Data Scientist, Novasenta, who was a primary investigator on this research as part of his doctoral dissertation in Pitt’s Department of Mechanical Engineering and Materials Science
“The long-term restructuring is akin to a scar forming after a cut has healed, while this first phase that we observed can be thought of as having a role similar to clotting immediately after the cut—the body’s first response to protect itself,” added Dr. Robertson, who has a secondary appointment in the Swanson School’s Department of Bioengineering. “Now that we know about this first phase, we can begin to investigate how to promote it in patients with aneurysms, and how factors like age and preexisting conditions affect this ability and may place a patient at higher risk for aneurysm rupture.”
Illustration: Collagen fibers restructure themselves in multiple directions to reinforce aneurysm walls. Credit: Anne Robertson and Sang Chao.
Dr. George Michalopoulos Awarded R01

McGowan Institute for Regenerative Medicine affiliated faculty member George Michalopoulos, PhD, Professor and Chairman of the Department of Pathology at the University of Pittsburgh, received NIH funding to study the impact of inhibition of EGF receptor on non-alcoholic fatty liver disease. This proposal, which is funded for 5 years, will study an important role of EGFR signaling in the pathogenesis of NASH/NAFLD and has high translational impact. Other Pittsburgh Liver Research Center members supporting the study include Ramon Bataller, MD, PhD, and Aatur Singhi, MD, PhD.
An abstract of the project follows:
In the last two decades, there has been an increased appreciation of a long-term threat, that of non-alcoholic fatty liver disease (NAFLD), evolving into non-alcoholic steatohepatitis (NASH), which can further advance into cirrhosis and hepatocellular carcinoma (HCC). Treatment of NAFLD and NASH is primarily through attempts at nutritional modification, often unsuccessful due to poor compliance and socioeconomic factors. There is currently no accepted single-agent pharmacologic treatment for NAFLD. The current proposal is based on findings from our work on liver regeneration, in which we discovered a unique role for EGFR for control of steatosis in replicating hepatocytes. We have now extended these studies in mice fed a high fat diet supplemented with fructose in the drinking water (“Fast Food Diet”, FFD). We found that in mice on FFD, concurrent EGFR inhibition completely prevented and eliminated any fat deposition in hepatocytes. Furthermore, EGFR inhibition reversed severe hepatocyte steatosis established after FFD feeding for 4 months. Detail analysis of the mechanisms revealed widespread effects of EGFR inhibition on multiple transcription factors related to lipid metabolism and subsequent consequences to specific enzymes associated with lipid biosynthesis and degradation. No such findings occur when signaling from the other of the two hepatocyte-mitogenic receptor tyrosine kinases, MET (the HGF receptor), was eliminated. The purpose of this proposal is to explore translational applications of this finding. This will be done by exploring effects of EGFR inhibitors established in human pharmacology. In addition, we will conduct parallel analysis between EGFR and MET signaling inhibition on their effects of NAFLD, aiming to uncover specific pathways unique to EGFR that may reveal new pharmacologic approaches more focused than the inhibition of the entire EGFR signaling with its potentially adverse complications. In parallel studies, we will also use available human NAFLD/NASH tissue material available in the Biorepository of the Pittsburgh Liver Research Center (PLRC). This material will be used under the established rules of IRB obtained by PLRC for such studies. We will explore activation of EGFR dependent pathways and will correlate with the histology of NAFLD/NASH. A serious complication of progression of NAFLD to NASH is development of fibrosis. We have uncovered EGFR-controlled signaling molecules in steatotic hepatocytes, which have been associated with activation of hepatic stellate cells and enhanced production of collagens. These also offer opportunities for selective pharmacology for fibrosis and their relevance will be assessed in the studies proposed.
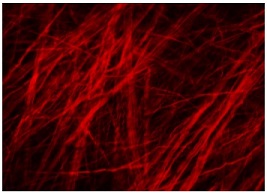
Pitt Scientists Find the Key to Viral-Bacterial Co-infection
The mechanism by which acute viral respiratory infections promote secondary bacterial growth and infection in the airways depends on iron-carrying extracellular sacs secreted by the cells lining the host’s airways, report researchers from the University of Pittsburgh School of Medicine in a paper published in Cell Reports. McGowan Institute for Regenerative Medicine faculty member Donna Stolz, PhD, associate director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and an associate professor in the Departments of Cell Biology and Pathology at the University of Pittsburgh, is a co-author on the paper.
The sacs, or “vesicles,” which carry iron bound to a protein called transferrin, associate with bacterial cells and supply them with essential nutrients, promoting the growth of expansive bacterial communities. The finding gives us a glimpse into how bacteria exploit the host’s defense system against pathogens and can offer a new way for creating therapies to prevent secondary bacterial infections in the clinical setting.
“The development of chronic bacterial infections often is preceded by acute viral infections, and such co-infections increase patients’ likelihood of death or lifelong disability,” said senior author Jennifer Bomberger, PhD, associate professor in the Department of Microbiology & Molecular Genetics at Pitt. “We wanted to understand what it is that the virus is doing that allows bacteria to get a foothold in the patient’s airways.”
Preventing and controlling secondary lung infections by increasingly antibiotic-resistant bacteria, such as Staphylococcus aureus and Pseudomonas aeruginosa, remains a challenging problem in health care. According to reviews of historic autopsy reports, more than 90% of deaths during the 1918 influenza pandemic likely resulted from secondary bacterial pneumonia and, to this day, up to 30% of adults hospitalized with viral community-acquired pneumonia develop bacterial co-infections.
To study the mechanism of viral-bacterial interactions in chronic lung disease, the Pitt researchers used a model of respiratory syncytial virus, or RSV, and P. aeruginosa co-infection, the severity of which depends on P. aeruginosa’s ability to form biofilms—large communities of bacteria encased in a polymeric matrix.
Using in vitro studies and fluorescent imaging, scientists showed that the production and secretion of extracellular vesicles—two processes that routinely occur in different cell types in the body, including the respiratory epithelium—are boosted by an acute viral infection. Crucially, these vesicles directly associate with P. aeruginosa biofilms and promote their growth.
“Extracellular vesicles naturally occur in the body and are used by the organism as a communication tool,” said Dr. Bomberger. “It seems that bacteria co-opted this process for their own benefit.”
While the exact mechanism of how extracellular vesicles attach to bacteria remains to be explored, researchers found that vesicles carry protein-bound iron on their surface, supplying bacteria with necessary nutrients.
“It would be interesting to see the implications this mechanism has for the host’s immune response,” said Matthew Hendricks, PhD, a lead author of the paper and a former graduate student in Bomberger’s laboratory. “If extracellular vesicles can shield bacteria from the immune cells, that could decrease the host’s ability to detect the infection and help bacteria evade the immune response.”
It also appears that the mechanism of extracellular vesicle-dependent interaction between viruses and bacteria is universal for different types of viruses, including other respiratory viruses and viruses that attack other mucosal locations, such as the gastrointestinal tract.
Illustration: Fluorescent extracellular vesicles associated with bacterial biofilms: red fluorescently labelled extracellular vesicles associate with biofilms formed by clusters of P. aeruginosa (in green). Jennifer Bomberger.
Using a Machine Model to Predict Risk of Human Aneurysms
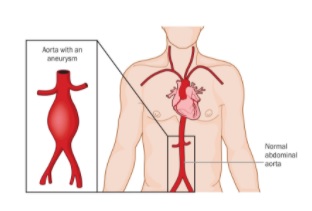
An abdominal aortic aneurysm (AAA) can be a ticking time bomb if undiscovered in time. However, researchers at the University of Pittsburgh are developing a new model to better predict at-risk patients. And the tools they are using apply mechanical testing to the human body – which is itself a complex machine.
An AAA occurs when the aorta weakens and begins to irreversibly dilate, like a slowly inflating balloon. If left untreated, the risk of rupture increases and has a 90 percent rate of mortality, making AAA the 15th leading cause of death in the United States with more than 15,000 deaths reported annually.
Once diagnosed, clinicians must determine whether the aorta requires surgery, using the AAA diameter to decide if an aneurysm is clinically relevant. A diameter 5.5 centimeters or larger typically calls for surgical intervention, barring other contraindications, but this one-size-fits-all approach misses nearly 25 percent of patients who experience a rupture at a smaller size.
Pitt bioengineer David Vorp, PhD, a McGowan Institute for Regenerative Medicine affiliated faculty member, received an award from the National Institutes of Health to track the natural evolution of small AAA and develop a predictive model to improve patient prognosis. His Vascular Bioengineering Lab at Pitt’s Swanson School of Engineering is focused on finding novel diagnoses and treatments for these silent killers.
“It’s a ticking time bomb,” explained Timothy Chung, PhD, a post-doctoral associate in Dr. Vorp’s lab. “Once you diagnose an abdominal aortic aneurysm, you don’t know when or if it’s going to rupture.
“Imagine you’re blowing up a balloon, and it pops. This event involves the mechanics and forces that are interacting with the wall of the balloon,” continued Dr. Chung, who will help lead the project. “We’re interested in the biomechanics of why elevated pressure or a weakening of the aneurysm wall might lead to rupture or accelerated growth.”
The research team hopes that CT scans and other data from a rare, longitudinal clinical trial (“Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial”) will help them identify the risks of elevated growth rate or eventual rupture.
Dr. Vorp’s lab group will create 3D geometric reconstructions and perform biomechanical simulations on patient datasets at each imaging scan interval (every six months) to learn how small AAA progresses over time. They will then use the scans and unique software tools from their lab to perform shape analyses that will determine which geometries may lead to poor patient outcomes.
“Currently, clinicians are simply applying a one-dimensional shape analysis, using diameter as a threshold for clinical intervention,” said Dr. Chung. “The tools developed in the Vascular Bioengineering Lab can help us extract more than one-dimensional measurements. They allow us to create two- and three-dimensional shape indices derived from image-based surface reconstructions, allowing for a more robust analysis.”
The team will then feed data from the shape analysis and biomechanical simulations to train a machine learning algorithm to classify different types of aneurysm outcomes. This will be used to develop a predictive model that can help guide clinicians and determine the need for surgical intervention.
“Early in my career, the advent of finite element analysis – a computational method to predict mechanical wall stress distribution in complex shapes both biological and human-made – provided a game-changing tool to better understand the role of biomechanics in AAA disease,” said Dr. Vorp, Associate Dean for Research and John A. Swanson Professor of Bioengineering. “Now, machine learning technologies can not only help us better understand the combination of factors that lead toward rupture or clinical intervention, but also package that knowledge into a true, personalized health tool for those afflicted with this potentially lethal condition.”
Illustration: Location and comparison of a normal aorta and abdominal aortic aneurysm. If left untreated the aneurysm will irreversibly grow increasing risk of rupture for a patient. Shutterstock.
Saving Baby Teeth to Treat Future Medical Needs

McGowan Institute for Regenerative Medicine affiliated faculty member Giuseppe Intini, DDS, PhD, Associate Professor of Periodontics and Preventive Dentistry at the University of Pittsburgh, School of Dental Medicine, recently talked with Nicole Fabian-Weber of Care.com about the potential benefits of saving your child’s baby teeth for future medical needs. What Ms. Fabian-Weber learned is that, at this point, few experts are completely sold on the practice.
Why would parents want to bank their child’s baby teeth? The thoughts are that those teeth contain valuable stem cells which one day may be used to treat a future disease afflicting that child. Currently, there are no treatments available using the stem cells from baby teeth, but that could change in 20 years or so.
As Dr. Intini explains, regardless of their origin, stem cells are either totipotent, pluripotent, or multipotent. “Totipotent stem cells can generate any type of cell,” Dr. Intini says. “Pluripotent cells can generate all cells except the placenta, the amniotic sac, and the umbilical cord (meaning a cell from the embryo can become anything from liver to hair cells but cannot fully generate another human being if transplanted in a host womb), and multipotent stem cells have the ability to develop into a limited type of cells. Teeth, it looks like, are multipotent.”
According to Dr. Intini, research is currently suggesting that there are stem cells in baby teeth — “suggesting” being key. “Right now, it appears — meaning, science is showing some evidence — that there are multipotent stem cells in teeth,” says Dr. Intini. “Keep in mind, though, this is all preclinical, and research has been done mostly with mice and rats. To really say teeth can produce stem cells that can be used for clinical application, we need clinical trials and right now there are very few.”
StemSave, a New York City-based baby teeth bank, offers families the opportunity to save doctor-extracted baby teeth for future use. Fees include an initial recovery and processing fee and an annual storage fee.
A Potential New Therapeutic Target for Kidney Cancer
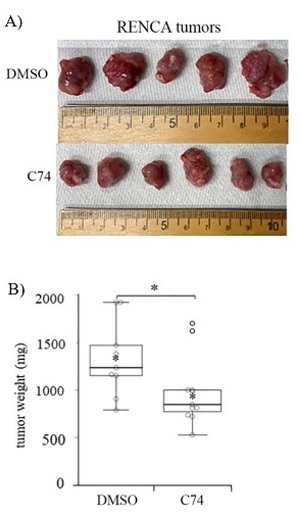
Kidney cancer will have been newly diagnosed in over 73,000 patients in the United States in 2020, resulting in nearly 15,000 deaths.1 Clear cell renal cell carcinoma (ccRCC) represents the most commonly diagnosed subtype of kidney cancer, accounting for more than 75% of new cases. Approximately 30% of ccRCC patients have metastatic disease at the time of diagnosis, and about 33% will develop recurrent or metastatic disease following treatment, indicating poor prognosis for the majority of ccRCC patients. The signature genetic lesion in ccRCC is the loss of the tumor-suppressing Von-Hippel Lindau (VHL) gene, which results in tumors marked by an abundant vascular microenvironment. With funding from an FY18 Kidney Cancer Research Program (KCRP) Idea Development Award – Established Investigator Option, McGowan Institute for Regenerative Medicine affiliated faculty member Partha Roy, PhD, Associate Professor of Bioengineering, Cell Biology, and Pathology at the University of Pittsburgh, set out to develop an in-depth molecular understanding of ccRCC-associated vascularization in an effort to exploit this phenomenon as a therapeutic target.
As described recently in the Journal of Biological Chemistry, Dr. Roy’s team—which included McGowan Institute affiliated faculty member Michael Lotze, MD, Professor of Surgery and Bioengineering, University of Pittsburgh, and Vice Chair of Research, Department of Surgery, University of Pittsburgh School of Medicine—analyzed data from The Cancer Genome Atlas and determined that increased levels of several cytoskeleton-regulating genes, including profilin1 (Pfn1), associated strongly with poor prognosis in ccRCC patients. The group observed that expression of Pfn1 is elevated primarily within the vascular endothelial cells (VEC; cells that form the walls of blood vessels) in the tumor microenvironment but also at a lower frequency within the tumor cells themselves. Using established VHL-deficient RCC cell line models, Dr. Roy’s team found that both proliferation and migratory behavior are reduced when Pfn1 is suppressed directly in tumor cells. In VEC, forced expression of Pfn1 caused the cells to export and deposit the protein in the growth media, suggesting that high Pfn1 expression in the VEC component of ccRCC tumors may affect the behavior of adjacent tumor cells. Indeed, when the team exposed RCC cells to recombinant Pfn1 protein in culture, the cells were stimulated to increase their migratory activity. Importantly, co-culture of RCC cells with VEC cells also led to increased migratory behavior, which could be inhibited by suppressing Pfn1 in the VEC population. These findings indicate that expression of Pfn1 within the tumor cells, and/or the associated VEC can elicit a pro-tumorigenic effect.
Dr. Roy’s team reasoned that Pfn1 expression in VEC and/or tumor cells, or released into the tumor microenvironment by VECs, may be an important and accessible therapeutic target for ccRCC patients. As Pfn1 acts primarily by promoting the growth of the actin-based cytoskeleton, the group developed a new small molecule inhibitor (C74) of the Pfn1-actin interaction and observed that RCC cells exhibit reduced proliferation and migration when treated with C74. In pilot preclinical testing, mice bearing RCC tumors were treated with localized injections of C74, which resulted in a significant reduction of tumor size compared to control treated animals. Notably, daily dosing with C74 did not result in discernible weight loss or other measurable side-effects in the treated mice.
Early results of this KCRP-funded research have demonstrated a proof of concept for specific targeting of Pfn1 in ccRCC based on the highly vascularized nature of the disease. The disruption of actin cytoskeletal dynamics by inhibition of Pfn1-binding reduces tumor cell growth capacity and cell motility, hallmarks of more aggressive kidney cancers, and may serve to slow or prevent metastatic progression. Dr. Roy’s work has the potential to translate into a promising new therapy to improve outcomes for the vast majority of patients diagnosed with ccRCC.
Illustration: C74 inhibits kidney tumor growth in vivo. Mice bearing subcutaneous RENCA tumors, a mouse model of ccRCC, were treated daily with either control (DMSO) or the Pfn-1/Actin inhibitor C74. After 20 days, C74-treated tumors were found to be significantly smaller in size (A) and in weight (B), indicating a reduction in tumor aggressiveness. Cell Biology Molecular Bases of Disease, Volume 295, Issue 46, P15636-15649, November 13, 2020.
Will the Science of Aging Reversal Come into Its Own in 2021?

As reported by Heather McKenzie from BioSpace, when our backs were against the wall, the biotech/biopharma community came together to create not one, but two COVID-19 vaccines currently approved for emergency use in the U.S. The lessons learned from the global pandemic are expected to translate to existing and emerging therapeutic areas – particularly, oncology; more efficient regulatory-industry relationships; mRNA is a word we will continue to hear a lot about; and home health care is here to stay. These are just a few of the insights Ms. McKenzie discovered when she recently spoke with 12 executives from the biotech/biopharma space.
One of those individuals was McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Rando, MD, PhD, Professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, the Director, Glenn Laboratories for the Biology of Aging, Stanford University, and the Deputy Director, Stanford Center on Longevity, also at Stanford University. This is what he had to say:
“The biggest risk factor for all these diseases that we want to treat–cancer, heart disease, neurodegenerative disease–the biggest risk factor is aging,” said Dr. Rando, co-founder of Fountain Therapeutics, a biopharmaceutical company focused on reversing aging to treat disease.
“I think that that will continue to be a real focus. Can we develop…almost a preventative therapeutic approach? There’s been a lot of movement over the last several years in terms of therapeutics targeted for the basic biology of aging, and I think that’s something that has just continued to gain steam increasingly over the last 5-10 years for sure, and even more so over the last few years I think that’s a big shift, and I think it’s moving from the academic world into the biotech and pharma world.”
In the treatment space, Dr. Rando believes that the overall viewpoint is changing for “a couple of reasons. Really, a lot of the drugs that we use in clinical practice are actually preventative medicines. So, it’s not that different. I think, number one, they’ve come to recognize that we already do this, disease by disease. Another thing is, there’s been so much investment in biotech in this area, that I think they’re just realizing there’s something here, and no one wants to miss the boat.”
Drs. Alejandro Almarza and Juan Taboas Awarded NIH R01 Grant

McGowan Institute for Regenerative Medicine affiliated faculty member Alejandro Almarza, PhD, Associate Professor in Oral Biology in the School of Dental Medicine with a secondary appointment in the Department of Bioengineering in the Swanson School of Engineering, University of Pittsburgh, recently received an NIH R01 Grant for his project titled “Polymer Scaffolds for Mandibular Condyle Cartilage Regeneration.” The project is in collaboration with McGowan Institute affiliated faculty member Juan Taboas, PhD, Associate Professor in Oral Biology in the School of Dental Medicine with a secondary appointment in the Department of Bioengineering in the Swanson School of Engineering, at Pitt. The 4-year grant awards over $2.5 million towards their research.
The objective of this study is to regenerate fibrocartilage-bone interface of the mandibular condyle in skeletally mature goats using a comprehensive tissue engineering approach. A condylar defect will be treated with novel multilayer scaffold implant designed to promote site-specific tissue regeneration. Successful completion of this proposal is the critical step to provide a regenerative therapy to treat temporomandibular joint (TMJ) mandibular cartilage degeneration and is a basis for successful osteochondral tissue regeneration in other sites.
The abstract for the project reads:
The most severe cases of TMJ disorders consist of mandibular condyle degeneration. Unfortunately, no regenerative options exist, and current treatments do not restore full function. The articulating tissue of the condyle is a fibrocartilage that consists of an intricate interface between fibrous, cartilaginous, and boney tissue that is essential for normal function and that is lost in severe TMJ disorders. The objective of this study is to regenerate fibrocartilage-bone interface of the mandibular condyle in skeletally mature goats using a comprehensive tissue engineering approach. A condylar defect will be treated with novel multilayer scaffold implant designed to promote site-specific tissue regeneration. We have strong pilot in-vivo data showing that our scaffolds components regenerate fibrous and cartilage tissue in our novel goat model, and bone in a segmental defect model. We will implant the scaffolds in a mediolateral grove-shaped condylar defect. We hypothesize that a multilayer scaffold will allow for site- specific fibrous-cartilage-bone regeneration of the mandibular condyle cartilage when compared to a homogenous sponge scaffold and untreated control defects. First, we will study the properties of a multilayer scaffold design in-vitro. We will characterize the permeability and release of TGFβs from the scaffold. Second, we will assess the functional healing of condylar defects treated with the multilayer scaffolds. We will assess mechanical properties, regenerate tissue composition, and condylar architecture formation using terminal assays at 1-, 3-, and 6-months post-surgery. Third, we will study the regeneration potential of three cell subpopulations found on the condyle. Successful completion of this proposal is the critical step to provide a regenerative therapy to treat TMJ mandibular cartilage degeneration and is a basis for successful osteochondral tissue regeneration in other sites.
Liver Research Papers Published
McGowan Institute for Regenerative Medicine affiliated faculty members who are also members of the Pittsburgh Liver Research Center (PLRC) recently published several articles. The authors include:
- Andrew Duncan, PhD, Associate Professor in the Department of Pathology at the University of Pittsburgh with a secondary appointment in the Department of Bioengineering of the Swanson School of Engineering at Pitt
- Satdarshan Paul Monga, MD, Endowed Research Chair in Experimental Pathology and Vice-Chair of Experimental Pathology, Department of Pathology at the University of Pittsburgh, Professor of Pathology (Division of Experimental Pathology), Professor of Medicine (Division of Gastroenterology, Hepatology & Nutrition), and Founding Director of the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded PLRC
- Michael Oertel, PhD, Assistant Professor of Pathology, Division of Experimental Pathology, Department of Pathology
The papers include:
- Scaffolding protein IQGAP1 is dispensable but its overexpression promotes hepatocellular carcinoma via YAP1 signaling. Delgado ER, Erickson HL, Tao J, Monga SP, Duncan AW, Anakk S. Molecular and Cellular Biology, 2021 Feb 1;MCB.00596-20.
- Nrf2 and β-catenin coactivation in hepatocellular cancer: biological and therapeutic implications. Tao J, Krutsenko Y, Moghe A, Singh S, Poddar M, Bell A, Oertel M, Singhi AD, Geller D, Chen X, Lujambio A, Liu S, Monga SP. Hepatology, 2021 Feb 2.
- A Fbxo48 inhibitor prevents pAMPKα degradation and ameliorates insulin resistance. Liu Y, Jurczak MJ, Lear TB, Lin B, Larsen MB, Kennerdell JR, Chen Y, Huckestein BR, Nguyen MK, Tuncer F, Jiang Y, Monga SP, O’Donnell CP, Finkel T, Chen BB, Mallampalli RK. Nature Chemical Biology, 2021 Jan 25.
Illustration: Healthy liver. NIH/NIDDK.
Dr. Heather Szabo-Rogers Receives Grant from American Association for Anatomy

Academics, researchers, and scientists are being recognized for their significant contributions to the anatomical sciences and the future of anatomy education and research by the American Association for Anatomy (AAA), the U.S.-based international society representing 2,300 members in anatomy and anatomy-related disciplines. McGowan Institute for Regenerative Medicine affiliated faculty member Heather Szabo-Rogers, PhD, Assistant Professor in the Department of Oral Biology, Center for Craniofacial Regeneration, School of Dental Medicine, University of Pittsburgh, recently was awarded funding through the AAA Fellows Grant Award Program (FGAP).
Dr. Szabo-Rogers’ project is Prickle1 protein-protein interactions are required for craniofacial chondrocyte signaling and polarity. The FGAP award will expand an NIH R03 application to a competitive R01. The new application will focus on the development of the nasal capsule cartilages, in addition to determining the role of Prickle1 in primary ciliopathies and Robinow Syndrome (RS). FGAP funding will support hiring a trainee to analyze existing cutting-edge images and generate data from RS-patient cells and a mouse model. Once collected, the new data generated with FGAP funding will strengthen Dr. Szabo-Rogers’ “strong preliminary data.”
Through these awards, grants, and scholarships, the AAA continues to elevate, celebrate, and advance—even in a pandemic—the foundational science of anatomy and its application in healthcare, allied health, and beyond.
Congratulations, Dr. Szabo-Rogers!
Pitt-McGowan Institute Center for Preclinical Studies Supports Total Artificial Heart Study

Carmat, a developer of an artificial heart which offers a bridge to transplant in patients with end-stage biventricular heart failure, recently reported its total artificial heart (TAH) received the European CE mark. The artificial heart provides an alternative for individuals for whom maximal medical therapy and left ventricular assist device are insufficient or contraindicated. It is designed for patients expected to receive a heart transplant within 180 days.
Carmat anticipates launching its Early Feasibility Study (EFS) for their Investigational Device Exemption (IDE) in the U.S. shortly as they enroll 10 patients by the end of 2021.
The University of Pittsburgh McGowan Institute for Regenerative Medicine Center for Preclinical Studies (MIRM-CPCS) is contributing in the preparations for the feasibility study.
The MIRM-CPCS is serving as the U.S. training center for surgeons supporting the FDA-approved clinical trial to be conducted at Carmat U.S. trial sites. As required, surgeons (and their supporting teams) must establish competencies in the use of the Carmat TAH prior to the implantation, operation and management of the TAH device system in a human patient.
In February and November 2020, MIRM-CPCS hosted clinical personnel from the 5 U.S. trial sites providing the platform by which surgeons were familiarized and qualified in the implant and operational use of the TAH. McGowan Institute affiliated faculty member Peter Wearden, MD, PhD, Director Nemours Cardiac Center—Florida, Chair of the Department of Cardiovascular Surgery, and Chief of Cardiothoracic Surgery at the Nemours Children’s Hospital, Orlando, Florida, is Carmat’s U.S. proctoring/instructor surgeon.
The technology underlying the company’s total heart arose from the combination of the expertise of cardiac surgeon Alain Carpentier, MD, PhD, who pioneered mitral valve repair and invented the Carpentier-Edwards heart valves, and the technological capabilities of the aerospace company Matra Défense (Airbus Group).
Carmat’s electro-hydraulic system mimics the action of a human heart, restoring normal blood circulation throughout the body.
The system includes an implantable component shaped like a human heart. The bioprosthetic heart includes four biological valves that keep blood pulsing through the unit in one direction, two ventricular cavities separated by a membrane, a motor pump unit, and integrated electronics and sensors that enable the unit to respond to the patient’s physiological needs. One ventricular cavity contains blood, and the other holds actuation fluid. An outer bag also contains actuation liquid.
Portable external equipment includes a controller and lithium-ion batteries that provide four hours of mobility. A hospital-based console gives physicians control of the heart prosthesis during implantation and enables monitoring of the device.
Heart failure affects at least 26 million people worldwide, about 5% of whom have end-stage disease that does not respond to current medical treatments. Fewer than half of individuals diagnosed with heart failure survive five years. While heart transplantation is the gold standard treatment for biventricular end-stage heat failure, an acute lack of donors limits transplants to about 5,500 per year.
Illustration: Carmat.
Pitt Researchers Solve Mystery of Metabolic Dysfunction in Psychiatric Patients

Why do patients who receive antipsychotic medications to manage schizophrenia and bipolar disorder quicky gain weight and develop prediabetes and hyperinsulemia? The question remained a mystery for decades, but in a paper published in Translational Psychiatry, researchers from the University of Pittsburgh School of Medicine finally cracked the enigma. Zachary Freyberg, MD, PhD, is senior author on the study and is a McGowan Institute for Regenerative Medicine affiliated faculty member.
Antipsychotic drugs, scientists showed, not only block dopamine signaling in the brain but also in the pancreas, leading to uncontrolled production of blood glucose-regulating hormones and, eventually, obesity and diabetes.
“There are dopamine theories of schizophrenia, drug addiction, depression and neurodegenerative disorders, and we are presenting a dopamine theory of metabolism,” said lead author Despoina Aslanoglou, PhD, a postdoctoral fellow at Pitt’s Department of Psychiatry. “We’re seeing now that it is not only interesting to study dopamine in the brain, but it is equally interesting and important to study it in the periphery.”
Dopamine is a neurotransmitter that acts as a chemical messenger between neurons and is commonly known to play a role in pleasure, motivation, and learning. And antipsychotic medications—such as clozapine, olanzapine, and haloperidol—relieve hallucinations and delirium by blocking a subtype of dopaminergic receptors in the brain called D2-like receptors and preventing dopamine molecules from causing neurological effects.
But, as Dr. Aslanoglou and Dr. Freyberg, assistant professor of psychiatry and cell biology at Pitt, found, it’s not so simple.
“We still don’t really understand how dopamine signals biologically,” said Dr. Freyberg. “Even decades after dopamine receptors have been discovered and cloned, we still deploy this ’magical thinking’ approach: something happens that’s good enough. We use drugs that work on dopamine receptors, but how they intersect with this ’magical system’ is even less understood.”
The human pancreas contains miniature structures called pancreatic islets, which are made up of alpha and beta cells whose function is to produce and secrete hormones that regulate blood glucose. Alpha cells produce glucagon to raise blood glucose, and beta cells produce insulin to lower blood glucose back to normal.
If even one player in the glucose-regulating machinery breaks, our bodies begin to suffer. Low blood glucose makes us feel dizzy and faint, while high blood glucose—when sustained for a long time—causes diabetes and other complications in the cardiovascular system.
And, as it turns out, dopamine can tip the scales.
Dr. Freyberg’s team found that both pancreatic alpha and beta cells can make their own dopamine, confirming that its effects aren’t limited to the brain. What’s more, while beta cells primarily rely on the uptake of the dopamine precursor L-DOPA, alpha cells can make L-DOPA from scratch and ramp up its production in response to glucose. This raises the possibility that alpha cells can use dopamine to not only signal at their own receptors, but also supply it to beta cells, where it acts on D2-like receptors and inhibits secretion of glucose-lowering insulin.
And unexpectedly, the researchers discovered that pancreatic dopamine also can act on receptors designed to recognize other molecules, such as “fight-or-flight” messengers adrenaline and noradrenaline.
At a low concentration, scientists showed, dopamine primarily binds to inhibitory D2-like dopamine receptors and blocks insulin or glucagon release. At high concentrations, however, dopamine also can bind to beta-adrenergic receptors and become stimulatory, pushing hyperglycemic effects of glucagon release in alpha cells while at the same time inhibiting insulin release in beta cells through inhibitory alpha-adrenergic receptors.
Together, these findings finally explain how psychiatric patients develop metabolic syndrome after getting treatment. Blocking inhibitory dopamine receptors with antipsychotics causes a vicious circle—the brake comes off and insulin and glucagon release become unchecked, quickly desensitizing the body and further propagating hyperinsulimia, hyperglycemia and, eventually, obesity and diabetes.
“When you identify something so important, you have to make sure you find an application for it and improve people’s lives,” said Dr. Aslanoglou. “Our discovery can inform us of how to better formulate drugs to target dopamine signaling. This might be a novel pathway to therapeutics in both psychiatry and metabolism.”
Illustration: University of Pittsburgh.
AWARDS AND RECOGNITION
Dr. MaCalus Hogan Joins Orthopaedic Foot & Ankle Foundation Board of Directors

Foot and ankle orthopaedic surgeon, UPMC Vice Chair and Residency Program Director, and McGowan Institute for Regenerative Medicine affiliated faculty member MaCalus Hogan, MD, MBA, has been installed as a member-at-large for the Orthopaedic Foot & Ankle Foundation Board of Directors. The Foundation is the philanthropic arm of the American Orthopaedic Foot & Ankle Society (AOFAS)® that advances the Society’s mission by funding and promoting education, research, and humanitarian endeavors.
“With the evolving dynamics of healthcare, it is an honor to contribute to the mission of the Orthopaedic Foot & Ankle Foundation Board of Directors,” said Dr. Hogan. “I am looking forward to being a part of the Board’s efforts and progress in the days ahead.”
Dr. Hogan earned his medical degree from Howard University College of Medicine in Washington, D.C., and his Master of Business Administration degree from University of Pittsburgh Joseph M. Katz School of Business. He completed his orthopaedic surgery residency at the University of Virginia Medical School in Charlottesville, and a fellowship in foot and ankle surgery at Hospital for Special Surgery in New York.
Dr. Hogan began his involvement with the AOFAS in 2010 as a participant in the AOFAS Resident Scholarship Program. In 2017, he received funding through the AOFAS Research Grants Program for his project entitled, “The Role of the Syndesmosis in Ankle Joint and Tibiofibular Kinematics.” He is a two-time recipient of the prestigious AOFAS J. Leonard Goldner Award for best basic science research, earning the honor in 2013 and 2020.
Dr. Ted Sakai to Join Editorial Board

McGowan Institute for Regenerative Medicine affiliated faculty member Tetsuro “Ted” Sakai, MD, PhD, MHA, FASA, has been invited to serve as an Associate Editor of Clinical Transplantation: The Journal of Clinical and Translational Research. Clinical Transplantation is the official journal of the American Society of Transplantation and the American Society of Transplant Surgeons and will become the official journal of the Society for Advancement of Transplant Anesthesia. The journal is considered essential reading for researchers and clinicians in the multidisciplinary field of transplantation and targets readers who manage patients needing or having organ or tissue transplants, including those of the kidney, intestine, liver, pancreas, islets, heart, heart valves, lung, bone marrow, cornea, skin, bone, and cartilage.
Dr. Sakai is Professor of the Department of Anesthesiology and Perioperative Medicine and Professor of the Clinical Translational Science Institute, University of Pittsburgh School of Medicine. He is Vice Chair for Faculty Promotion and Development and Associate Program Director of the Anesthesiology Residency Program in the Department of Anesthesiology and Perioperative Medicine. He is also a Staff Anesthesiologist (Hepatic and Intestinal Transplantation Anesthesia) in the UPMC Department of Anesthesiology and Perioperative Medicine. His research interests include: Education in anesthesiology (resident research education); liver transplantation anesthesiology; and quality improvement in anesthesiology.
Congratulations, Dr. Sakai!
Genprex Strengthens Scientific Advisory Board with Appointment of Dr. George Gittes

Genprex, Inc., a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, announced the addition of George Gittes, MD to its Scientific Advisory Board (SAB). Dr. Gittes is the inventor of the Company’s licensed diabetes gene therapy technology that is currently in development and serves as the Chief of Pediatric Surgery and Surgeon-in-Chief Emeritus at the UPMC Children’s Hospital of Pittsburgh.
In February 2020, Genprex signed an exclusive license agreement with the University of Pittsburgh for Dr. Gittes’ diabetes gene therapy technology. Type 1 and Type 2 diabetes together affect approximately 30.3 million people in the U.S., or about 9 percent of the U.S. population. By replacing or repairing damaged or missing genes, gene therapy offers a potential cure for patients suffering from diabetes, as opposed to merely treating symptoms.
As a member of Genprex’s SAB, Dr. Gittes will leverage his highly impressive expertise to provide strategic support and drive strategy for the Company’s diabetes clinical development program.
“We are delighted to have Dr. Gittes join our Scientific Advisory Board as Genprex continues to advance its diabetes program,” said Rodney Varner, Genprex’s President and Chief Executive Officer. “It is imperative that we rely on the guidance and direction of esteemed individuals with a wealth of expertise in research and gene therapy, and I expect that Genprex will benefit greatly from Dr. Gittes’ experience. He will play an instrumental role in our efforts to bring new hope to patients who suffer from the devastating complications of this chronic illness.”
In addition to his role as Chief of Pediatric Surgery and Surgeon-in-Chief Emeritus at UPMC Children’s Hospital of Pittsburgh, one of the busiest pediatric surgical programs in the U.S., he was appointed Director of the Richard King Mellon Foundation Institute for Pediatric Research and Co-Scientific Director at UPMC Children’s Hospital in 2018. Prior to UPMC, Dr. Gittes served as the Director of Surgical Research at Children’s Mercy Hospital in Kansas City and held the Thomas M. Holder and Keith W. Ashcraft Chair in Pediatric Surgical Research. During his time in Kansas City, he also was elected to the position of President of the Society of University of Surgeons.
He is an active member of numerous professional and scientific society memberships, including the American Society of Clinical Investigators, the American Surgical Association, the American Diabetes Association, and the Association of American Physicians. His research has been published in several peer-reviewed scientific publications, and he is the recipient of several research grants, including a recent $2.59 million grant awarded by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases to assist his ongoing preclinical research for important proof-of-principle non-human primate studies of his diabetes gene therapy.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#218 –– Mr. Stephen Winowich discusses his pioneering work at Procirca and the Artificial Heart Program at UPMC.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Evan R Delgado, Hanna L Erickson, Junyan Tao, Satdarshan P Monga, Andrew W Duncan, Sayeepriyadarshini Anakk
Title: Scaffolding Protein IQGAP1 is Dispensable But Its Overexpression Promotes Hepatocellular Carcinoma via YAP1 Signaling
Summary: IQ motif-containing GTPase-activating protein 1 (IQGAP1) is a ubiquitously expressed scaffolding protein that is overexpressed in a number of cancers, including liver cancer, and is associated with pro-tumorigenic processes such as cell proliferation, motility, and adhesion. IQGAP1 can integrate multiple signaling pathways and could be an effective anti-tumor target. Therefore, we examined the role for IQGAP1 in tumor initiation and promotion during liver carcinogenesis. We found that ectopic overexpression of IQGAP1 in the liver is not sufficient to initiate tumorigenesis. Moreover, the tumor burden and cell proliferation in the DEN-induced liver carcinogenesis model in Iqgap1-/- mice maybe driven by MET signaling. In contrast, IQGAP1 overexpression enhanced YAP activation and subsequent NUAK2 expression to accelerate and promote hepatocellular carcinoma (HCC) in a clinically relevant model expressing activated (S45Y) β-catenin and MET. Here, increasing IQGAP1 expression in vivo does not alter β-catenin or MET activation; instead, it promotes YAP activity. Overall, we demonstrate that although IQGAP1 expression is not required for HCC development, the gain of IQGAP1 function promotes the rapid onset and increased liver carcinogenesis. Our results show that an adequate amount of IQGAP1 scaffold is necessary to maintain the quiescent status of the liver.
Source: Molecular and Cellular Biology. 2021 Feb 1;MCB.00596-20. Online ahead of print.
GRANT OF THE MONTH
PI: George Michalopoulos
Title: Inhibition of EGF Receptor Prevents and Reverses Non-Alcoholic Fatty Liver Disease
Description: In the last two decades, there has been an increased appreciation of a long-term threat, that of non-alcoholic fatty liver disease (NAFLD), evolving into non-alcoholic steatohepatitis (NASH), which can further advance into cirrhosis and hepatocellular carcinoma (HCC). Treatment of NAFLD and NASH is primarily through attempts at nutritional modification, often unsuccessful due to poor compliance and socioeconomic factors. There is currently no accepted single-agent pharmacologic treatment for NAFLD. The current proposal is based on findings from our work on liver regeneration, in which we discovered a unique role for EGFR for control of steatosis in replicating hepatocytes. We have now extended these studies in mice fed a high fat diet supplemented with fructose in the drinking water (“Fast Food Diet”, FFD). We found that in mice on FFD, concurrent EGFR inhibition completely prevented and eliminated any fat deposition in hepatocytes. Furthermore, EGFR inhibition reversed severe hepatocyte steatosis established after FFD feeding for 4 months. Detail analysis of the mechanisms revealed widespread effects of EGFR inhibition on multiple transcription factors related to lipid metabolism and subsequent consequences to specific enzymes associated with lipid biosynthesis and degradation. No such findings occur when signaling from the other of the two hepatocyte-mitogenic receptor tyrosine kinases, MET (the HGF receptor), was eliminated. The purpose of this proposal is to explore translational applications of this finding. This will be done by exploring effects of EGFR inhibitors established in human pharmacology. In addition, we will conduct parallel analysis between EGFR and MET signaling inhibition on their effects of NAFLD, aiming to uncover specific pathways unique to EGFR that may reveal new pharmacologic approaches more focused than the inhibition of the entire EGFR signaling with its potentially adverse complications. In parallel studies, we will also use available human NAFLD/NASH tissue material available in the Biorepository of the Pittsburgh Liver Research Center (PLRC). This material will be used under the established rules of IRB obtained by PLRC for such studies. We will explore activation of EGFR dependent pathways and will correlate with the histology of NAFLD/NASH. A serious complication of progression of NAFLD to NASH is development of fibrosis. We have uncovered EGFR-controlled signaling molecules in steatotic hepatocytes, which have been associated with activation of hepatic stellate cells and enhanced production of collagens. These also offer opportunities for selective pharmacology for fibrosis and their relevance will be assessed in the studies proposed.
Source: National Institute of Diabetes and Digestive and Kidney Diseases
Term: 2/3/21 – 01/31/25
Amount: $473,996 (one year)
