December 2019 | VOL. 18, NO. 12| www.McGowan.pitt.edu
Two Steps Forward, One Step Back: Dr. Stephen Badylak at TEDx Fairfield University

With its theme of Inspiration and Innovation, Fairfield University, Fairfield, Connecticut, hosted a recent TEDx on October 28, 2019.
McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, was one of the nine speakers for this event.
During his talk, Dr. Badylak spoke of efforts in regenerative medicine in early 1993 to today. He explained that research from 1993 paved with way for experiments and theories today, and today’s milestones will continue to pave the way for future efforts. He spoke of —
- Regenerative medicine concepts in 1993 believed that it was possible to generate functional tissues outside of the body and with them replace virtually any body part. That has not happened yet, but much was learned during this period.
- In 1996 there was an explosion of knowledge in stem cell biology. Through this knowledge, it was thought this was THE answer. Although today there are over 2,800 active, registered clinical stem cell trials, there are no approved surgical uses for stem cells.
- In his research, Dr. Badylak and his team have learned that the extracellular matrix (ECM) is the ideal microenvironmental niche for stem cell production. With this knowledge, one specific treatment for esophageal cancer is being tested. Fourteen patients have undergone the replacement of their abnormal esophagus lining cells with ECM. The ECM promotes the regeneration of a new, normal esophagus lining and these patients are now living with the positive results of this treatment.
Every one of the above examples has advanced the field of regenerative medicine. In closing, Dr. Badylak leaves viewers with one last thought: The field of regenerative medicine is not about living to be 120 years old. Regenerative medicine is focused on a better quality of life vs. a quantity of life.
Dr. Badylak is a professor in the Department of Surgery at the University of Pittsburgh. He has practiced both veterinary and human medicine and holds more than 65 U.S. patents and 300 patents worldwide. He has authored more than 380 scientific publications and 55 book chapters. He has served as chair of several study sections at the National Institutes of Health (NIH), and is currently a member of the College of Scientific Reviewers for NIH. Dr. Badylak has either chaired or been a member of the Scientific Advisory Board to several major medical device companies, and presently serves as chief scientific officer for ECM-Therapeutics in addition to his professorial role at the University. More than ten million patients have been treated with bioscaffolds developed in Dr. Badylak’s laboratory.
In the spirit of ideas worth spreading, TEDx is a program of local, self-organized events that bring people together to share a TED-like experience. These local, self-organized events are branded TEDx, where x = an independently organized TED event.
Illustration: TEDx Fairfield University
In Memoriam: James Funderburgh, PhD
 It is with sadness that the McGowan Institute for Regenerative Medicine announces the passing of affiliated faculty member James Funderburgh, PhD.
It is with sadness that the McGowan Institute for Regenerative Medicine announces the passing of affiliated faculty member James Funderburgh, PhD.
Dr. James Funderburgh served as a Professor of Ophthalmology at the University of Pittsburgh and the Associate Director of the Louis J. Fox Center for Vision Restoration. He was also the Director of the Gene Expression Core Module and a Member of the Graduate Faculty in Biochemistry and Molecular Genetics. In addition, he held a secondary appointment as a Professor of Cell Biology and Physiology.
Dr. Funderburgh earned his undergraduate degree in Chemistry at the University of Texas, Austin. He then got his MS in Biological Chemistry from the University of Minnesota and his PhD in Physiological Chemistry from the University of Wisconsin. He did postdoctoral work at the University of Geneva, Department of Pathology in Switzerland, and was a senior fellow in the Department of Ophthalmology, University of Washington. Prior to coming to Pittsburgh, he was a Research Professor at Kansas State University.
Dr. Funderburgh’s research interests included:
- extracellular matrix biochemistry
- proteoglycans
- tissue engineering
- corneal cell biology
- wound healing
- stem cell biology
He was a member of the American Society for Biochemistry and Molecular Biology, the Association for Research in Vision and Ophthalmology, and the American Society for Cell Biology. During his career he received the honor of becoming a Fellow (Gold Level) of the Association for Research in Vision in Ophthalmology. Dr. Funderburgh was on the Editorial Review Board of Molecular Vision.
Dr. Funderburgh is survived by his wife, Martha; sister, Susan Jarratt of Irvine, CA; brother, John Funderburgh of Dallas; three children – Dan Funderburgh of Brooklyn (Jenny), Anna Funderburgh (Mikael), St. Paul, and Eva Funderburgh Hollis (Ben) of Seattle; and four grandchildren, Jonas, Sebastian, Niko, and Alice.
The family is planning a service in early 2020 to celebrate Dr. Funderburgh’s life. Donations may be made to Allegheny Land Trust, Eye and Ear Foundation of Pittsburgh, or to your favorite musical organization. Condolences may be left at www.mccabebrothers.com.
RESOURCES AT THE MCGOWAN INSTITUTE
January Histology Special
The McGowan Histology Core Laboratory is open to all university of Pittsburgh and outside the university, researchers. At the Histology laboratory you will find a friendly and knowledgeable staff. The Histology lab offers routine staining, special staining, and antibody work-up services. Lori Walton has worked for the University and UPMC, in histology for over 20 years, and is available to troubleshoot, or help to work up special circumstance staining for your individual needs. The lab utilizes many automated staining systems including an immuno-stainer, for uniform reproducible results.
Trichrome Stains are intended for use in the study of connective tissue, muscle and collagen fibers. Trichrome stains are used primarily for distinguishing collagen from muscle tissue.
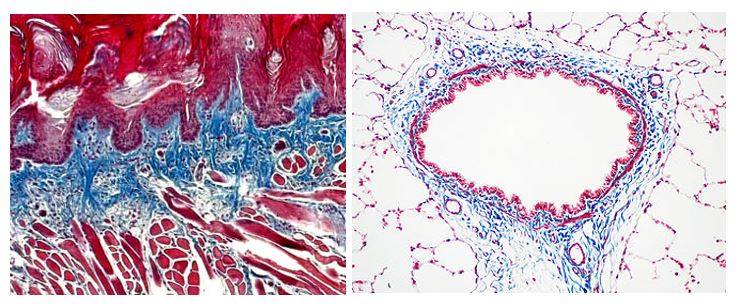
The McGowan Institute Histology Core Laboratory offers Masson Trichromes, automated staining results in one week or less, beautifully reproducible.
Through the whole month of January, you will receive 25% off your trichrome stains, when you mention this ad. Contact Lori at the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology work with the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Save the Dates!
Ophthalmology Networking Session
 The McGowan Institute and the University of Pittsburgh Department of Ophthalmology is organizing a Faculty Networking Session on Tuesday, February 18, 2020. This even will be held in Alumni Hall Connolly Ballroom, 4227 Fifth Avenue, Pittsburgh. There will be mini-presentations by scientific and clinical faculty. Areas of consideration include devices, tissue engineering, cell therapy, biologics, and gene therapy. Do not miss this opportunity to share your needs and ideas or to introduce your lab’s capabilities. Registration is now open.
The McGowan Institute and the University of Pittsburgh Department of Ophthalmology is organizing a Faculty Networking Session on Tuesday, February 18, 2020. This even will be held in Alumni Hall Connolly Ballroom, 4227 Fifth Avenue, Pittsburgh. There will be mini-presentations by scientific and clinical faculty. Areas of consideration include devices, tissue engineering, cell therapy, biologics, and gene therapy. Do not miss this opportunity to share your needs and ideas or to introduce your lab’s capabilities. Registration is now open.
To register, please click here.
McGowan Institute Annual Retreat
 Registration is now open for the 2020 McGowan Institute Scientific Retreat. The retreat will be held March 9-10, 2020 at Oglebay Resort in Wheeling, WV.
Registration is now open for the 2020 McGowan Institute Scientific Retreat. The retreat will be held March 9-10, 2020 at Oglebay Resort in Wheeling, WV.
Program highlights include:
- Regenerative Medicine Technology Pitch Competition
- Team Formation to Address Grand Challenges in Regenerative Medicine
- Screening of Burden of Genius
- Fireside Chat with Local Entrepreneurs
- Commercialization Office Hours
- Invention Disclosure Sprints
- Emerging Themes in Regenerative Medicine
- Poster Teasers and Poster Presentations
The program committee under the leadership of Julie Phillippi, PhD is developing an exciting program. For more information and to register, please click here.
SCIENTIFIC ADVANCES
Dr. Dennis McNamara to Chair Clinical Steering Committee for Trial to Treat Acute Myocarditis

Cardiol Therapeutics Inc., a leader in the production of pharmaceutical cannabidiol (CBD) products and in the development of innovative cannabidiol medicines for heart disease, announced the formation of the Clinical Steering Committee (CSC) for a Phase 2 international trial in acute myocarditis using its CardiolRx™100 cannabidiol formulation.
McGowan Institute for Regenerative Medicine affiliated faculty member Dennis McNamara, MD, Professor of Medicine at the University of Pittsburgh and the Director of the Heart Failure/Transplantation Program at the University of Pittsburgh Medical Center, is the chair of Cardiol’s Acute Myocarditis CSC.
The role of the CSC is to advise on the trial design, provide overall supervision of the trial, and ensure that it is being conducted in accordance with the principles of Good Clinical Practice. The CSC has oversight of the protocol, any protocol amendments, and provides advice to the investigators on all aspects of the trial.
Acute myocarditis is characterized by inflammation of the heart muscle (myocardium). The most common cause is viral infection of the heart tissue which is initially responsible for the inflammation. In a significant number of cases, perhaps due to an autoimmune process, the inflammation persists with ongoing myocardial damage and depressed heart function. Although the symptoms are often mild, myocarditis remains an important cause of acute and fulminant heart failure and is the most common cause of sudden cardiac death in people less than 35 years old.
CardiolRx™100 is Cardiol Therapeutics’ pure pharmaceutically (cGMP) produced high concentration cannabidiol formulation that is THC free (<10ppm). Based on the large body of experimental evidence of the anti-inflammatory and cardioprotective properties of cannabidiol in models of cardiovascular disease, Cardiol believes there is an opportunity to develop a potential breakthrough therapy for acute myocarditis that would be eligible for designation as an orphan drug. In the United States, an orphan drug designation is granted for pharmaceuticals being developed to treat medical conditions affecting fewer than 200,000 people. These conditions are referred to as orphan diseases.
Pancreatic Cancer: Not All Doom and Gloom

Pancreatic cancer happens when cells that aren’t normal grow and start to form tumors in the pancreas, a small organ located deep in the belly, behind your stomach. The pancreas makes juices that help your body digest food. It also makes insulin and other hormones that help control your blood sugar.
Experts don’t know what causes pancreatic cancer. But they do know that changes in the body’s DNA play a role in many cancers.
There are two main types of pancreatic tumors: exocrine and endocrine. The type of tumor depends on which type of cells are involved. Exocrine cells make digestive juices. Endocrine cells make insulin. Most people with pancreatic cancer have exocrine tumors, which grow faster than endocrine tumors.
Treatments are more successful when cancer is found early. But in most cases, pancreatic cancer has already spread by the time it is found.
Still, it’s not all doom and gloom, says McGowan Institute for Regenerative Medicine affiliated faculty member Nathan Bahary, MD, PhD, to Denise Mann, MS, from MSN. “Survival is now 9 percent, up from 4 percent. That’s progress.”
Cancer specialists are experimenting with chemotherapy combinations and treating the cancer before surgery, says Dr. Bahary, associate professor in the Department of Medicine, Division of Oncology at the University of Pittsburgh. He also holds a secondary appointment as an Associate Professor in Microbiology and Molecular Genetics. “In the next five years, I hope we will double the survival rate, and in 10 to 20 years, I hope we find a way to detect it early and find better ways to treat spreading disease,” he says.
And the Beep Goes On: CMU/UPMC Collaborative Effort Unravels Medical Mysteries

Artur Dubrawski, PhD, is not a critical care physician, but his best friend is. Dr. Dubrawski, a research professor in Carnegie Mellon University’s Robotics Institute, loves talking about disease symptoms with McGowan Institute for Regenerative Medicine affiliated faculty member Michael Pinsky, MD, a professor of critical care medicine, cardiovascular disease, bioengineering and more at the University of Pittsburgh Medical Center. They also love talking about data and, more important, what the data means.
The pair have been collaborating for a decade, combining the power of CMU artificial intelligence research with the clinical expertise at UPMC to unravel medical mysteries. Their skilled clinician investigator team, including University of Pittsburgh professors Gilles Clermont, MD (also a McGowan Institute affiliated faculty member) and Marilyn Hravnak, RN, PhD, have been leading the way for bedside predictive analytics. In 2014, they began to identify and predict which patients would go into shock. They hung around in intensive care units, building algorithms to make sense from the data contained in thousands of patients’ medical records.
Spending so much time in ICUs, they couldn’t help but notice the “acoustic wallpaper” of constant beeps from machines trying to alert staff to a problem.
“Very often,” Dr. Dubrawski said, “those alerts, even in the best clinical hospitals, do not really reflect a medically relevant change in health status.” In other words, 85% of the time, nurses are listening to false alarms.
Whether there is a real emergency, an oxygen sensor that slipped from someone’s finger or a tangled ECG electrode, the machines not only signal into the semi consciousness of beep-fatigued employees, but they are not collecting data until the problem is rectified. Drs. Pinsky and Dubrawski analyzed the alerts and categorized the urgency of response, which led to them designing a protocol to help save lives.
Along the way, they found something else unexpected: they could predict tachycardia.
A cardiac condition as dangerous as it is difficult to spell, tachycardia is defined as a rapid heartbeat. Left unchecked, it can lead to any number of heart conditions for a “regular” patient. Dr. Dubrawski, in collaboration with CMU colleague Lujie Chen, PhD student, became interested specifically in tachycardia for ICU patients, where a rapid heartbeat leads to longer hospital stays, and can be the precursor to heart failure, coronary artery disease or even death.
Drs. Dubrawski and Pinsky paired up again to see if they could build another algorithm to predict which ICU patients might experience tachycardia and whether they could adapt nursing protocols to better help these patients.
They studied patient data collected over 25,000 ICU visits from a nationwide database, and together with their partners at UPMC, identified 42 vital sign features to use when developing their predictive algorithms. They backed up in the data from the time of the tachycardia events in one-minute intervals, to see if there’s a “magic window” when this condition could be identified and prevented.
They looked at everything from blood pressure to the use of norepinephrine to see what combination of factors might lead to rapid heartbeat.
“Some of these patients are at risk hours before the event,” Dr. Dubrawski said. “And some of these signs are likely to be missed by nurses.”
While patients might not demonstrate visible symptoms of an impending, massive crisis, the researchers found, with reasonable accuracy, some patterns in the data do predict its emergence. Drs. Pinsky and Dubrawski created algorithms that allowed a computer to “learn” the signals that an injured patient’s cardio-respiratory system is deteriorating before the damage becomes irreversible. They also created a mechanism to tell a robotic system to administer the best treatments and therapies to save that person’s life.
And the friends are just getting started. Their bedside data exploration also led to discoveries around cardio-respiratory insufficiency, a grave condition Dr. Dubrawski says is more general—meaning their work in this area can be more impactful. After tackling acute crises in the ICU, they’re exploring internal bleeding—another common and dangerous occurrence in intensive care. Dr. Pinsky said the team recently discovered this new secret hiding within the data recorded from commonly used intravenous catheters placed in the superficial vein to draw blood, deliver fluids or medications.
While it’s in there, the catheter records central venous pressure (CVP), which Dr. Pinsky said is “useless in defining the onset of bleeding.” But when the data was compared to the CVP data collected when the patient was stable, the researchers were able to identify bleeding as well as the most advanced monitoring devices. “That finding is simply amazing for all of medicine,” Dr. Pinsky said, “because it opens up a window into instability without the need for more invasive monitoring.”
Dr. Dubrawski explained that routine clinical care and simple data review by a human could hardly identify all the patterns and symptoms that something urgent will happen medically. The data is simply too complex, and it comes at the clinicians too rapidly. Artificial intelligence and machine learning can handle these complexities and paint a picture much more reliable.
“These tools are within our grasp,” Dr. Dubrawski said, “and we are limited only by our imagination.”
BioHybrid Solutions Awarded $30M Contract by the US Dept. of Defense

McGowan Institute for Regenerative Medicine affiliated faculty member Krzysztof Matyjaszewski, PhD, is the Co-Founder and CSO of BioHybrid Solutions, LLC (BHS), a Pittsburgh-based protein engineering company, which recently received a contract worth up to $30 million from the United States Department of Defense to develop a next-generation prophylactic medical countermeasure. The project was sponsored by the U.S. Government through the Medical CBRN Defense Consortium (MCDC). Dr. Matyjaszewski is the J.C. Warner Professor of Natural Sciences at Carnegie Mellon University.
The U.S. Defense Threat Reduction Agency (DTRA) selected BHS through a request for prototype proposal under the MCDC to apply its proprietary NanoArmored TM protein engineering technology to improve pharmacokinetics of the prophylactic while reducing the potential for immunogenic side effects. Over the five-year program, BHS will develop NanoArmored TM drug candidates, conduct studies to demonstrate functionality, scale manufacturing, and complete regulatory activities towards approval of the countermeasure by the U.S. Food and Drug Administration. BHS is leading the program in collaboration with FLIR Systems, Battelle, Ology Bioservices, the Allegheny Health Network, BTG PLC (now part of Boston Scientific) and the US Army.
In order to expedite development, BHS will utilize its specialized high-throughput discovery technology to rapidly select the NanoArmor needed and then manufacture that drug candidate.
“Our technology allows us to rationally tune protein performance through precision modification of proteins with a wide variety of synthetic polymers,” noted Dr. Matyjaszewski. “To date, we have created thousands of different NanoArmored proteins for a variety of industrial and therapeutic indications. We are well positioned to apply our technology to this important national security and public health application.”
“We have developed a unique way to protect against the toxic effects of chemical warfare agents and are honored to have been selected by DTRA to lead this very important program.” said Alan Russell, PhD, Co-Founder and CEO of BioHybrid Solutions and former director of the McGowan Institute.
NeuBase Prepares to Face the Challenges of a Rare Disease Trial

NeuBase Therapeutics, Inc. is developing the next generation of gene silencing therapies with its flexible, highly specific synthetic antisense oligonucleotides. The proprietary NeuBase peptide-nucleic acid (PNA) antisense oligonucleotide (PATrOL™) platform allows for the rapid development of targeted drugs, increasing the treatment opportunities for the hundreds of millions of people affected by rare genetic diseases, including those that can only be treated through accessing of genomic loci or secondary and tertiary RNA structures. Using PATrOL technology, NeuBase aims to first tackle rare, genetic neurological disorders.
As reported by Ed Miseta, Chief Editor, Clinical Leader, NeuBase Therapeutics is hoping to help patients overcome Huntington’s disease, myotonic dystrophy, familial Parkinson’s disease, and other rare diseases. While a small number of patients are impacted by any rare disease, collectively these diseases impact 10 percent of the population, or 700 million people across the globe. While gene defects cause many of these patients to suffer and die, only around five percent of them have an available therapy. And some of those are not effective.
“The Human Genome Project really got things rolling with the publication of the draft human genome sequence in 2001 which promised the development of new treatments for many of these patients,” says Dietrich Stephan, PhD, CEO of NeuBase. Dr. Stephan is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
The first disease NeuBase is attempting to address is Huntington’s disease, a genetic ailment inherited in a dominant way. Generally, one parent is affected, and 50 percent of their kids will inherit that gene. A child that inherits the gene will start having trouble walking, talking, and thinking when they reach middle age. The disease will eventually take their life, and there is no treatment.
“These children will often grow up not knowing if they have the gene,” says Dr. Stephan. “Sometimes they will visit an underground genetic testing facility because they don’t want the diagnosis to impact their ability to get health or life insurance. Although a federal law prevents insurance companies from denying coverage to patients with a gene-positive mutation, the diagnosis can still present insurance issues for patients. And, for a healthy 20-year-old person, this is essentially a death sentence.”
A patient diagnosed with Huntington’s disease may be able to enroll in a clinical trial to test a NeuBase treatment by the end of 2021. Between now and then, there are several challenges the company will need to overcome. The first, as is usually the case with any rare disease, is patient density and access. Most rare diseases will have a small patient population that is geographically dispersed, which means recruitment takes place globally, across designated centers of excellence specializing in the disease. Patients often need to fly into those clinics, which makes patient recruitment difficult at best.
The second layer of complexity has to do with the diagnosis. NeuBase would like to eventually run trials on patients who are not yet showing symptoms. For pre-symptomatic patients, a test will reveal if they have the gene mutation. Getting a patient on the treatment before they begin to show symptoms would allow them to be treated prior to cells and tissues being damaged irreversibly.
NeuBase has settled on the clinical end point it will pursue. The company will also measure the mutant Huntington protein levels over a period of time and determine if those levels decline.
“We are all searching for a biomarker which, if you knock down that mutant protein through therapy, will predict whether a patient will get the disease, or will get better if they already have it,” says Dr. Stephan. “In addition to standard clinical endpoints, using biomarkers is a second level of determining if the treatment is effective. For Huntington’s disease, there is a biomarker (mutant Huntington protein) that is of interest, as well as several early brain imaging ligands which we hope will evolve rapidly to show correlation with clinical progression and be accepted by regulators. Many researchers working on rare diseases could rely on biomarkers as surrogate measures for a clinical readout.”
Dr. Stephan believes cells in the brain of a Huntington’s patient will start to die a decade before the onset of symptoms. With that in mind, he would like to see a patient start on a therapy while in their 30s and 40s, to stop the progression of the disease and prevent the symptoms from manifesting. That would be his ideal therapy, and the one NeuBase is attempting to develop. Post approval, he would also like the treatment to be something patients or caregivers could purchase from a pharmacy and administer at home.
Provost Inaugural Lecture: Dr. John Kellum

In celebration of his appointment, McGowan Institute for Regenerative Medicine affiliated faculty member John Kellum, MD, Endowed Chair in Critical Care Medicine Research, presented the December 9, 2019, Provost Inaugural Lecture. His presentation was entitled “The Inconvenient Truth About Acute Kidney Injury.”
Dr. Kellum is a Professor in the Departments of Critical Care Medicine (primary), Medicine, Bioengineering, and Clinical and Translational Science at the University of Pittsburgh. He is also the Director of the Center for Critical Care Nephrology in the Department of Critical Care Medicine, and Associate Director for Acute Illness in the Institute for Personalized Medicine at Pitt. Dr. Kellum also serves as an Intensivist at UPMC.
Dr. Kellum received his MD at the Medical College of Ohio at Toledo in 1988 and then completed his residency at the University of Rochester. Dr. Kellum completed a Fellowship in Critical Care Medicine at UPMC Presbyterian Hospital.
Dr. Kellum’s research interests span various areas, including:
- Mechanisms of organ failure in sepsis and strategies to reduce organ injury
- Acute renal failure/acute kidney injury
- Hemofiltration in the treatment of multiple organ dysfunction and systemic inflammatory response
- Acid-base balance
- Shock and resuscitation
- Biomarkers
- Bioengineering
- Organ donor management
A recipient of a $3.4 million award from the National Institute of Diabetes and Digestive and Kidney Diseases, Dr. Kellum will study patients with acute kidney injury (AKI) including obtaining biopsies for interrogation, careful clinical and molecular phenotyping, and long-term follow-up. This information is important to design future treatment plans toward improved health outcomes.
How Dr. Rory Cooper Engineered a Better Life

Each month, the U.S. Patent and Trademark Office’s (USPTO’s) Journeys of Innovation series tells the stories of inventors or entrepreneurs whose groundbreaking innovations have made a positive difference in the world.
Recently, their featured inventor was McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, FISA & Paralyzed Veterans of America Professor and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh. Dr. Cooper is Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories, a VA Rehabilitation R&D Center of Excellence in partnership with Pitt. Dr. Cooper is an adjunct professor in the Robotics Institute of Carnegie Mellon University, and PM&R of the Uniformed Services University of Health Sciences, and he was awarded Honorary Professor at Hong Kong Polytechnic University and Xi’an Jiatong University where he was awarded an Honorary Doctorate.
Dr. Cooper has always enjoyed tinkering and competition. As a kid growing up in Southern California, he ran track and cross country, and he often worked in his parents’ garage, making improvements to skateboards and bicycles. He brought these skills to his work in the U.S. Army, but then an accident changed his life forever and set him on a path to become an inventor, engineer, and bronze medalist.
Dr. Cooper was interviewed by USPTO’s Linda Hosler. Ms. Hosler’s interview takes Dr. Cooper back to his childhood, to the accident, and to the results of his career path and research focus. When asked about the importance of accessibility and the role it’s played in the focus of his work, he responded:
Accessibility helps drive social inclusion. If you think about it, the wheelchair I use, the adaptive vehicle I use, the home modifications I use, they all allow me to be productive and creative and contribute to society. What’s important is to create a world where everyone belongs and everyone can contribute. Accessibility is a tool to facilitate that.
Read and/or listen to the entire interview here.
AWARDS AND RECOGNITION
Dr. William Federspiel Named a National Academy of Inventors Fellow

William Federspiel, PhD, Director of the Medical Devices Laboratory of the McGowan Institute for Regenerative Medicine and the William Kepler Whiteford Professor in the Department of Bioengineering with secondary appointments in Chemical Engineering and Critical Care Medicine at the University of Pittsburgh, has been named a Fellow of the National Academy of Inventors (NAI). Election to NAI Fellow status is the highest professional distinction accorded solely to academic inventors who have demonstrated a prolific spirit of innovation in creating or facilitating outstanding inventions that have made a tangible impact on quality of life, economic development, and the welfare of society. Academic inventors and innovators elected to the rank of NAI Fellow status have been nominated by their peers for outstanding contributions to innovation in areas such as patents and licensing, innovative discovery and technology, significant impact on society, and support and enhancement of innovation. The 2019 Fellows Gala and Induction Ceremony will take place on April 10, 2020, at The Heard Museum in Phoenix, AZ.
Dr. William Federspiel joined the faculty of the University of Pittsburgh in 1995 as an Associate Professor. He received his PhD in Chemical Engineering from the University of Rochester in 1984 and has held academic positions in biomedical engineering at Johns Hopkins University and Boston University. His past industrial experience includes being a Principal Staff Scientist at ABIOMED Inc., a Boston based artificial heart company, and a Research Scientist at the Biomechanics Institute in Boston, a nonprofit bioengineering think tank. In addition to his current academic position, Dr. Federspiel is a Founder of ALung Technologies, a Pittsburgh-based medical start-up company, for which he serves as the head of the scientific advisory board.
The major research theme of the Medical Devices Laboratory is the development of medical devices whose therapeutic function stems from biotransport and bioseparation processes and which can be translated for near-term clinical use in critical care medicine. A span of Dr. Federspiel’s research interests include:
- Design and development of novel artificial lung devices, including respiratory support catheters and paracorporeal assist lungs, for near-term clinical use in the treatment of respiratory failure in patients with acute, acute on chronic, or chronic lung insufficiencies.
- Design and development of membrane and particle-based blood purification devices for the selective or semi-selective and patterned removal of pathogenic antibodies, inflammatory mediators, and other blood borne solutes for near-term clinical use in critical care settings.
- Advancing the development of novel artificial lung platforms for future applications by combining microfabrication and fiber technology with cellular and biomolecular components to create biohybrid artificial alveolar capillary units and bioactive hollow fibers with improved gas exchange efficiency and capacity.
- Developing improved transport models and understanding of polymer degradation and drug delivery from nanoparticles and microparticles.
- Advanced application of fluid mechanics and mass transport principles to model and optimize artificial lungs and other membrane-based medical devices where functional performance depends on underlying transport or separation principles that dictate the device characteristics.
- Development of mathematical and computer simulation models related to respiratory and cardiovascular fluid mechanics and mass transport.
- Development of oxygen depletion devices for blood storage systems that will extend the shelf life of red cell units and deliver red cells of higher efficacy and lower toxicity for transfusion therapy.
Dr. Federspiel has over 100 peer reviewed journal articles or book chapters published or in press, over 100 proceedings and abstracts, and over 75 invited talks at national and international universities and meetings.
Congratulations, Dr. Federspiel!
Dr. Stephen Badylak Receives 2019 Lifetime Achievement Award from TERMIS-AM

McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor in the Department of Surgery and director of the Center for Pre-Clinical Tissue Engineering within the McGowan Institute, was honored with the 2019 Lifetime Achievement Award from Tissue Engineering and Regenerative Medicine International Society-Americas (TERMIS-AM). The TERMIS-AM Lifetime Achievement Award was established to recognize an individual who has contributed immensely to the tissue engineering and regenerative medicine field. The award is presented to an individual whose work has impacted and assisted with laying the foundation stones for the field.
Dr. Badylak directs a laboratory focused upon the use of biologic scaffolds composed of extracellular matrix (ECM) to facilitate functional tissue and organ reconstruction. Dr. Badylak is the past President of TERMIS, the author of more than 300 peer reviewed publications, and holds more than 50 issued U.S. patents and 300 patents worldwide. Dr. Badylak is the editor of the textbook “Host Response to Biomaterials” (Academic Press, 2015). The focus of Dr. Badylak’s work has been the mechanisms by which ECM signals host tissues to promote and support functional tissue reconstruction. Dr. Badylak places high emphasis upon clinical translation of all activities in the laboratory and work conducted within the laboratory spans the full spectrum from basic science at the subcellular level to patient care at the bed side.
The award was presented to Dr. Badylak in Orlando, FL, during the TERMIS-AM Annual Conference and Exhibition. In addition to his plaque, Dr. Badylak received a $2000 honorarium and $1000 towards travel expenses to the conference including a complimentary conference registration.
Congratulations, Dr. Badylak!
Illustration: John Fisher, Chair TERMIS-AM (left) presents Stephen Badylak, DVM, PhD, MD (right) with the 2019 TERMIS-AM Lifetime Achievement Award.
Dr. Morgan Fedorchak Receives 2019 Emerging Innovator Award

The Innovation Institute celebrated the University of Pittsburgh faculty, students and staff who are working to make their ideas and research discoveries have a real-world impact at the 14th Annual Celebration of Innovation, on November 20 at the Petersen Events Center Campus View Club.
During this celebration, McGowan Institute for Regenerative Medicine affiliated faculty member Morgan Fedorchak, PhD, assistant professor of ophthalmology, bioengineering, chemical and petroleum engineering, and clinical and translational science, received the 2019 Emerging Innovator Award. This honor is presented to an early- to mid-career faculty member who has shown a keen interest in innovation commercialization and is dedicated to achieving impact through commercial translation of research.
Dr. Fedorchak leads the Ophthalmic Biomaterials Laboratory. In her lab’s first four years, it has demonstrated an intense focus on translational science and has launched two projects on a commercialization pathway. She has taken advantage of a range of educational programming and funding opportunities to move her innovations from the lab towards the market. These include the First Gear commercialization program, the Michael G. Wells Competition, and Chancellor’s Innovation Commercialization Funds, as well as consulting regularly with licensing managers and entrepreneurs in residence.
Congratulations, Dr. Fedorchak!
McGowan Institute Affiliated Faculty Members Receive Swanson School Professorship and Fellowship Appointments
In recognition of outstanding productivity as a faculty member, the University of Pittsburgh Department of Bioengineering appointed two faculty professorships and one faculty fellowship in 2019. Lance Davidson, PhD, was appointed William Kepler Whiteford Professor of Bioengineering; William Federspiel, PhD, was appointed John A. Swanson Professor of Bioengineering; and Warren Ruder, PhD, was appointed William Kepler Whiteford Fellow of Bioengineering.
“In addition to conducting cutting-edge research, each of these individuals is a dedicated teacher and mentor, which is critical for developing the next generation of knowledge creators and professional practitioners,” said McGowan Institute for Regenerative Medicine faculty member Sanjeev Shroff, PhD, Distinguished Professor and Gerald E. McGinnis Chair of Bioengineering. “I am very proud to have Lance, Bill, and Warren as a part of our bioengineering family.”
Lance Davidson, PhD, William Kepler Whiteford Professor of Bioengineering
 Dr. Davidson’s MechMorpho Lab carries out interdisciplinary research at the interface of physics, engineering, mathematics, and cell biology. They study the mechanics of morphogenesis – the central process of tissue self-assembly that couples physical processes that move cells and tissues with the biological processes that give cells their identity, establish tissue architecture and physiological function.
Dr. Davidson’s MechMorpho Lab carries out interdisciplinary research at the interface of physics, engineering, mathematics, and cell biology. They study the mechanics of morphogenesis – the central process of tissue self-assembly that couples physical processes that move cells and tissues with the biological processes that give cells their identity, establish tissue architecture and physiological function.
Using these tools they have uncovered how mechanics lays out and shapes key germ layers in the embryo that give rise to the central nervous system, how mechanical cues guide cells that form the heart and the cardiovascular system, and how mechanical cues can drive regeneration of skin cells. This work is supported by the United States National Institutes of Health including the National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Together with their basic research objectives their long-term engineering objective is to turn the principles of morphogenesis into technology to advance cell and tissue engineering.
William Federspiel, PhD, John A. Swanson Professor of Bioengineering
 Dr. Federspiel directs the Medical Devices Lab in the McGowan Institute of Regenerative Medicine where researchers develop clinically significant devices for the treatment of pulmonary and cardiovascular ailments by utilizing engineering principles of fluid flow and mass transfer. In particular, Dr. Federspiel’s lab has created next-generation artificial lung devices, including portable, wearable devices for adults and children suffering from lung disease.
Dr. Federspiel directs the Medical Devices Lab in the McGowan Institute of Regenerative Medicine where researchers develop clinically significant devices for the treatment of pulmonary and cardiovascular ailments by utilizing engineering principles of fluid flow and mass transfer. In particular, Dr. Federspiel’s lab has created next-generation artificial lung devices, including portable, wearable devices for adults and children suffering from lung disease.
His research in artificial lung technology eventually led Dr. Federspiel to co-found ALung Technologies, a Pittsburgh-based medical device startup company that develops technology for treating respiratory failure. He serves as head of the scientific advisory board for the company, which is currently involved in clinical trials for their Hemolung® Respiratory Assist System (RAS), a dialysis-like alternative for or supplement to mechanical ventilation which removes carbon dioxide directly from the blood in patients with acute respiratory failure.
Warren Ruder, PhD, William Kepler Whiteford Fellow and Associate Professor of Bioengineering
 Dr. Ruder directs the Synthetic Biology and Biomimetics laboratory where his team focuses on applying synthetic biology constructs, methods, and paradigms to solve a range of biomedical problems. His group aims to both understand the fundamental biology of natural systems as well as re-engineer these systems with synthetic gene networks.
Dr. Ruder directs the Synthetic Biology and Biomimetics laboratory where his team focuses on applying synthetic biology constructs, methods, and paradigms to solve a range of biomedical problems. His group aims to both understand the fundamental biology of natural systems as well as re-engineer these systems with synthetic gene networks.
They have expertise in multiple fields including gene network engineering, cell physiology and biomechanics, microfluidics, mechanical engineering and biomaterials. They are currently developing new approaches to embed synthetic gene networks within biomimetic systems that mimic cell, tissue, and organism physiology.
Dr. Ruder recently received a prestigious $2.2 million NIH Director’s New Innovator Award to develop magnetically activated gene networks in human cells.
Dr. David Finegold Named the John C. Mascaro Faculty Fellow in Sustainability

The University of Pittsburgh’s Mascaro Center for Sustainable Innovation named eight faculty awardees for the 2020 John C. Mascaro Faculty Program in Sustainability.
One of the 2020 honorees included McGowan Institute for Regenerative Medicine affiliated faculty member David Finegold, MD, Professor, Human Genetics, and Director, Multidisciplinary Master of Public Health, who was named the John C. Mascaro Faculty Fellow in Sustainability.
Dr. Finegold has a major research focus on lymphatic vascular biology and genetic variation underlying primary and secondary lymphedema.
The one-year awards, created to enhance the University’s mission of interdisciplinary excellence in sustainability research and education, go to faculty members from all disciplines, who may apply as faculty fellows, scholars or lecturers.
Congratulations, Dr. Finegold!
Dr. Warren Ruder Attends 2019 Israeli-American Kavli Frontiers of Science Symposium

McGowan Institute for Regenerative Medicine affiliated faculty member Warren Ruder, PhD, Associate Professor and William Kepler Whiteford Fellow of Bioengineering at the University of Pittsburgh, attended the 4th Israeli-American Kavli Frontiers of Science Symposium in Jerusalem, Israel, on September 16-18, 2019. The program’s participants are selected from recipients of prestigious fellowships, awards, and other honors, as well as from nominations by members of the National Academy of Sciences and other participants.
Dr. Ruder directs the Synthetic Biology and Biomimetics laboratory in the Swanson School of Engineering where his team focuses on applying synthetic biology constructs, methods, and paradigms to solve a range of biomedical problems.
“Our lab’s goal is to both understand the fundamental biology of natural systems as well as re-engineer these systems with synthetic gene networks,” said Dr. Ruder. “We aim to be a leader in engineering interactions between living systems and machines.”
Dr. Ruder’s lab has expertise in multiple fields including gene network engineering, cell physiology and biomechanics, microfluidics, mechanical engineering and biomaterials. They are currently developing new approaches to embed synthetic gene networks within biomimetic systems that mimic cell, tissue, and organism physiology.
The meeting brings together young scientists to discuss advances and opportunities in a broad range of disciplines, and the structure of the program allows for one-on-one conversations and group discussions on cutting-edge research in a variety of fields. The meeting also includes formal presentations during the eight themed sessions, which provide insight into topics relevant to participants.
According to the Kavli Frontiers of Science, in addition to the knowledge gained at the meeting, the collaborative environment at the Israeli-American Kavli Frontiers of Science Symposium is meant to “create a network of connections that can be maintained as participants advance in their careers.”
Brown Lab Student Announced as Finalist for the George J. Mitchell Scholarship Program

The US-Ireland Alliance recently announced the 21st Class of George J. Mitchell Scholars and among the finalists was McKenzie Sicke, a bioengineering undergraduate at the University of Pittsburgh who works in the lab of McGowan Institute for Regenerative Medicine Bryan Brown, PhD, associate professor of bioengineering. In Dr. Brown’s lab, she studies angiogenic response to polypropylene mesh implants in rabbit models. The George J. Mitchell Scholarship Program is a national, competitive scholarship that awards one year of postgraduate study in any discipline offered by institutions of higher learning in Ireland and Northern Ireland to up to 12 students annually.
A native of upstate New York, Ms. Sicke joined the Swanson School of Engineering at Pitt in 2016 and has spent her time studying bioengineering and reaching beyond the classroom to discover how this work can be applied to the real world. Upon graduation, she hopes to continue her studies and was attracted to Ireland’s medical device design community. She said, “I am driven by the desire to create empathetic healthcare solutions that have a big impact.”
“Being able to take my project from start to finish over the past year and a half has been an invaluable experience,” she said. “With the help of my research mentor, Aimon Iftikhar, I developed protocols and took them through each phase of testing. I’ve learned a lot working with her and contributing to her thesis work has made me more confident of my place in a lab setting.”
She also spent the past summer participating in Pitt’s SERIUS study abroad program at the National University of Singapore where she worked in the SINAPSE Lab on a neurotechnology project focused on nanoparticle-aided stem cell therapy for ischemic stroke.
In addition to pursuing her degree and research projects, Ms. Sicke has worked as a teaching assistant and peer advisor for freshman engineering students and volunteers as a 3D printing mentor in the bioengineering department’s student-run makerspace. She is also the current publicity chair for the undergraduate chapter of the Biomedical Engineering Society.
“This is indeed a magnificent accomplishment. Students like Kenzie are a source of delight to the Department of Bioengineering,” said Arash Mahboobin, PhD, assistant professor of bioengineering and director of the undergraduate program. “I always feel fortunate and privileged to have the opportunity to get to know these young individuals and, perhaps, have some influence on their development and progression as professionals and people. I will certainly watch Kenzie’s career develop with great interest and high expectations.”
The Mitchell Scholars Program is named in honor of George J. Mitchell, the former United States senator who served as chairman of the peace negotiations in Northern Ireland. Under his leadership the Good Friday Agreement, a historic accord ending decades of conflict, was agreed to by the governments of Ireland and the United Kingdom and the political parties of Northern Ireland.
Illustration: University of Pittsburgh Swanson School of Engineering (Ms. Sicke) and McGowan Institute for Regenerative Medicine (Dr. Brown).
Happy Holidays from the McGowan Institute

We wish a safe and happy holiday to all!
To the 250+ scientists, engineers and clinicians that are part of the team of professionals who are committed to the development and translation of regenerative medicine and medical device-based therapies;
To the dedicated support staff who make it possible for the scientists, engineers and clinicians to pursue the goals and objectives of the Institute;
To the hundreds of student trainees who are learning the fundamentals and developing the applications that continue to make regenerative medicine the promise of the future in improved health care and quality of life;
To the many agencies, foundations, companies, and individual donors without whose fiscal support and encouragement the outcomes that we give thanks for would not have been possible, and;
To the many patients who have trusted our team. These individuals are the true pioneers and the heart of the achievements we recognize…
Since the formation of the McGowan Center for Artificial Organ Development (1992) and the McGowan Institute for Regenerative Medicine (2001) there have been many accomplishments advancing the science and clinical achievements in regenerative medicine-based therapies. We see progress in the areas of medical devices, tissue engineering and cell-based therapies, and the prospects for the future are many. Thanks for the opportunities and outcomes created by all who participate in or support the initiatives of the McGowan Institute. Best wishes for another successful year!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#204 –– Dr. Alejandro Soto-Gutierrez discusses his research in the treatment of liver diseases using regenerative and disruptive approaches.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Cramer MC and Badylak SF
Title: Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior
Summary: Biologic scaffold materials composed of allogeneic or xenogeneic extracellular matrix (ECM) are commonly used for the repair and remodeling of injured tissue. The clinical outcomes associated with implantation of ECM-based materials range from unacceptable to excellent. The variable clinical results are largely due to differences in the preparation of the material, including characteristics of the source tissue, the method and efficacy of decellularization, and post-decellularization processing steps. The mechanisms by which ECM scaffolds promote constructive tissue remodeling include mechanical support, degradation and release of bioactive molecules, recruitment and differentiation of endogenous stem/progenitor cells, and modulation of the immune response toward an anti-inflammatory phenotype. The methods of ECM preparation and the impact of these methods on the quality of the final product are described herein. Examples of favorable cellular responses of immune and stem cells associated with constructive tissue remodeling of ECM bioscaffolds are described.
Source: Ann Biomed Eng. 2019 Nov 18. [Epub ahead of print]
GRANT OF THE MONTH
PI: Jeffrey Gross
Title: Elucidating the Molecular Underpinnings of Endogenous RPE Regeneration
Description: Diseases resulting in degeneration of the retinal pigment epithelium (RPE) are among the leading causes of blindness worldwide and no therapy exists that can replace RPE or restore lost vision. Age-related macular degeneration (AMD) is one such disease and is the third leading cause of blindness in the world. While there are some effective treatments for exudative (wet) AMD, ~90% of AMD cases are atrophic (dry) and these are currently untreatable. Transplantation of stem-cell derived RPE has emerged as a possibility for treating geographic atrophy and clinical trials are underway. However, little is known about the fate of transplanted RPE and whether their survival and integration can be improved. An intriguing alternative approach to treating AMD and other RPE diseases is to develop therapies focused on stimulating endogenous RPE regeneration. For this to be possible, we must first gain a deeper understanding of the mechanisms underlying RPE regeneration. In mammals, RPE regeneration is extremely limited and, in some contexts, RPE cells overproliferate after injury, such as during proliferative vitreoretinopathy, where proliferative RPE cells invade the subretinal space and lead to blindness. Recently, a subpopulation of quiescent human RPE stem cells was identified that can be induced to proliferate in vitro and differentiate into RPE or mesenchymal cell types, suggesting that the human RPE contains a population of cells that could be induced to regenerate. Despite these studies, little is known about the process by which RPE cells respond to injury to elicit a regenerative, rather than pathological, response. Indeed, no studies have demonstrated regeneration of a functional RPE monolayer following severe RPE damage in any model system. The development of such a model is a critical first step to acquiring a deeper understanding of the molecular mechanisms underlying RPE regeneration. This knowledge gap is a major barrier to developing effective strategies to restore RPE lost to disease or injury and is the focus of our proposal. We developed a transgenic zebrafish model to study RPE injury and regeneration and demonstrate that the zebrafish RPE regenerates after severe injury. We further demonstrate i) that RPE regeneration involves a robust proliferative response during which proliferative cells move to the injury site and differentiate into RPE, ii) that the source of regenerated cells is likely uninjured peripheral RPE, iii) using this system, we can identify the molecular underpinnings of the regenerative response, and iv) the innate immune system plays a critical role in RPE regeneration. Experiments in this proposal build off of these strong preliminary data to test the hypothesis that RPE regeneration is affected by a population of injury-activated resident RPE cells that proliferate upon injury and regenerate lost RPE tissue. Understanding how injury-responsive RPE cells proliferate in vivo and the signals/pathways active during the injury response holds significant promise to identify strategies to stimulate or reactivate this ability in the human eye, which would be transformational for treating AMD and other diseases that affect the RPE.
Source: National Eye Institute
Term: June 2019 – May 2024
Amount: $323,875 (2019)
