August 2021 | VOL. 20, NO. 8| www.McGowan.pitt.edu
ISSRDC Highlights Opportunities Within Biomanufacturing in Space
The 2021 International Space Station Research and Development Conference (ISSRDC) featured a fireside chat on biomanufacturing in space. Gary Rodrigue, MBA, director of programs and partnerships at the Center for the Advancement of Science in Space (CASIS), moderated a discussion with William Wagner, PhD, director of the University of Pittsburgh’s McGowan Institute for Regenerative Medicine. The discussion focused on the value of space-based biomanufacturing and the critical role of the orbiting laboratory in advancing this research area.
Spaceflight studies over the last decade have shown that microgravity can enable a better understanding of fundamental biology and accelerate advancements in health care and medical technologies. Utilizing the low Earth orbit environment for biomedical research could lead to discoveries not possible on Earth. One area that has potential to provide both benefits to Earth and economic value is biomanufacturing in space. Biomanufacturing is the use of biological and nonbiological materials to produce commercially relevant biomolecules and biomaterials for use in preclinical, clinical, and therapeutic applications.
In 2020, CASIS, manager of the International Space Station (ISS) U.S. National Laboratory, hosted a Biomanufacturing in Space Symposium together with the McGowan Institute for Regenerative Medicine. The symposium brought together thought leaders in the areas of regenerative medicine, tissue engineering, and space-based research. The goal of the symposium was to identify the most promising opportunities to leverage the ISS for research and development (R&D) to advance space-based biomanufacturing. The symposium identified multiple opportunities in three key areas: disease modeling using microphysiological systems (also called tissue chips) and organoids, stem cells and stem-cell-derived products, and biofabrication. A perspective paper developed from the symposium was recently published in Preprints. In addition to Dr. Wagner, McGowan Institute for Regenerative Medicine strategy and business development officer Patrick Cantini is a co-author on the paper.
The ISSRDC session with Dr. Wagner reviewed the current state of biomedical research on the space station and highlighted outcomes from the symposium. The session also discussed how the ISS National Lab is uniquely positioned to enable R&D to advance space-based biomanufacturing and drive a robust biomanufacturing market in low Earth orbit.
The 10th annual ISSRDC was held virtually on August 3-5. To view the agenda and speakers, please visit www.issconference.org.
Illustration: U.S. International Space Station Research Laboratory.
RESOURCES AT THE MCGOWAN INSTITUTE
September Histology Special – Safranin O Staining
Safranin O staining is used for the detection of cartilage, mucin, and mast cell granules on formalin-fixed, paraffin-embedded tissue sections or frozen sections. The cartilage and mucin will be stained orange to red, and the nuclei will be stained black. The background is stained bluish green.
You will receive 30% off your Safranin O staining in September when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
UPMC-Pitt Researchers Receive Grant to Advance Liver Organoids

Researchers from the University of Pittsburgh Liver Research Center, McGowan Institute for Regenerative Medicine, and Carnegie Mellon University’s (CMU) Computational Biology Department have been awarded almost $1.5 million from the National Science Foundation (NSF) to develop new approaches for producing liver organoids — tiny, lab-grown human organs that have potential for disease modeling, drug discovery, and transplantation.
“Mouse models are often used for research on human diseases, but there is a gap between studies in cells and animals and translation to humans,” said principal investigator Mo Ebrahimkhani, MD, associate professor of pathology and bioengineering at Pitt and member of the Pittsburgh Liver Research Center and the McGowan Institute. “Organoids help fill that gap because they can capture the complexity of our human organs and tissues.”
To grow these mini organs, researchers start with induced pluripotent stem cells (iPSCs), which are made from adult cells. iPSCs can turn into almost any cell type, and given the right signals at the right time, they can grow into complex 3D organoids of multiple tissue types.
Traditionally, organoid development is directed by adding a cocktail of chemicals at different times. But this trial-and-error approach is hard to optimize and can introduce variability among batches, hampering reproducibility and scalability of organoid production and making it difficult to compare findings across labs. Another problem is that development can stall, leaving cells with immature or abnormal characteristics or lacking blood vessels, which are important for transplantation applications.
These challenges prompted the NSF’s call for research proposals that apply multidisciplinary approaches to understand processes of cellular differentiation during organ development and improve production of mature, functional cells or organoids.
Dr. Ebrahimkhani, in collaboration with McGowan Institute affiliated faculty member Samira Kiani, MD, co-principal investigator and associate professor of pathology at Pitt, and Dr. Ziv Bar-Joseph, PhD, professor of computational biology and machine learning who heads the Systems Biology Group at CMU, received funding to develop liver organoids that can be produced consistently and reliably. The researchers aim to program iPSCs with genetic instructions that guide cells to produce liver tissues with specified structure and function, rather than relying on external addition of growth factors.
“We will develop programmable organoids with genetic circuits and switches, allowing them to sense the cell types and states, so they know when it’s the right time to turn on and turn off their programs,” Dr. Ebrahimkhani explained.
“The cells can be frozen, stored, and shipped to different labs,” he said. “This will improve reproducibility, reliability, and sharing of the science.”
To generate genetic programs for liver organoids, the researchers aim to understand the processes that control organ development by combining two different tactics.
“Our lab uses synthetic biology to manipulate genetic pathways in cells. By building these pathways, we understand the process,” said Dr. Ebrahimkhani. “It’s like building a car from all the individual components. As I build the car, I understand how it functions.”
Dr. Bar-Joseph’s lab will use computational analyses to reconstruct models that help explain how cells differentiate and interact with one another during organ development, an approach termed systems biology.
“Systems biology is like trying to understand the car by taking it apart and looking at its individual components,” said Dr. Bar-Joseph. “In this research, we aim to combine synthetic biology and systems biology to understand organ development.”
As part of the grant, the researchers will also develop an outreach program to help educate the public about stem cell and organoid research. They will create videos and a website on the Tomorrow Life platform, a science communication filmmaking initiative directed by Dr. Kiani.
Although the grant will focus on liver organoids, the researchers say that their findings could also inform the development of other types of human organoids, such as brain, kidney, or retina.
Dr. Peter Rubin Leads Clinical Trial Assessing Treatment of Partial Thickness Burns

Synedgen, a biotechnology company using glycopolymer chemistry to develop therapeutics that enhance and control signaling in the innate immune system, announced the initiation of a randomized controlled trial (RCT) to test the safety and effectiveness of SynePure™ Wound Cleanser (SynePure) in combination with Catasyn™ Advanced Technology Hydrogel (Catasyn) for the treatment of superficial partial thickness burn wounds.
This study is an investigator-initiated randomized trial comparing SynePure and Catasyn (intervention group) to the current gold standard treatment, Silvadene (control group). Both groups will receive the same care other than cleansing treatment. Subjects will be recruited from the University of Pittsburgh Medical Center (UPMC) Mercy Burn Center adult patient population who have sustained superficial partial thickness burn wounds. McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, FACS, Chair of Plastic Surgery at UPMC, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh, is the principal investigator for this study. The trial is being funded by the Defense Health Agency (DHA) through the US Army Medical Research and Development Command’s (USAMRDC) Small Business Innovation Research (SBIR) program to improve the current treatment of burn wounds, particularly to meet a gap in the early acute phase of treatment and will potentially increase the technological readiness level of these products.
“We’re excited to announce that the first patient has received treatment in the burn study,” said Shenda Baker, PhD, President and Chief Executive Officer of Synedgen. “We believe the combination of our two wound care products is not only safe but improves time-to-heal and infection rates in superficial partial-thickness burn wounds versus the current standard of care treatment. We are thankful to our partners at the DHA and USAMRDC for funding this important study, which we hope will introduce an improved standard of care for partial thickness burns.”
“Burn wounds are associated with significant morbidity and mortality as well as quality of life impairments for the patient. Burn wounds are particularly problematic, as they can compromise skin integrity, which can lead to infection,” said Dr. Rubin. “Moreover, thermal destruction of the skin provides an ideal environment for microbial colonization where, if left untreated, infection can spread to a systemic level. I am pleased to be leading this study and very much look forward to its findings.”
SynePure and Catasyn are Food and Drug Administration (FDA) 510(k) cleared wound care medical devices, formulated with a novel glycopolymer designed to be biocompatible and protective of dermal surfaces. SynePure is optimized for the cleansing and debridement of wounds and thermal injuries, while Catasyn is a protective gel dressing that provides an environment that supports wound healing. SynePure and Catasyn are biocompatible, shelf-stable up to two years, non-staining, and may be used in any care setting from the battlefield to a tertiary hospital.
Current Defense standard-of-care offers an array of antimicrobial, silver-based, stem cell, or autologous graft therapies which are normally deployed in the day(s) or week(s) following initial trauma. SynePure and Catasyn are first-in-class products that can be used to treat initial trauma within the acute care gap of the minutes or hours immediately following traumatic injury. Immediate use following injury reduces time to healing, provides a protective barrier against infiltration by damage-causing microbes, and in some cases, reduces scar formation. Results from this RCT are expected not only to enhance choice of care available to warfighters, but also permit a significant enhancement in civilian care by bringing cutting-edge technologies to the patient bedside.
SynePure and Catasyn were developed with grants from the Army and DARPA (W81XWH-13-C-0053 and N66001-12-C-4053), with the RCT funded through SBIR contract W81XWH-16-C-0023 with the USAMRDC SBIR office.
Blood Thinners Reduce Need for Cardiovascular and Respiratory Support in Certain COVID-19 Patients

Giving moderately ill, hospitalized COVID-19 patients a full dose of a blood thinner improved their chances of leaving the hospital without needing mechanical ventilation. But this strategy did not yield the same results for COVID-19 patients who were critically ill, who needed intensive care-level support at the time of enrollment.
These are the findings of two new studies (critically ill patients and noncritically ill patients) published in The New England Journal of Medicine (NEJM). The studies incorporated data from three platform trials as part of a global collaboration to identify possible treatments during the height of the pandemic. The trials are:
- “Accelerating COVID-19 Therapeutic Interventions and Vaccines-4” (ACTIV4a): A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19,
- “Antithrombotic Therapy to Ameliorate Complications of COVID-19” (ATTACC), and
- “Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia” (REMAP-CAP) Therapeutic Anticoagulation.
McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, Executive Vice President, UPMC, and Chief Innovation Officer; Associate Vice Chancellor for Healthcare Innovation at the University of Pittsburgh Schools of the Health Sciences; Distinguished Professor and the Mitchell P. Fink Endowed Chair in Critical Care Medicine at the University of Pittsburgh, with secondary appointments in Medicine, Health Policy and Management, and Clinical and Translational Science, is a senior investigator and member of the International Trial Steering Committee of REMAP-CAP and a co-author on both publications.
Led by researchers from the University of Pittsburgh School of Medicine, NYU Grossman School of Medicine, and global collaborators, ACTIV4a was launched after researchers observed that patients who died from COVID-19 had blood clots throughout their bodies, including in their smallest blood vessels. Doctors saw antithrombotics—also known as blood thinners or anticoagulants—as potential treatment because they reduce the risk of clotting. But clinicians did not know whether a full therapeutic dose that is used to treat blood clots or a low dose typically used to prevent blood clots would be most effective.
“Early on in the pandemic, doctors observed that blood clots in both large and small vessels were associated with severe disease in hospitalized patients with COVID-19,” said both NEJM studies’ co-first author Bryan McVerry, MD, associate professor of medicine and associate division chief for strategy and development in Pitt’s Division of Pulmonary, Allergy and Critical Care Medicine. “The unprecedented global collaboration of multiple clinical trial networks demonstrated that early intervention targeting clotting can improve outcomes in COVID-19 and rapidly changed the landscape of treatment for the disease.”
Pitt’s Epidemiology Data Center is coordinating the ACTIV4a trial, under the leadership of Stephen Wisniewski, PhD, Pitt’s vice provost for budget and analytics, and Maria Mori Brooks, PhD, professor of epidemiology and biostatistics at Pitt’s Graduate School of Public Health.
As part of the research effort, the lead researchers of the three platform trials synchronized their study protocols to study the effects of using full and low doses of the anticoagulant heparin in patients hospitalized with COVID-19. Researchers grouped the patients according to whether they had severe or moderate COVID-19 and by their levels of D-dimer, a blood protein that may indicate clotting.
Moderately ill hospitalized COVID-19 patients were defined as those who did not receive “organ support,” including high-dose oxygen therapy, mechanical ventilation, life support, medicines that increase blood pressure or medicines that change the force of the heart’s contraction. Hospitalized COVID-19 patients who did require such support were defined as severe or critically ill.
In April 2020, the research teams started randomly assigning half of their hospitalized COVID-19 patients to receive either a low or full dose of heparin for up to 14 days after enrollment. By December 2020, oversight boards stopped enrollment of critically ill patients in the trial when interim results showed that full-dose anticoagulation did not reduce the need for organ support, and may cause harm, in severe and critically ill patients. One month later, oversight boards also stopped enrollment of moderately ill patients in the trial when interim results indicated that full doses of blood thinners likely did offer a benefit. The trial enrolled 1,098 critically ill and 2,219 moderately ill patients, and researchers measured how long patients were free of organ support, up to 21 days after enrollment in both cohorts.
Among moderately ill patients, the study authors found a 99% chance that full-dose heparin increased the probability of survival to hospital discharge with reduced need for organ support compared to those who received low-dose heparin. However, a small number of patients experienced major bleeding, though this happened infrequently. For critically ill patients, full-dose heparin also decreased the number of major thrombotic events, but it did not result in a greater chance of survival to hospital discharge, or a greater number of days free of organ support than usual care.
“These results are very exciting and lead us to better understand the impact of applying the right therapies at the right time in the course of this challenging disease,” says ACTIV4a study co-chair Judith Hochman, MD, the Harold Snyder Family Professor of Cardiology, and senior associate dean for clinical sciences at NYU Grossman School of Medicine and a co-corresponding author of the moderately ill study. “Our results will help clinicians utilize known and easily available medical therapies to better treat moderately ill COVID-19 patients.”
ACTIV4a Antithrombotics Inpatient is conducting further research to test the effects of adding an anti-platelet agent to anticoagulation.
“More work needs to be done to continue to improve outcomes in patients with COVID-19,” said ACTIV4a study co-chair Matthew D. Neal, MD, the Roberta G. Simmons Associate Professor of Surgery at Pitt, co-first author of the moderately ill study and co-senior author of the critically ill study. “Given what we know about the type of blood clots in patients with COVID-19, testing anti-platelet agents is a particularly exciting approach.”
The trials are supported by several funding organizations, including the National Institutes of Health (U.S.), the Canadian Institutes of Health Research, the National Institute for Health Research (U.K.), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (EU). The ClinicalTrials.gov identifier(s) for the two published studies are NCT04505774 and NCT04359277.
Finding Inspiration to Rebuild Human Heart Muscle

Advances in cardiac tissue engineering offer hope for an array of useful applications, from heart repair to disease modelling. As part of active, ongoing research related to bioengineering functional human organs, McGowan Institute for Regenerative Medicine affiliated faculty member Adam Feinberg, PhD, a Professor of Biomedical Engineering and Materials Science and Engineering at Carnegie Mellon University, and his team are finding inspiration from the developing heart to rebuild human heart muscle.
Inside the adult human heart, the myocardium (muscular wall) is organized in a complex structure with layers of aligned cardiomyocytes (heart cells), wrapped in different orientations to support its pumping ability. It is formed during embryonic morphogenesis, where the heart starts as a linear tube and transforms into its four-chambered structure.
To better understand how cardiomyocytes interact with each other while they’re reorganizing inside of the embryo, Dr. Feinberg’s group engineered two-dimensional tissues using fibronectin micropatterns to identify and observe cell–cell and cell–extracellular matrix (ECM) interactions. Previous research has explored how cardiomyocytes organize into an aligned tissue; however, the individual physical, mechanical, and biochemical factors that drive cardiomyocyte alignment remain largely unknown.
“We were interested in exploring the multiple factors that influence structure formation, and ultimately function, in human cardiac muscle tissue,” explained Ivan Batalov, PhD, leader of the research recently published in Scientific Reports, and former Materials Science and Engineering PhD student in Dr. Feiburg’s lab. “In order to assess how heart muscle cells interact with each other versus how they interact with the ECM, we had to decouple their interactions, because they naturally happen together.”
The group’s research required a large amount of data, sophisticated analysis techniques and the development of a custom algorithm to process the data and extract useful information. Their results ultimately showed that both cell-cell and cell-ECM interactions play an important role in the formation of aligned myocardium.
“These results are exciting because they show how we can use insights from heart development to create biologically-inspired engineering design principles to rebuild human heart muscle,” said Dr. Feinberg. “This is important because normally, the adult heart cannot repair itself following injury or disease. We need to understand why this happens and determine how we can potentially direct regeneration.”
Big picture, these findings support ongoing efforts to engineer human heart tissue outside of an organism. Such tissues could be used to repair damaged or dead tissues for patients who’ve experienced a heart attack or are suffering from heart disease, the most common cause of death worldwide. They could also be leveraged during drug development to ensure more accurate testing (as opposed to animal testing) of a drug’s toxicity and effectiveness.
“Creating quality, functional heart muscle tissues requires an in-depth understanding of how cardiomyocytes interact,” added Dr. Batalov. “We need to understand what drives the alignment of these cells to ultimately be successful.”
Published in Scientific Reports, the paper was co-authored by Dr. Feinberg’s current and former students, Ivan Batalov, PhD, Quentin Jallerat, PhD, Sean Kim, and Jacqueline Bliley.
Illustration: McGowan Institute for Regenerative Medicine.
Vascular Bioengineering Lab Receives Funding to Track Aneurysms and Predict Rupture
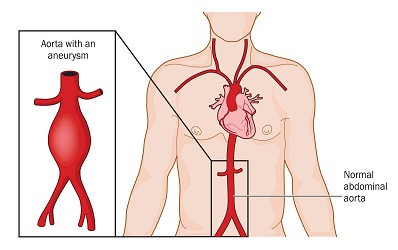
The Vascular Bioengineering Lab (VBL) at the University of Pittsburgh’s Swanson School of Engineering—led by McGowan Institute for Regenerative Medicine affiliated faculty member David Vorp, PhD, Associate Dean for Research, Swanson School of Engineering, and the John A. Swanson Professor of Bioengineering—seeks to understand and develop solutions to the causes and effects of disease in tubular tissue and organs. Part of this research includes a closer look at abdominal aortic aneurysms (AAA)—the 15th leading cause of death in the United States.
An AAA occurs when the aorta weakens and begins to irreversibly dilate, like a slowly inflating balloon. If left untreated, the risk of rupture increases and has a 90 percent rate of mortality.
The VBL team is working to develop a new model to better predict at-risk patients and are using tools from the lab to perform shape analysis and biomechanical simulations. They will use these data to train a machine learning algorithm to classify different types of aneurysm outcomes. This classifier will be used to develop a predictive model that can help guide clinicians and determine the need for surgical intervention.
“Most medical treatments are based on how they will affect the average patient, but in fact, each of us is very different from one another. Being able to understand and predict how a specific person’s aneurysm will grow based on their own unique characteristics is a big leap forward,” said Nathan Liang, MD, MS, assistant professor of surgery in Pitt’s School of Medicine, vascular surgeon at UPMC, and co-investigator on the project.
“This wasn’t possible in the past, but now with better machine learning algorithms and increasingly powerful computers, we are close to making this a reality. Our classifier will allow clinicians and patients to work together, create individualized management plans, and improve the care for those with abdominal aortic aneurysms.”
The team received a $100,000 award from Precision Medicine Initiative for Commercialization (PreMIC), a collaboration between Pitt’s Institute for Precision Medicine, sciVelo, and the Innovation Institute. Funding for PreMIC comes from a RK Mellon Foundation grant to the Institute for Precision Medicine that in part provides critical funding to early-stage translational science projects.
“Our initial pilot study to develop the aneurysm prognosis classifier revealed the importance of additional interrogation of medical images using biomechanical and morphological analyses,” said Timothy Chung, PhD, a postdoctoral associate in the Vascular Bioengineering Lab who will help lead the project. “Utilizing a strong research team and collaborations offered at Pitt, we aim to improve and personalize AAA patient healthcare.”
Illustration: Location and comparison of a normal aorta and abdominal aortic aneurysm. If left untreated the aneurysm will irreversibly grow increasing risk of rupture for a patient. Credit: Shutterstock.
SURGIMESH Benefits Hernia Patients

As much as 10% of the population develops some type of hernia during life.1 More than 1 million abdominal hernia repairs are performed each year, with inguinal hernia repairs constituting nearly 800,000 of these cases.2 According to a February 2012 Wall Street Journal article, more than 30% of patients may, unfortunately, suffer from long-term chronic pain and restricted movement after surgery to fix a hernia.3
BG Medical, the manufacturer and distributor of the 21st Century SURGIMESH® Platform, announced recently it is honoring Hernia Awareness Month by partnering with CQInsights to pioneer a new approach that delivers better outcomes for patients, with a larger mission to share sustainable solutions that improve healthcare for everyone. SURGIMESH is the only non-woven polypropylene matrix mesh that promotes rapid and complete vascularized incorporation in just 12 days. Because of its low profile and ability to be trimmed, SURGIMESH is also easier to deploy for all types of open and laparoscopic procedures, including robotics. SURGIMESH is a proprietary 21st century alternative to the use of older knitted or woven meshes and has the potential to significantly reduce chronic pain and recurrence.
“This novel configuration of polypropylene and its unique production style and structural architecture will benefit patients clinically as it mimics the organization of the ECM in its random orientation,” said McGowan Institute for Regenerative Medicine affiliated faculty member Jarrod Kaufman, MD, Chairman of Surgery at Monmouth Medical Center Southern Campus. His practice, Premier Surgical, is based in Brick, NJ. “ Innovative products and the ongoing multicenter clinical research will help better understand the magnitude of benefit this mesh provides to our patients undergoing hernia repair surgery.”
CQInsights is the first healthcare data consulting and analytics company focused on digital health transformation by applying Systems and Data Science principles, including value-based continuous quality improvement, to real patient care, benefiting all facets of the healthcare industry.
1Ruhl CE, Everhart JE. Risk factors for inguinal hernia among adults in the US population. Am J Epidemiol. 2007 May 15. 165(10):1154-61.
2Fitch MT, Manthey DE. Abdominal hernia reduction. Roberts JR, Custalow CB, Thomsen TW, et al., eds. Roberts and Hedges’ Clinical Procedures in Emergency Medicine. 6th ed. Philadelphia: Elsevier Saunders; 2014. 873-9.
3Landrow, L. (2012, February 28). A Secret for Patients Undergoing Hernia Repair. The Wall Street Journal. Retrieved from https://www.wsj.com/.
Roy Lab Student Abigail Allen Receives NIH F31 for Her Cardiovascular Research

Cardiovascular disease is a leading cause of death in the United States and the contributing factor for one in every four deaths. An improved understanding of the mechanisms behind atherosclerosis, a common contributor to cardiovascular disease, may help guide new therapeutic strategies to combat this deadly disease.
University of Pittsburgh graduate student Abigail Allen—who works in the laboratory of McGowan Institute for Regenerative Medicine affiliated faculty member Partha Roy, PhD, Associate Professor of Bioengineering, Cell Biology, and Pathology at the University of Pittsburgh—received an F31 award from the National Institutes of Health for her work that examines the effect of actin-binding protein Profilin-1 (Pfn1) perturbation in atherosclerosis. Her studies involve taking a close look at angiogenesis and how Pfn1 may play an important role in its function.
“Angiogenesis is the process in which new blood vessels sprout from pre-existing vasculature, and it is facilitated by complex interaction between endothelial cells (EC), extracellular matrix (ECM) and various stromal cells (immune cells, fibroblasts),” Ms. Allen explained. “This process is central to both normal physiology and life-threatening pathologies, like cancers, atherosclerosis, or diabetic retinopathy.”
In addition to facilitating nutrient delivery, new vessel formation from angiogenesis also promotes infiltration of immune cells into the vascular system during inflammation and diseases involving inflammation.
“Actin cytoskeleton remodeling in EC not only plays pivotal role in cellular restructuring, cell motility and sprouting angiogenesis, but is also important for features that impact vascular infiltration of immune cells, such as regulation of EC barrier function and EC-immune cell interactions,” Ms. Allen added.
Studies from the Roy Lab have demonstrated that actin-binding protein Pfn1, an important regulator of actin cytoskeletal dynamics, is an indispensable endothelial mediator of angiogenesis in both physiological and abnormal settings.
Other studies report that in preclinical setting of atherosclerosis, an overall decrease in Pfn1 expression—by modifying the gene at the embryonic level—leads to less vascular infiltration of pro-atherogenic immune cells, specifically macrophages. This effect reduces the disease burden.
“These taken together with my preliminary findings that endothelial Pfn1 depletion dramatically alters the circulating levels of immune-modulatory cytokines in vivo, underscore the importance of endothelial Pfn1 function in vascular biology with potential impact on vascular-immune cell interactions,” Ms. Allen said.
Illustration: Roy Lab (Allen)/McGowan Institute for Regenerative Medicine (Roy).
Dr. Tracy Cui Receives Chancellor’s Gap Project Funding

A sign of the strength of University of Pittsburgh’s innovation and entrepreneurship ecosystem is the reauthorization earlier in 2021 of the Chancellor’s Gap Fund, which provides grants of $25,000 to $75,000 for innovators with promising discoveries to explore the commercial potential of their ideas.
Proposals went through a multi-step process involving a team drawn from the Office of Innovation and Entrepreneurship and the Clinical and Translational Science Institute, as well as an internal faculty review board.
Three projects were selected this year and one of those included the work of McGowan Institute for Regenerative Medicine faculty member Tracy Cui, PhD, a William Kepler Whiteford Professor of Bioengineering at the University of Pittsburgh and the Director of the Neural Tissue/Electrode Interface and Neural Tissue Engineering Lab. The project is entitled “Biodegradable Nerve Stimulator.” In addition to Dr. Cui, the principal investigators are Trent Emerick, MD, Department of Anesthesiology and Perioperative Medicine, and Raj Kubendran, PhD, Department of Electrical and Computer Engineering.
A description of the project follows:
A biodegradable nerve stimulator to treat acute and chronic pain. The stimulator is an injectable wire that is placed under the skin near the nerve of interest using ultrasound. The device does not require an incision or surgery to be implanted and is considered minimally invasive. The electrical stimulation would produce a soothing vibration in the area of interest. The device degrades over 3-6 months, however ample evidence exists in the medical literature that shows that temporary nerve stimulation can lead to long-term pain relief due to brain plasticity and central nervous system changes at the level of the spinal cord.
Currently, many patients with refractory pain who have failed conservative therapies turn to permanent steel surgical stimulators as an option. These devices are costly and have an overall complication rate of 30-40%. These steel stimulators require surgery and an incision, which leads to higher health care costs from surgery/anesthesia, and many other complications that a biodegradable lead avoids. Compliance is also an issue with the surgical devices.
A prototype of the biodegradable device has been developed. The Gap Fund award will be used to develop a pain model to test the biodegradable stimulator versus a control. Additionally, it will be used to develop the conceptual framework and prototype for the external battery pack and circuitry design. A company, Vanish Therapeutics, was spun out of the University in April 2021.
Congratulations, Dr. Cui!
Double Lung Transplant Done Twice

McGowan Institute for Regenerative Medicine affiliated faculty member Pablo Sanchez, MD, PhD, is an Associate Professor of Cardiothoracic Surgery; Vice Chairman, Benign Lung Diseases; Chief, Division of Lung Transplant and Lung Failure; Surgical Director, Lung Transplantation and ECMO; Director, Lung Transplant Research; and, Director, Ex Vivo Lung Perfusion (EVLP) Program, all within the Department of Cardiothoracic Surgery at the University of Pittsburgh. The UPMC Lung Transplant Program is one of the most recognized and experienced centers in the world. Physicians of this team evaluate many high-risk patients, including those needing re-transplantation. Beth Slack was one of these patients. Dr. Sanchez and Mrs. Slack were interviewed recently by Meghan Schiller, KDKA. Watch the interview here; part of their conversation transcript follows:
Double lung transplants are not uncommon, but it’s extremely rare to need a double lung transplant done twice. It was last April, when Mrs. Slack got the news: her body was rejecting her new lungs. After undergoing a double lung transplant again, she was asked how’s she doing today:
“I’m actually feeling great,” Mrs. Slack said, a mother of two. “My children just started football and cheerleading, for once I can step in with them and help out and run alongside the field. I volunteer with the other parents, we ride bikes up and down our street, it’s a lot, there’s a lot I can do now.”
Mrs. Slack’s situation is unique, and it was a certain health condition that caused her to need a second double lung transplant, according to Dr. Sanchez.
“She has something that is called ‘Kartagener’s syndrome,’ which is a genetic disorder which pretty much [means] all her organs are flipped,” he explained. “So, her liver is actually on the wrong side, her lungs are flipped, everything is completely the opposite of what you’d see in a normal person.”
So, how does a doctor deal with someone with such a unique situation?
“Well, that’s actually the only two organs she had in the right place. Her right lung was where the right lung should be and the left lung is where the left lung should be,” Dr. Sanchez said. “Pretty much [we] have to retrofit normal lungs to her anatomy, and that’s what we did twice, actually.”
Mrs. Slack’s need for a second double transplant is extremely rare, according to Dr. Sanchez. In a good year, there are 40 patients out of 3,000 transplants (or less than 5%) they see that need a re-transplantation.
With a new lease on life, Mrs. Slack has plenty to look forward to.
“Honestly, I’m just looking forward to hopefully having simplicity, normalcy, to give my kids a normal life and for them not to have a sick mom,” she said. “And to be able to do everything every other mother can do.”
She also has an inspiring message to those waiting for the same call she waited for.
“Don’t lose hope and keep up the fight because it’s worth it,” she said. “It’s not easy, I warn everybody that asks me, and that’s even what Dr. Sanchez told me – that if I want it, I got to work for it. Still to this day, I work, it’s not easy, there’s still challenges that I’ve gone through.”
AWARDS AND RECOGNITION
Dr. Jonathan Vande Geest Elected Fellow of ASME

McGowan Institute for Regenerative Medicine affiliated faculty member and Professor of Bioengineering Jonathan Vande Geest, PhD, was elected Fellow of the American Society of Mechanical Engineers (ASME), recognizing his “exceptional engineering achievements and contributions to the engineering profession and to ASME.” This election puts him in company with the 3,462 other Fellows of ASME, an organization with nearly 80,000 members.
Dr. Vande Geest directs the Soft Tissue Biomechanics Lab at the University of Pittsburgh’s Swanson School of Engineering. The goal of their work is to develop and utilize novel experimental and computational bioengineering approaches to study the structure-function relationships of soft tissues in human growth, remodeling, and disease. The results of this research are then used to understand human disease and develop innovative medical devices and drug therapies.
He earned his BS in biomedical engineering from the University of Iowa in 2000 and his PhD in bioengineering from Pitt in 2005. He received his first academic appointment at the University of Arizona in the Department of Aerospace and Mechanical Engineering and later joined their Department of Biomedical Engineering in 2009. He returned to Pitt as Professor in the Department of Bioengineering in January 2016.
A member since 2000, Dr. Vande Geest has a long history with ASME. In 2013 he received the Y. C. Fung Young Investigator Award – a society-wide medal awarded by the Bioengineering Division of ASME to recognize those demonstrating significant potential to make substantial contributions to the field of bioengineering. He chaired the ASME Biosolids Technical Committee from 2016-2019 and more recently chaired two ASME-sponsored virtual summer bioengineering meetings in 2020 and 2021.
As part of this election, ASME recognized “his service to the field of bioengineering; contributions in aneurysm, glaucoma, and vocal fold paralysis research; and leadership in ASME’s Bioengineering Division.”
In addition to being a Fellow of ASME, Dr. Vande Geest also became a Fellow of the American Institute for Medical and Biological Engineering in 2018. He is a member of the Biomedical Engineering Society, the International Society of Applied Cardiovascular Biology, the Association of Research in Vision and Ophthalmology, the American Heart Association, and the American Physiological Society.
Dr. Vande Geest also holds appointments in Pitt’s Department of Mechanical Engineering and Material Science, Department of Ophthalmology, Louis J. Fox Center for Vision Restoration, and Vascular Medicine Institute.
“I am proud to have nominated Dr. Vande Geest for this well-deserved honor,” said David Vorp, PhD, Associate Dean for Research and John A. Swanson Professor of Bioengineering at Pitt, who is also a Fellow of ASME and an affiliated faculty member of the McGowan Institute. “He is not only a leader of impact in the field of bioengineering and biomechanics, but a wonderful colleague and Pitt faculty member as well. His career trajectory is certainly one that will be associated with many other awards and honors in the future.”
Congratulations, Dr. Vande Geest!
Dr. Mary Amanda Dew Honored by Society for Health Psychology

McGowan Institute for Regenerative Medicine affiliated faculty member Mary Amanda Dew, PhD, Professor of Psychiatry, Psychology, Epidemiology, Biostatistics, Clinical and Translational Science, and Acute and Tertiary Care (Nursing), has received the American Psychological Association (APA) Division 38: Society for Health Psychology 2021 Excellence in Health Psychology Research Award.
The Award highlights the accomplishments of psychologists who have made significant contributions to the body of academic literature, demonstrating the value of psychological science and inquiry toward the betterment of health and healthcare. Dr. Dew is an expert in evaluating risk factors for mental health and behavior problems in organ transplantation populations including organ recipients, living donors and families, as well as in testing interventions to improve psychosocial and medical outcomes.
“I was surprised and humbled to learn that I would receive the award, and I am so appreciative that my health psychology colleagues view my work as making an important and timely contribution to the field,” said Dr. Dew. “The American Psychological Association is the preeminent U.S. society for psychologists, and I was previously honored to become a Fellow in the APA. I devote considerable time to my position as an associate editor for Health Psychology, the flagship APA journal for Division 38/Society for Health Psychology. I believe that the opportunities I have had to not only conduct research but participate in the development of public policies for behavioral health evaluation and care in the field of organ transplantation have helped me to contribute to the advancement of this field.”
Congratulations, Dr. Dew!
Dr. Ellen Gawalt Named Interim Dean of Duquesne’s Bayer School

Ellen Gawalt, PhD, has been named interim dean of Duquesne University’s Bayer School of Natural and Environmental Sciences (BSNES), effective Thursday, July 1, 2021. She will step in for Philip Reeder, PhD, who will be rejoining the faculty to pursue his research.
A professor at Duquesne since 2003, Dr. Gawalt is a Hillman Distinguished Professor and chair of the school’s chemistry and biochemistry departments. An affiliated faculty member of the McGowan Institute for Regenerative Medicine, her research group focuses on the surface chemistry of biomaterials to improve tissue-material interaction.
“I’m excited for the opportunity to work directly with Dr. Gawalt. She has an impressive academic background and has been a stellar leader of the chemistry and biochemistry department,” said Provost David Dausey, PhD. “She will be an excellent interim dean who will continue to advance and grow the Bayer School of Natural and Environmental Sciences for a new generation of students.”
Dr. Gawalt is a mentor for the Bayer Scholars Program, which provides full-tuition scholarships and research opportunities for underrepresented groups in chemistry. Since 2013, 18 students have graduated from the program under Dr. Gawalt’s supervision and gone on to work in industry or attend graduate school. The program serves as another example of Duquesne’s long-standing commitment to creating equity and opportunity in the region.
“Dr. Gawalt’s students have graduated from Duquesne prepared for anything and have gone on to success in an array of professions. Her teaching acumen, research expertise and commitment to all students position her well to step into this leadership role at the Bayer School,” said Duquesne President Ken Gormley, JD. “The faculty, staff and students at Bayer will benefit greatly from her service as interim dean.”
A prolific researcher, Dr. Gawalt has authored or co-authored more than 100 scientific papers and presentations. Her research has generated more than $3.5 million in funding, with a focus on scientific discovery and student education. Dr. Gawalt is a two-time recipient of the BSNES Excellence in Teaching Award and also has been honored with the University’s Creative Teaching Award.
“Science plays such an important role in our society, so it’s an honor to help educate the next generation of scientists,” Dr. Gawalt said. “As interim dean, I look forward to working alongside our excellent faculty and staff to help students explore new discoveries and reach their goals.”
Dr. Gawalt received her bachelor’s degree in chemistry from Duke University and her master’s and doctoral degrees in chemistry from Princeton University.
Top Dentists Honored in Pittsburgh Magazine

“If you had a patient in need of a dentist, which dentist would you refer them to?”
This is the question asked to thousands of dentists to help determine who the topDentists should be. Dentists and specialists are asked to take into consideration years of experience, continuing education, manner with patients, use of new techniques and technologies, and of course physical results. Pittsburgh Magazine’s annual list, which in 2021 contains more than 420 dentists and specialists, includes three McGowan Institute for Regenerative Medicine affiliated faculty members:
Endodontics
- Herbert Ray, Jr., DMD, Associate Professor of Endodontics and the Department Chair and Director, Graduate Endodontic Residency Program, University of Pittsburgh School of Dental Medicine
Oral and Maxillofacial Surgery
- William Chung, MD, DDS, Professor in the Department of Oral and Maxillofacial Surgery, School of Dental Medicine, University of Pittsburgh
- Bernard Costello, MD, DMD, FACS, Dean of the University of Pittsburgh School of Dental Medicine, Professor of Oral and Maxillofacial Surgery, Chief of Pediatric Oral and Maxillofacial Surgery at the Children’s Hospital of Pittsburgh, and a surgeon with the Cleft and Craniofacial Center
The nomination pool of dentists consists of dentists listed online with the American Dental Association as well as all dentists listed online with their local dental societies, thus allowing virtually every dentist the opportunity to participate. Dentists are also given the opportunity to nominate other dentists that they feel should be included in the list.
topDentists was started with the intent to identify the best dentists and specialists in the country and is the only list of its kind, chosen by the dental profession themselves. topDentists has over fifty years in combined experience compiling peer-review referral guides in the legal, dental, and medical fields. Using this experience along with the input of several prominent dentists in the United States, topDentists has created a selection process that has been refined and improved over previous superlative guides. Because dentists must be voted in strictly by their peers, and virtually every dentist is given an opportunity to participate, and listings cannot be purchased (and no payment is required to be listed), inclusion in topDentists is considered a distinct honor.
Congratulations, all!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#224 –– Dr. Eric Lagasse and Dr. Michael Hufford discuss their work in ectopic organogenesis.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Sarkis Hamalian, Robert Güth, Farhana Runa, Francesca Sanchez, Eric Vickers, Megan Agajanian, Justin Molnar, Tuan Nguyen, Joshua Gamez, Jonathan D Humphries, Anupma Nayak, Martin J Humphries, Julia Tchou, Ioannis K Zervantonakis, Jonathan A Kelber
Title: A SNAI2-PEAK1-INHBA stromal axis drives progression and lapatinib resistance in HER2-positive breast cancer by supporting subpopulations of tumor cells positive for antiapoptotic and stress signaling markers
Summary: Intercellular mechanisms by which the stromal microenvironment contributes to solid tumor progression and targeted therapy resistance remain poorly understood, presenting significant clinical hurdles. PEAK1 (Pseudopodium-Enriched Atypical Kinase One) is an actin cytoskeleton- and focal adhesion-associated pseudokinase that promotes cell state plasticity and cancer metastasis by mediating growth factor-integrin signaling crosstalk. Here, we determined that stromal PEAK1 expression predicts poor outcomes in HER2-positive breast cancers high in SNAI2 expression and enriched for MSC content. Specifically, we identified that the fibroblastic stroma in HER2-positive breast cancer patient tissue stains positive for both nuclear SNAI2 and cytoplasmic PEAK1. Furthermore, mesenchymal stem cells (MSCs) and cancer-associated fibroblasts (CAFs) express high PEAK1 protein levels and potentiate tumorigenesis, lapatinib resistance and metastasis of HER2-positive breast cancer cells in a PEAK1-dependent manner. Analysis of PEAK1-dependent secreted factors from MSCs revealed INHBA/activin-A as a necessary factor in the conditioned media of PEAK1-expressing MSCs that promotes lapatinib resistance. Single-cell CycIF analysis of MSC-breast cancer cell co-cultures identified enrichment of p-Akthigh/p-gH2AXlow, MCL1high/p-gH2AXlow and GRP78high/VIMhigh breast cancer cell subpopulations by the presence of PEAK1-expressing MSCs and lapatinib treatment. Bioinformatic analyses on a PEAK1-centric stroma-tumor cell gene set and follow-up immunostaining of co-cultures predict targeting antiapoptotic and stress pathways as a means to improve targeted therapy responses and patient outcomes in HER2-positive breast cancer and other stroma-rich malignancies. These data provide the first evidence that PEAK1 promotes tumorigenic phenotypes through a previously unrecognized SNAI2-PEAK1-INHBA stromal cell axis.
Source: Oncogene. 2021 Aug;40(33):5224-5235. Epub 2021 Jul 8.
GRANT OF THE MONTH
PI: Mo Ebrahimkhani and Samira Kiani
Title: Collaborative Research: RECODE: Directed Differentiation of Human Liver Organoids via Computational Analysis and Engineering of Gene Regulatory Networks
Description: Organoids are group of cells produced from stem cells that mimic closely structure and functions of human organs. Organoids can be used for modeling the development of human diseases and for testing newly produced medicines. However, there is a need for generation of human organoids with better function and less variability. This project aims to use genetic based analysis and engineering to control stem cell differentiation towards human liver cells and improve the final manufacturing outcome of liver organoids. As part of the activities in this project, graduate and undergraduate students will be trained and short educational video clips will be made for online education and public engagement.
This RECODE project will address biotechnology challenges of in vitro liver organoid engineering such as maturity, vascular formation, and reproducibility. To this end, synthetic biology, stem cell engineering, and systems biology will be integrated to develop a platform for autonomous differentiation of human induced pluripotent stem cells to liver organoids. Studies will be performed in two independent objectives to: 1) develop synthetic gene circuits for multistep differentiation in liver organoids and 2) identify lineage plasticity regulators during differentiation to control final cell fates in engineered organoids. The understanding gained through this work will address knowledge gaps applicable to cellular differentiation, human liver maturation, and vascular formation. This project will also provide support for the development of a strong STEM workforce through novel online education and public outreach activities surrounding stem cell engineering.
This RECODE award is co-funded by the Systems and Synthetic Biology Cluster in the Division of Molecular and Cellular Biosciences and the Engineering Biology and Health Cluster in the Division of Chemical, Bioengineering, Environmental, and Transport Systems.
This award reflects NSF’s statutory mission and has been deemed worthy of support through evaluation using the Foundation’s intellectual merit and broader impacts review criteria.
Source: National Science Foundation
Term: January 1, 2022 – December 31, 2025 (Estimated)
Amount: $1,064,877
