August 2019 | VOL. 18, NO. 08| www.McGowan.pitt.edu
New Alliance with the International Space Station U.S. National Laboratory

The McGowan Institute has formed an alliance with the International Space Station (ISS) U.S. National Laboratory to develop and demonstrate the how micro-gravity can improve regenerative medicine-based therapies. The ISS provides a unique platform to conduct studies in a microgravity environment.
This alliance — a core element of the ISS National Laboratory Industrial Biomedicine Program — was unveiled at the 8th annual ISS Research and Development Conference held in Atlanta earlier this month. This new partnership will serve as a benchmark for how the ISS National Laboratory develops similar programs in the future involving research and development activities aboard the space station.
The ISS National Laboratory and MIRM will collaborate with partners from industry, other academic research centers, and government agencies to drive the progress of regenerative medicine research onboard the ISS. As part of this alliance, Pitt will develop Earth-based facilities on campus to advance research and meet with potential partners, while working in coordination with the ISS National Laboratory on flight opportunities to the orbiting laboratory. The program will focus on microgravity life sciences research and development, with a line of sight toward products and services for clinical application on Earth.
“As the premier partner for the Industrial Biomedicine Alliance with the ISS National Laboratory, we look forward to using the space station as a testbed for regenerative medicine advances and product development in Low Earth Orbit,” said MIRM Director William R. Wagner, Ph.D.
The products of the ISS Industrial Biomedicine Program and this research partnership will help build the fundamental business case for the industrialization of crewed platforms in low Earth orbit. In future alliances, the ISS National Laboratory will work with companies and research partners who seek to better understand and find solutions to common problems on Earth through space-based experimentation on the ISS National Laboratory.
Illustration: Wikipedia
RESOURCES AT THE MCGOWAN INSTITUTE
September Histology Special
Make no Bones about it! The McGowan Histology Core has a Bone to pick FOR YOU!
The Histology lab offers decalcification, processing and embedding, cutting and staining of those difficult boney samples. Our staff has years of experience with bone samples that include special staining and IHC on sometimes difficult to work with, large and small bone samples. We have even developed a technique to insure your sample will not lift from the slide during aggressive antigen retrieval protocols.
Enjoy these beautiful examples, of just some of the special stains we offer at the McGowan Histology Core.
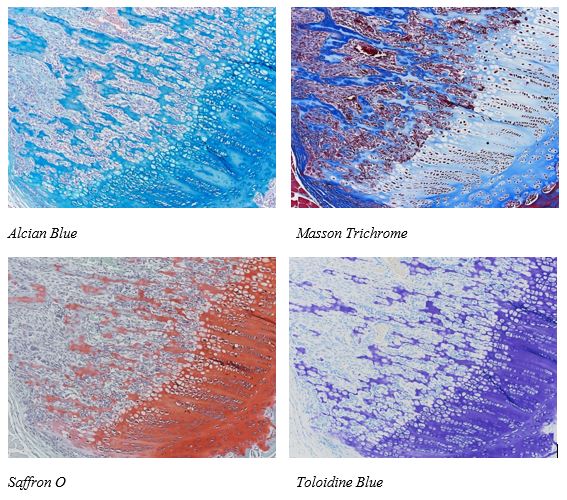
You’ll receive 25% off bone samples in September when you mention this ad. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology work with the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
Pitt First to Grow Genetically Engineered Mini Livers in the Lab to Study Disease and Therapies

Researchers at the University of Pittsburgh School of Medicine—including McGowan Institute for Regenerative Medicine affiliated faculty members Alejandro Soto-Gutierrez, MD, PhD, associate professor of pathology at Pitt’s School of Medicine and faculty member of the Pittsburgh Liver Research Center, and Ira Fox, MD, professor of surgery and pediatric transplantation at UPMC Children’s Hospital of Pittsburgh—are the first to grow genetically modified miniature human livers in the laboratory, to emulate human liver disease progression and test therapeutics.
In a proof-of-concept paper published in Cell Metabolism, Pitt researchers chronicle how they transformed genetically engineered human cells into functional, 3D liver tissue that mimics non-alcoholic fatty liver disease (NAFLD)—a condition involving fat buildup in the liver, which can lead to cirrhosis or even liver failure. With obesity rates in America climbing, NAFLD is quickly becoming the leading cause of chronic liver disease.
“This is the first time we can create genetically engineered human mini livers with a disease using stem cells in the lab,” said senior author Dr. Soto-Gutierrez.
That’s important not only for understanding what causes the disease and how it progresses, but also for testing therapeutics. It’s common for drugs to fail in clinical trials, despite promising results in mice.
For instance, the drug Resveratrol, which acts on SIRT1 proteins commonly associated with NAFLD, was effective in mouse models, but failed in human clinical trials.
“Mice aren’t humans,” Dr. Soto-Gutierrez said. “We are born with certain mutations, polymorphisms, that will predispose us to certain diseases, but you can’t study polymorphisms in mice, so making a mini customized human liver is advantageous.”
First, the researchers genetically engineered normal human skin cells to express a chemically activated switch that could tamp down the SIRT1 gene. Then, they reprogrammed the cells back to their stem cell state and turned them into liver cells. After that, they seeded the genetically engineered human liver cells into rat livers stripped of their own cells, where they blossomed into functional 3D mini livers, with blood vessels and other structural features of a normal organ.
That structure is part of what distinguishes mini livers from ‘organoid’ cultures—tiny balls of cells that self-assemble to replicate simplified organ function—although the mini livers lacked the distinct zones of metabolic function that normal livers have.
Once the mini livers were mature, the researchers flipped the genetic switch to suppress the SIRT1 gene, and the bioengineered mini livers started to mimic the metabolic dysfunction observed in tissues from patients with fatty liver disease.
But just like the clinical trials, Resveratrol wasn’t effective in the lab-grown livers either.
The key, Dr. Soto-Gutierrez explained, is that Resveratrol boosts the activity of SIRT1 proteins, not SIRT1 genes. If SIRT1 gene expression is suppressed—like it is in his bioengineered livers, and perhaps also NAFLD patients—there isn’t any protein to act on, so the drug won’t work. It’s targeting the wrong step.
“That’s an insight that could only come from studying functional human tissue,” Dr. Soto-Gutierrez said.
Genetically engineered, lab-grown mini livers provide a ready and reliable test bed for drugs at all stages of disease progression.
“These mini livers aren’t ready for clinical applications like transplantation anytime soon, but I imagine in the future we can make human livers where you can order what kind of function you want, or even enhance function,” Dr. Soto-Gutierrez added.
Several of the authors have patents on technology used in this paper (WO/2011/002926m, WO/2015/168254, PCT/US2018/018032, PCT/US2017/044719) as well as financial interests in Von Baer Wolff, Inc., a company aiming to produce iPS-derived human liver cells and treating liver failure by reprogramming therapy. The current study is not associated with Von Baer Wolff, Inc.
The co-first authors of this study are Alexandra Collin de l’Hortet, PhD, and Kazuki Takeishi, MD, PhD. Additional authors include Jorge Guzman-Lepe, MD, Kazutoyo Morita, Branimir Popovic, DVM, Yang Wang, MD, PhD, Kan Handa, MD, PhD, of Pitt; Michael Salomon, PhD, of Sirion Biotech; Chu-Xia Deng, PhD, of the University of Macau; and Deepak Nagrath, PhD, Abhinav Achreja, PhD, Anjali Mittal, Noah Meurs, Ziwen Zhu, and Frank Weinberg, MD, PhD, of the University of Michigan.
This research was supported by grants from the National Institutes of Health (DK099257, DK117881, CA227622, CA204969 and CA222251) and from the American Liver Foundation.
Grant: Role of Extracellular Matrix in Age-Related Declines of Muscle Regeneration

McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, MPT, Director of Rehabilitation for UPMC International and Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh with secondary appointments in the Departments of Physical Therapy, Bioengineering, Orthopaedic Surgery, and Microbiology & Molecular Genetics, and Carnegie Mellon University’s Philip LeDuc, PhD, William J. Brown Professor of Mechanical Engineering with appointments in Biological Sciences, Computational Biology, and Biomedical Engineering, the Founding Director of the Center for the Mechanics and Engineering of Cellular Systems, and a McGowan Institute affiliated faculty member, are the co-principal investigators on a recently awarded National Institutes of Health R01 grant entitled “Role of Extracellular Matrix in Age-Related Declines of Muscle Regeneration.”
Co-investigators of this work include McGowan Institute affiliated faculty member Antonio D’Amore, PhD, Research Assistant Professor in the Departments of Surgery and Bioengineering, and Pitt’s Aaron Barchowsky, PhD, and Claudette St. Croix, PhD.
The project abstract reads:
Skeletal muscle trauma resulting from an injury or surgery often results in significant functional declines in older adults. These declines are at least partially attributed to failed muscle healing. Muscle regeneration is predominantly dictated by the action of muscle stem, or “satellite”, cells (MuSCs), a reserve cell population that typically demonstrates considerable dysfunction with increasing age. According to the stem cell “niche” concept, stem cell responses are largely determined by biophysical and biochemical cues that emanate from the surrounding microenvironment. Indeed, expanding recognition of the influence of the microenvironment on stem cell behavior has led to a recent surge in the development of bioinspired and engineered extracellular matrix (ECM) approaches for the treatment of skeletal muscle injuries. Still lacking, however, is an in-depth knowledge of whether and how pathogenic instructional characteristics of the native ECM disrupt MuSC function and skeletal muscle regeneration. While it is evident that MuSC activation, self-renewal, proliferation and differentiation are influenced by physical and dynamic niche interactions, a mechanistic understanding of the direct impact of age-related ECM alterations on skeletal muscle regenerative capacity is unknown.
The over-arching goal of this project is to test the central hypothesis that age-related biophysical alterations in the skeletal muscle ECM promotes a fibrogenic conversion in MuSCs, ultimately driving impaired skeletal muscle regeneration. Further, the team hypothesizes that these pathogenic biophysical changes may be reverted, at least partially, by mechanical stimulation. To achieve this goal, they will employ an integrated approach that encompasses cutting-edge super-resolution imaging and 3-D tissue engineering methods to address two specific aims.
Aim 1 studies will measure, manipulate, and mimic the biophysical properties of young and aged skeletal muscle ECM in order to dissect the effect of age-related architectural and elastic ECM modifications on MuSC fate. Aim 2 studies will identify mechanisms by which mechanical stimulation modulates biophysical properties of the aged ECM to promote MuSC myogenicity and muscle regeneration. Successful achievement of these aims will further our understanding of 1) the instructional capabilities of the native ECM on MuSC lineage specification, 2) how these instructional capabilities change over time, and 3) the molecular mechanisms controlling age-related declines in skeletal muscle regenerative potential. Taken together, successful completion of these studies may provide a foundation for the identification of novel ECM targets in the treatment of skeletal muscle injuries for a geriatric population. More broadly, an improved insight into how age-associated alterations in biomechanical, architectural and dynamic ECM properties direct MuSC function will expand the fundamental understanding of aging and stem cell biology.
Congratulations, all!
New Study Highlights Advantages of Living-Liver Donation

Living-donor liver transplant offers numerous advantages over deceased-donor transplant, including better three-year survival rates for patients and lower costs, according to new research from the UPMC Thomas E. Starzl Transplantation Institute and the University of Pittsburgh School of Medicine.
The findings, published online in the Annals of Surgery, highlight living donation as a viable, if not preferable, option for the more than 14,000 people currently on the waiting list, as well as many more who never qualify to be on the list under current allocation rules.
About 8,000 liver transplants are performed each year, according to the Organ Procurement & Transplantation Network, and living-donor liver transplant comprises less than 5% of that total. Additionally, about 25% of people on the waiting list die each year waiting for a transplant, and those who eventually receive a transplant often have a lengthy period on the waiting list, resulting in poorer health at the time of transplant.
“The consequences for patients on the waiting list can mean the difference between life and death because the longer they are waiting, the sicker they become,” said Abhinav Humar, MD, chief of transplant services at UPMC, clinical director of the Thomas E. Starzl Transplantation Institute and lead author of the study. “Living-donor liver transplants, in tandem with deceased-donor liver transplants, represents an opportunity to significantly decrease the risk of wait-list mortality, and gives us the ability to transplant a person sooner.”
The retrospective review of 245 adult living-donor liver transplants and 592 deceased-donor liver transplants performed over the last 10 years at UPMC—which has the country’s largest living-donor liver transplant program—compared survival rates and other outcomes such as recovery times, complications, costs and resource utilization. The patients were followed for at least two years post-transplant.
Of those comparisons, three-year patient survival outcomes were superior in living-donor liver transplant recipients—86% versus 80%. Living-donor liver transplant recipients overall had about a 5% survival advantage over deceased-donor recipients. Patients who received a liver from a living donor had a hospital stay of 11 days as compared to 13 days for those who received a liver from a deceased donor, had less likelihood of intraoperative blood transfusion (53% compared to 78%) and less likelihood of the need for post-transplant dialysis (1.6% versus 7.4%). Hospital costs related to transplant also were 29.5% lower for living donor recipients. For the living-liver donor, there were no mortalities observed, and the overall complication rate was 20%.
“Living-donor liver transplant should be considered the first and best option for most patients with liver disease,” Dr. Humar said. “It is not only an option for those on the waiting list but could perhaps offset the fact that not everyone who may benefit from transplant qualifies to receive a deceased-donor transplant based on today’s current rules of allocation.”
Given the advantages, UPMC in recent years expanded its living-donor liver transplant program. In 2018, living-donor transplants comprised about 54% of the program’s total number of liver transplants compared to the national average of around 4%. UPMC’s transplant rate also increased from 45 out of every 100 persons on the program’s waiting list in 2015 to around 88 in 2018. The program has led the country in living-donor liver transplants the past two years and is the only center in the U.S. to perform more living- than deceased-donor transplants.
“It is unclear why the number of living-donor liver transplants is so low in the United States,” said Amit Tevar, MD, associate professor of surgery in the Pitt Department of Surgery and director of the Kidney and Pancreas Transplant Program at UPMC. “Fewer than 15 U.S. programs did more than 10 living-donor liver transplants in 2018, and this is surprising given the number of analyses nationwide like this that have now been reported.”
Additional authors on the study include Swaytha Ganesh, MD, Dana Jorgensen, PhD, MPH, Armando Ganoza, MD, Michele Molinari, MD, and Christopher Hughes, MD, all of Pitt.
Pitt Receives $6 Million for Vision Restoration Research

The University of Pittsburgh received a $6 million grant from the Richard King Mellon Foundation to support the development of a cortical vision research program in the Pitt School of Medicine Department of Ophthalmology. The program will aim to understand how the eye and the brain work together to help us see the world and use that knowledge to develop new ways to restore vision using various technologies such as brain computer interfaces and novel genetic technologies.
“The RK Mellon Foundation’s investment is a resounding vote of confidence in a world-renowned talent—Dr. José-Alain Sahel—and his team’s groundbreaking efforts to preserve and restore the gift of sight for millions of people across the world,” says Pitt Chancellor Patrick Gallagher. “I am deeply grateful for the Foundation’s support and excited to watch this next chapter in vision research and care unfold right here in Pittsburgh.”
“As the world’s population continues to grow and age, the number of individuals with visual impairments is expected to triple by the year 2050, and Pittsburgh, with its aging population, will be highly affected by this epidemic of vision loss,” said McGowan Institute for Regenerative Medicine affiliated faculty member José-Alain Sahel, MD, director of the UPMC Eye Center, and Eye and Ear Foundation chair of ophthalmology, Pitt School of Medicine. “We have established world class vision research and clinical care in Pittsburgh, and the cortical vision program will bring together the brightest minds to develop therapies that will directly benefit the people in our communities and around the world.”
Since 2016, Pitt and UPMC’s ophthalmology efforts have expanded significantly, adding 15 new clinical and research faculty. The vision research program also has established extensive collaborations with Carnegie Mellon University and an international partnership between the University of Pittsburgh and leading institutions such as the Institut de la Vision in Paris where Dr. Sahel serves as the director.
In March, UPMC broke ground on UPMC Vision and Rehabilitation at UPMC Mercy, which when completed, will provide advanced specialty clinical care to treat complex ocular diseases and innovative programs for the visually impaired, and be the home of the vision research program at Pitt and UPMC.
“This significant gift from the Richard King Mellon Foundation supports our continued efforts to make Pittsburgh a leader in vision care, and we are excited to partner with Dr. Sahel to take us toward that goal,” said Lawton Snyder, CEO of the Eye and Ear Foundation of Pittsburgh.
The new cortical vision program will have three major areas of focus—understanding the biological mechanisms of vision from the eye to the brain, developing new vision restoration therapies using cutting-edge technologies such as brain computer interfaces and optogenetics to directly stimulate the brain, and implementing vision rehabilitation programs following these restorative therapies to enhance their benefit and improve quality of life. The grant will help support the recruitment of new faculty who will establish and run research laboratories in pursuit of these goals.
In the US, Children Die Waiting for a Liver Transplant — There Is a Better Way

By Dr. George V. Mazariegos, Opinion Contributor, The Hill
“Children are the world’s most valuable resource and its best hope for the future,” President John F. Kennedy told the U.S. Committee for UNICEF in 1963. That same year, Dr. Thomas Starzl, my mentor, performed the world’s first pediatric liver transplant.
I’m excited about the government’s recent announcement of initiatives to increase kidney transplantation and to raise efficiency at all levels of the transplant process. This initiative should raise awareness of the needs of children on the waitlist and improve outcomes for patients for all organ transplants.
Working with children was a defining moment in my career. While every life is precious, to me, saving children is about preserving our future. Yet currently, one in 10 infants and one in 20 children who are awaiting liver transplants in the U.S. die while on the waitlist.
Annually, about 600 pediatric patients receive liver transplants, a fraction of the liver transplants received by adult recipients. In a five-year period, when 316 children died waiting for a liver, more than 1,500 adults were transplanted with a liver that came from a child. Almost half of all livers from children are transplanted into adults; nearly 25 percent of these livers given to adults are never offered to a child. We must do better for our children.
Children and their families awaiting transplants saw noteworthy progress in 2018, when after more than a decade of advocating for change in the liver allocation policy – during which time more than 500 children died – the Organ Procurement and Transplantation Network (OPTN) Board of Directors passed a new liver distribution policy. Under the new policy, generally, all deceased donor pediatric livers would be offered to children first before being offered to less critically ill adults. This is a life-saving game-changer for children waiting for a liver transplant.
Just before the policy was due to be implemented, several individual transplant programs and adult candidates awaiting liver transplantation filed a lawsuit alleging that the approved 2018 policy would disadvantage patients in their specific geographic areas. It was silent on the plight of children.
The 2018 policy changes went into effect May 14, 2019. Within 24 hours, the federal court ordered OPTN to reprogram to the old system, a liver allocation system that all parties concede is noncompliant with the federal regulation for organ allocation policies.
In the brief time the new policy was in effect, one of my youngest pediatric patients received part of a liver—all she needed to survive and continue to fight her disease. An adult received the other portion of that liver.
This demonstrates what the new liver policy was designed to do—increase the number of pediatric transplants and decrease the number of patients who die each year waiting. Prioritizing national sharing of pediatric donor livers for children would significantly decrease mortality for infants and children on the waitlist, without significant change for adults. Allocating to children first on the waitlist has the added benefit of increasing the likelihood of splitting a liver; allowing two patients to benefit. Reinstituting the 2018 policy would save an estimated 114 lives in the next year alone.
Under today’s policy, children lack the chance to compete against adults for livers because the calculation used to decide their place in line for a liver significantly underestimates their risk of death. To make matters worse, although there are enough pediatric donor livers to transplant all children in this country who need liver transplants, current OPTN policy dictates that these pediatric livers first be offered to adults locally rather than to sicker, more critically ill children further away.
More than 60 pediatric transplant organizations, professionals—including me—and pediatric liver transplant families want to raise awareness of these unintended consequences to children. We signed an open letter and started a petition, joined by more than 13,875 signers, to encourage the re-institution of the 2018 policy. We want to help children live their intended life.
While this currently rests with the federal courts, as a pediatric transplant surgeon, I’m an optimist. I believe the liver transplant community will recognize that what unites us far outweighs what divides us; that we can find a path to put children first and resolution outside of the courthouse.
Study: Inflammation Persists in Sepsis Survivors

One out of four sepsis patients who survive their hospital stay have elevated levels of inflammation a year after discharge, and they are at higher risk for major health problems and death, according to a study led by physician-scientists at the University of Pittsburgh School of Medicine and the Veterans Affairs Pittsburgh Healthcare System. McGowan Institute for Regenerative Medicine affiliated faculty members who are authors on this study include:
- Derek Angus, MD, MPH, FRCP, FCCM, FCCP, professor and chair of Pitt’s Department of Critical Care Medicine and director of Pitt’s Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center, and
- John Kellum, MD, FACP, FCCM, professor in the Departments of Critical Care Medicine, Medicine, Bioengineering, and Clinical and Translational Science at Pitt, director of the Center for Critical Care Nephrology and the vice-chair for research, both appointments in the Department of Critical Care Medicine, and associate director for acute illness in Pitt’s Institute for Personalized Medicine.
The results, published in JAMA Network Open, give tantalizing clues to future treatments that may improve outcomes for sepsis survivors.
Sepsis is a life-threatening condition that arises when the body’s response to an infection injures its own tissues and organs and affects more than 30 million people worldwide every year, according to the World Health Organization.
“Sepsis is the leading cause of death among hospitalized patients. Patients discharged from the hospital aren’t out of the woods yet. Approximately 1 out of every 3 sepsis survivors will die in the following year,” said lead author Sachin Yende, MD, MS, professor of critical care medicine and clinical and translational science at Pitt’s School of Medicine, and vice president of critical care and deputy chief of staff at the Veterans Affairs Pittsburgh Healthcare System. “Our new findings about chronic inflammation post-discharge suggest that addressing this condition may be important to improve patients’ long-term outcomes.”
Nearly all patients with sepsis have increased inflammation in their bloodstream during the first few days of their hospitalization. Whether this inflammation resolves or persists is poorly understood. Dr. Yende and his team followed 483 people who had been hospitalized with sepsis at one of 12 U.S. hospitals between 2012 and 2017 and survived to be discharged. Detailed information was gathered on the study participants, and they were contacted by telephone and home visits three, six and 12 months after enrollment for health interviews and a blood sample.
Approximately a quarter of the participants showed persistently elevated levels of inflammation and a half showed elevated levels of immunosuppression biomarkers up to a year after hospitalization. These patients had higher rates of readmission — particularly due to heart disease and stroke — and death compared to their peers whose inflammation and immunosuppression biomarkers had returned to normal after hospitalization.
“The participants with increased inflammation had levels that were twice as high as levels in healthy individuals and that elevated inflammation persisted long after hospital discharge,” said senior author Dr. Angus. “Sepsis increases risk of heart disease and stroke, and, for the first time, we’ve linked these adverse outcomes to persistent inflammation. This opens the door to future studies into why high levels of inflammation persist for at least a year after hospital discharge and the development of treatments aimed at modifying the inflammation with the hope that will improve health.”
The researchers cautioned that they did not have blood tests on the study participants before their sepsis diagnosis. It is possible that they had elevated levels prior to hospitalization that may have contributed to the development of sepsis and continued to persist after hospitalization.
Pitt Launches Online Master of Science in Health Informatics with Noodle Partners

The University of Pittsburgh School of Health and Rehabilitation Sciences (SHRS) today launched its first-ever online Master of Science in Health Informatics (MSHI) degree program to address the growing need for professionals who can use data to improve the quality of care.
“We recognize the inherent logistical challenges that students face today when earning their education, and we want to help facilitate that with this program,” said Anthony Delitto, PhD, dean of Pitt SHRS and McGowan Institute for Regenerative Medicine affiliated faculty member. “We’re excited and very much looking forward to the first cohort of students for this program come this fall. This is an excellent foray as we begin to offer this kind of 21st Century education.”
This new program is part of a larger effort for SHRS to bring more graduate degrees online. The Master of Science in Health Informatics program will enroll its first cohort in January 2020. The school is poised to bring three more programs online with Noodle Partners through Fall 2021.
Pitt SHRS is addressing the growing national demand for health informatics professionals to analyze trends, make projections, monitor outcomes and help improve patient care. Jobs related to health informatics are expected to grow by 20% by 2026, according to the U.S. Bureau of Labor Statistics, with a salary of $58,000 to $183,000.
“With this expected rapid job growth, there will be high demand for top-notch professionals in this field,” said Bambang Parmanto, PhD, professor and interim chair in Pitt SHRS’ Department of Health Information Management. “The Online MSHI will train professionals who can then apply their healthcare business and clinical knowledge as well as skills in informatics and analytics to improve healthcare in the digital age.”
The Online MSHI Program will have three tracks and four certificates available for students to specialize their education in addition to earning their graduate degree. These tracks include Data Science, Registered Health Information Administrator and Health Supervision Management.
The fully online program features options for internships or in-person projects within a student’s home community to further explore specific research interests. There is no requirement for students to come to campus in Pittsburgh unless desired. The on-campus version of the HI program begins this fall.
Pitt SHRS has a pedigree of producing multiple top-10 health programs, and the University is well-connected to an expansive network of health systems in the Pittsburgh area.
Pitt’s Online MSHI is the first graduate Health Informatics program to be brought online by Noodle Partners, which currently partners with over 15 top universities to host online degrees in fields like Business, Social Work, Education, and Information Technology.
“The growing demand for Health Informatics professionals makes accessible online degrees from top institutions like Pitt so important,” Noodle Partners CEO John Katzman said. “We’re building an incredible learning environment for these students.”
MechMorpho Lab Brings Computation and Experimentation Closer Together
Study: A bioengineering group from the University of Pittsburgh Swanson School of Engineering is bringing the worlds of computational modeling and experimentation closer together by developing a methodology to help analyze the wealth of imaging data provided by advancements in imaging tools and automated microscopes.
The work was overseen by McGowan Institute for Regenerative Medicine affiliated faculty member Lance Davidson, PhD, Professor and Wellington C. Carl Faculty Fellow in the Department of Bioengineering, who runs the MechMorpho Lab in the Swanson School of Engineering. The study was led by Tracy Stepien, PhD, a Pitt mathematics graduate alumnus, and Holley Lynch, PhD, a former postdoctoral associate in the MechMorpho Lab.
Their study focuses on embryonic tissue spreading, a process that is critical during wound healing and the progression of many diseases. The article, recently published in PLOS ONE, shows how using approximate Bayesian computation (ABC) – a statistical inference method – can help derive useful quantitative information for experimental design.
Dr. Davidson’s group cultured tissue from the Xenopus embryo to uncover the mechanical properties behind embryonic morphogenesis – the biological process of an organism developing its shape. During the study, they discovered that small explants spread slower than larger ones so they began creating modeling approaches to find out why.
They collected time-lapse image sequences over the course a few weeks, but the challenge when integrating modeling with experiments is determining the best set of parameters.
“As models get more complex and the experimental systems produce more data, it is difficult to determine if the chosen parameters are the optimal set,” said Dr. Stepien, a postdoctoral associate at the University of Arizona. “This is where Bayesian computation is useful – for each dataset, you can run the model thousands of times to identify sets of parameters that best match the experiment itself.”
Once the group applied a Bayesian approach to their model, they found that there was no one unique parameter set. Instead, they identified distributions of ‘almost-best’ parameters and then used statistical methods to compare the different distributions.
From the statistical analysis, they predict tissue properties such as force production and adhesion are more likely to vary with initial tissue size than factors such as rates of cell division or shape change.
“Our work provides predictive methods that can help guide more general studies of morphogenesis to better understand how tissue spreading is regulated during development and potentially control spreading during wound healing and cancer,” said Dr. Lynch, who is currently an assistant professor of physics at Stetson University.
According to the research group, these parameters may also prove to be useful for improving models of other experiments.
“Though this paper focused on one specific type of tissue movement, it is more broadly impactful in that it is trying to identify a new way forward for the scientific community,” said Dr. Davidson.
“Over several years our model went from something simple to something more rigorous and robust. A paper that originally focused on tissue spreading eventually evolved to demonstrate a framework that very closely integrates modeling and experiment.”
In the short-term, Dr. Davidson’s lab will follow-up on the predictions made in this paper and further investigate the biophysics of tissue spreading. In the long-term, the group would like to apply an equally robust statistical approach to other computational models being developed in the lab.
“We really want to integrate our experimental approaches with computational models, as robust statistically as this approach offers,” said Dr. Davidson. “Using the power of computation in conjunction with advanced biomechanical experiments could really impact our knowledge of disease development and treatment.”
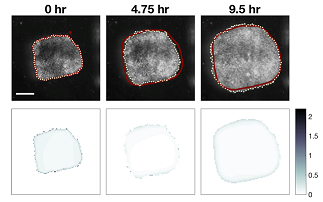
Illustration: Frames from a 10 hour long time-lapse sequence with model prediction (red line) and explant spreading (dotted line). “Good” models predict not only the extent of the movement but also the amount of mechanical strain as the tissue spreads. The images in the lower row report the error between predicted and actual strain (gradient blue) at the time-points above. Credit: MechMorpho Lab/University of Pittsburgh.
Working Towards 3D Printing the Human Heart

Researchers from Carnegie Mellon University (CMU) have published a paper in Science that details a new technique to 3D bioprint tissue scaffolds out of collagen, the major structural protein in the human body. This first-of-its-kind method brings the field of tissue engineering one step closer to being able to 3D print a full-sized organ such as an adult human heart. The work was done in the lab of Adam Feinberg, PhD, a professor of biomedical engineering (BME) and materials science & engineering at CMU. Dr. Feinberg is an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
The technique, known as Freeform Reversible Embedding of Suspended Hydrogels (FRESH), has allowed the researchers to overcome many challenges associated with existing 3D bioprinting methods, and to achieve unprecedented resolution and fidelity using soft and living materials.
Each of the organs in the human body, such as the heart, is built from specialized cells that are held together by a biological scaffold called the extracellular matrix (ECM). This network of ECM proteins provides the structure and biochemical signals that cells need to carry out their normal function. However, until now it has not been possible to rebuild this complex ECM architecture using traditional biofabrication methods.
“What we’ve shown is that we can print pieces of the heart out of cells and collagen into parts that truly function, like a heart valve or a small beating ventricle,” says Dr. Feinberg. “By using MRI data of a human heart, we were able to accurately reproduce patient-specific anatomical structure and 3D bioprint collagen and human heart cells.”
Over 4,000 patients in the United States are waiting for a heart transplant, while millions of others worldwide need hearts but are ineligible for the waitlist. The need for replacement organs is immense, and new approaches are needed to engineer artificial organs that are capable of repairing, supplementing, or replacing long-term organ function. Dr. Feinberg, who is a member of Carnegie Mellon’s Bioengineered Organs Initiative and the Next Manufacturing Center, is working to solve these challenges with a new generation of bioengineered organs that more closely replicate natural organ structures.
“Collagen is an extremely desirable biomaterial to 3D print with because it makes up literally every single tissue in your body,” explains Andrew Hudson, a BME PhD student in Dr. Feinberg’s lab and co-first author on the paper. “What makes it so hard to 3D print, however, is that it starts out as a fluid—so if you try to print this in air it just forms a puddle on your build platform. So, we’ve developed a technique that prevents it from deforming.”
The FRESH 3D bioprinting method developed in Dr. Feinberg’s lab allows collagen to be deposited layer-by-layer within a support bath of gel, giving the collagen a chance to solidify in place before it is removed from the support bath. With FRESH, the support gel can be easily melted away by heating the gel from room temperature to body temperature after the print is complete. This way, the researchers can remove the support gel without damaging the printed structure made of collagen or cells.
This method is truly exciting for the field of 3D bioprinting because it allows collagen scaffolds to be printed at the large scale of human organs. And it is not limited to collagen, as a wide range of other soft gels including fibrin, alginate, and hyaluronic acid can be 3D bioprinted using the FRESH technique, providing a robust and adaptable tissue engineering platform. Importantly, the researchers also developed open-source designs so that nearly anyone, from medical labs to high school science classes, can build and have access to low-cost, high-performance 3D bioprinters.
Looking forward, FRESH has applications in many aspects of regenerative medicine, from wound repair to organ bioengineering, but it is just one piece of a growing biofabrication field. “Really what we’re talking about is the convergence of technologies,” says Dr. Feinberg. “Not just what my lab does in bioprinting, but also from other labs and small companies in the areas of stem cell science, machine learning, and computer simulation, as well as new 3D bioprinting hardware and software.”
“It is important to understand that there are many years of research yet to be done,” adds Dr. Feinberg, “but there should still be excitement that we’re making real progress towards engineering functional human tissues and organs, and this paper is one step along that path.”
Other collaborators on the paper include co-first author Andrew Lee, a BME PhD student in Dr. Feinberg’s lab; BME postdoctoral researcher Dan Shiwarski, PhD; BME PhD students Joshua Tashman, TJ Hinton, Sai Yerneni, and Jacqueline Bliley; and BME Research Professor Phil Campbell, PhD, also a McGowan Institute affiliated faculty member.
Development of a Mantle Cell Lymphoma Cell Culture Model

Mantle cell lymphoma is an aggressive subtype of non-Hodgkin’s lymphoma that claims the lives of tens of thousands of people every year. Mantle cell lymphoma is considered incurable, even though new treatments have yielded promising results.
The combination chemotherapy regimen CHOP has a high response rate in patients. However, the cancer becomes resistant to treatment over time, and the duration of the response is often less than three years.
The development of effective treatments for patients with advanced disease is hampered by the lack of conventional cell culture and preclinical models of mantle cell lymphoma that recapitulate the chemoresistance seen in patients. The lack of chemoresistant models has also limited understanding of the mechanisms used by cells to acquire resistance.
In a study recently published in Experimental Biology and Medicine, McGowan Institute for Regenerative Medicine affiliated faculty member Kathryn Whitehead, PhD, Associate Professor in the Departments of Chemical Engineering and Biomedical Engineering at Carnegie Mellon University, and colleagues describe the generation of a chemoresistant mantle cell lymphoma cell line that mimics the low-level resistance observed in patients.
JeKO-1 cells treated with increasing concentrations of the chemotherapy cocktail CHOP developed tolerance to CHOP treatment and exhibited rapid proliferation when exposed to therapeutic levels of CHOP. These cells also exhibited increased expression of three oncogenes implicated in the development of mantle cell lymphoma–Cyclin D1, Mcl-1 and Bcl-1.
Dr. Whitehead said, “A significant challenge in cancer therapy today is a lack of models that are representative of true human disease. We are pleased to have developed a mantle cell lymphoma cell culture model that recapitulates typical levels of chemoresistance in patients.”
Vorp Lab Using Amazon Web Services to Diagnose/Treat Abdominal Aortic Aneurysms

McGowan Institute for Regenerative Medicine affiliated faculty member David Vorp, PhD, associate dean for research at Pitt’s Swanson School of Engineering and the John A. Swanson Professor of Bioengineering, and his team are using Amazon Web Services (AWS) resources to improve the diagnosis and treatment of abdominal aortic aneurysms, the 13th-leading cause of death in western countries. New machine learning technologies and advances in computing power, like those offered by Amazon SageMaker and Amazon EC2, are making it possible to rapidly translate insights discovered in the lab into treatments and services that could dramatically improve human health.
Currently, clinicians can use only the simple measurements of an aneurysm’s diameter and growth rate to predict the risk of a rupture. “With the latest advances in machine learning, we are developing an algorithm that will provide clinicians with an objective, predictive tool to guide surgical interventions before symptoms appear, improving patient outcomes,” said Dr. Vorp.
In the latest sign of Pittsburgh’s growing importance as a center of health care technology innovation, the Pittsburgh Health Data Alliance (PHDA)—a unique consortium formed four years ago by UPMC, the University of Pittsburgh and Carnegie Mellon University—announced recently that it is working closely with AWS, an Amazon.com company, through a machine learning research sponsorship, to advance innovation in areas such as cancer diagnostics, precision medicine, voice-enabled technologies and medical imaging.
The PHDA uses the “big data” generated in health care — including patient information in the electronic health record, diagnostic imaging, prescriptions, genomic profiles and insurance records — to transform the way that diseases are treated and prevented, and to better engage patients in their own care.
Introducing Regenerative Medicine to Future Scientists
Bryan Brown, PhD, Director of Educational Outreach at the McGowan Institute, had the opportunity during the summer recess to introduce twenty students from around the country to biomedical science and research methodology in regenerative medicine, and it looks like he “hit a home run.” Dr. Brown organized the 6th annual regenerative medicine summer school, and – new this year – the McGowan Institute also offered paid internships to ten of the students. These 8-week internships were in the lab of one of several McGowan affiliated faculty members. As a result of the generosity of the Bayer USA Foundation, there was no cost to the summer school students and stipends were provided for the interns.
The summer school is aimed to provide national and regional students with a week-long didactic and experiential learning experience addressing the science and engineering related to the multidisciplinary field of regenerative medicine. Dr. Brown describes the summer school as a “regenerative medicine boot camp” to introduce undergraduate students to the topics addressed as well as the multidisciplinary nature of regenerative medicine. The internships following the summer school week gave students the opportunity to work in an active lab. Some of the students spoke about their experiences and impressions.
 Ashley Kennedy is a junior at Northeastern State University in Tahlequah, Oklahoma. She is majoring in Cell and Molecular Biology, with a minor in Bioinformatics. She learned about the program through the Honors Department at her college.
Ashley Kennedy is a junior at Northeastern State University in Tahlequah, Oklahoma. She is majoring in Cell and Molecular Biology, with a minor in Bioinformatics. She learned about the program through the Honors Department at her college.
Ashley’s mentor for the internship was Kacey Marra, PhD, Professor in the Departments of Plastic Surgery, School of Medicine (primary) and Bioengineering, School of Engineering (secondary) at the University of Pittsburgh; and Vice Chair of Research, Department of Plastic Surgery. Under Dr. Marra’s supervision, Ashley worked on the project, Making Polycaprolactone Nerve Guides Reproducible. This was her third research lab internship, but it was both the first biomaterials lab and the largest lab that she had experienced.
“I found the experience of being in such a large lab and seeing how everyone collaborates on different projects to be the most rewarding part of the internship. I felt very included and enjoyed being able to design my own experiments,” Ashley said. “I feel like this will be a beneficial experience to take into my future schooling because it will help me recognize lab expenses, make me a better collaborator, and give me ideas for troubleshooting any engineering-based projects I may run into. All in all, I had a lot of fun this summer.”
 Matias Malkamaki is a sophomore at Mount Vernon Nazarene University in Mount Vernon, Ohio. He is a Chemistry and Biology double major. A mentor at his university suggested that he apply to the summer school/internship.
Matias Malkamaki is a sophomore at Mount Vernon Nazarene University in Mount Vernon, Ohio. He is a Chemistry and Biology double major. A mentor at his university suggested that he apply to the summer school/internship.
Matias worked in the lab of Nam Vo, PhD, Associate Professor in the Department of Orthopaedic Surgery with a secondary appointment in the Department of Pathology at the University of Pittsburgh. Under Dr. Vo’s guidance, Matias worked on three projects: Expanding Rat Behavioral Study Through Testing the Viability of a Homecage Monitoring System; Developing a Technique for Consistently Injecting One Microliter of Solution into a Rat Tail Disc; and Optimization of Cell Culture Conditions to Maintain Disc Nucleus Pulposus Cell Phenotype.
“The most rewarding experience was being a part of the ideation process in which people are simply bouncing ideas off of each other and seeing where those ideas take them. Also, having someone in the lab see me and decide that they would take time out of their day to impart knowledge and skills to me was rewarding,” Matias said. “I now have a better understanding of the value of the scientific process, and I also better understand what it takes to go from an idea to the bedside (in a medical situation) or other real-world application. I have also gained many team-based skills necessary in an academic setting.”
 Valerie Flores is a junior at Stephen F. Austin State University in Nacogdoches, Texas. She learned about the summer program through her health professions advisor and then received more information from Dr. Brown directly. Valerie worked in the lab of Jelena Janjic, PhD, Associate Professor of Pharmaceutics (with tenure) in the Graduate School of Pharmaceutical Sciences and Mylan School of Pharmacy at Duquesne University. Dr. Janjic is also the Founder and Co-Director of the Chronic Pain Research Consortium at Duquesne. Valerie worked on the project Effects of Nanoparticles on Macrophages in vitro.
Valerie Flores is a junior at Stephen F. Austin State University in Nacogdoches, Texas. She learned about the summer program through her health professions advisor and then received more information from Dr. Brown directly. Valerie worked in the lab of Jelena Janjic, PhD, Associate Professor of Pharmaceutics (with tenure) in the Graduate School of Pharmaceutical Sciences and Mylan School of Pharmacy at Duquesne University. Dr. Janjic is also the Founder and Co-Director of the Chronic Pain Research Consortium at Duquesne. Valerie worked on the project Effects of Nanoparticles on Macrophages in vitro.
“The most rewarding part about my summer would have to be the variety of skills I have gained from this experience. Before this lab exposure, I was not well rounded on lab techniques; however, I have broadened certain skills such as cell culturing, cell viability, and cell exposure,” Valerie said. “I know that this internship will be valuable to my studies not only for my newly attained skills but also for the exposure of what being a part of the research is all about. I have learned the process behind getting research started and the process of ending a research project. Many valuable lessons came from this summer, and I am very grateful to have had this opportunity.”
Dr. Brown extends his thanks and appreciation to all of the McGowan faculty, trainees, and staff for their contributions to the summer school and internship programs, and to the Bayer USA Foundation whose generosity permitted this year’s program to be offered to the participating students at no cost to them, Also our we extend our appreciation to the Society for Biomaterials and the Tissue Engineering and Regenerative Medicine International Society (TERMIS) for their endorsement of the program.
AWARDS AND RECOGNITION
Top Dentists Honored in Pittsburgh Magazine

“If you had a patient in need of a dentist, which dentist would you refer them to?”
This is the question asked to thousands of dentists to help determine who the topDentists should be. Dentists and specialists are asked to take into consideration years of experience, continuing education, manner with patients, use of new techniques and technologies, and of course physical results. Pittsburgh Magazine’s annual list, which contains 398 dentists across 12 specialties, includes three McGowan Institute for Regenerative Medicine affiliated faculty members:
Endodontics
- Herbert Ray, Jr., DMD, Assistant Professor of Endodontics and the Director, Graduate Endodontic Residency Program, University of Pittsburgh School of Dental Medicine
Oral and Maxillofacial Surgery
- William Chung, MD, DDS, Professor in the Department of Oral and Maxillofacial Surgery, School of Dental Medicine, University of Pittsburgh
- Bernard Costello, MD, DMD, FACS, Dean of the University of Pittsburgh School of Dental Medicine, Professor of Oral and Maxillofacial Surgery, Chief of Pediatric Oral and Maxillofacial Surgery at the Children’s Hospital of Pittsburgh, and a surgeon with the Cleft and Craniofacial Center
The nomination pool of dentists consists of dentists listed online with the American Dental Association as well as all dentists listed online with their local dental societies, thus allowing virtually every dentist the opportunity to participate. Dentists are also given the opportunity to nominate other dentists that they feel should be included in the list.
topDentists was started with the intent to identify the best dentists and specialists in the country and is the only list of its kind, chosen by the dental profession themselves. topDentists has over fifty years in combined experience compiling peer-review referral guides in the legal, dental and medical fields. Using this experience along with the input of several prominent dentists in the United States, topDentists has created a selection process that has been refined and improved over previous superlative guides. Because dentists must be voted in strictly by their peers, and virtually every dentist is given an opportunity to participate, and listings cannot be purchased (and no payment is required to be listed), inclusion in topDentists is considered a distinct honor.
Congratulations, all!
Dr. Mary Amanda Dew Honored with 2019 Bud Orgel Award from the Association of Psychologists in Academic Health Centers

McGowan Institute for Regenerative Medicine affiliated faculty member Mary Amanda Dew, PhD, Professor of Psychiatry, Psychology, Epidemiology, Biostatistics, and Clinical and Translational Science has been awarded the 2019 Bud Orgel Award for Distinguished Achievement in Research from the Association of Psychologists in Academic Health Centers.
The award recognizes the outstanding contributions of psychologists to the advancement of research and scholarship in medical school and health care settings.
Dr. Dew is the director of the clinical epidemiology program at UPMC Western Psychiatric Hospital (WPH). At the University of Pittsburgh Medical Center, she is Director, Quality of Life Research, Artificial Heart Program in Adult Cardiothoracic Transplantation.
Dr. Dew is known for an outstanding research career evaluating risk factors for mental health and behavior problems in organ transplantation populations including organ recipients, living donors and families, as well as testing interventions to improve psychosocial and medical outcomes. She has also mentored numerous trainees in transplantation psychiatry and psychology.
Congratulations, Dr. Dew!
Illustration: University of Pittsburgh Department of Psychiatry.
Dr. David Vorp Receives Research Leader Fellowship from APLU

The Association of Public and Land-Grant Universities (APLU) Council on Research (CoR) has named McGowan Institute for Regenerative Medicine affiliated faculty member David Vorp, PhD, as one of eight fellows in its third Research Leader Fellowship Program cohort selected nationwide. Dr. Vorp is Associate Dean for Research for Pitt’s Swanson School of Engineering, the John A. Swanson Professor of Bioengineering, and Professor of Cardiothoracic Surgery, Surgery, Chemical & Petroleum Engineering, and the Clinical and Translational Sciences Institute.
According to APLU, the CoR Research Leadership Program is designed to provide training and skill development necessary in the breadth and depth of the academic research enterprise. The APLU notes that because many universities have segmented research support organizations, rising research leaders often oversee relatively confined areas such as research administration, research development, research compliance, research communication, economic development, or sponsored programs.
The APLU CoR Fellowship is designed to allow rising research leaders to gain expertise outside of their respective portfolios and to foster connections with CoR’s extensive network of senior research officers through site visits and participation in CoR meetings. The fellowship is 18 months in duration.
“This is a tremendous opportunity for me that I will ensure also greatly benefits Pitt and the Swanson School,” Dr. Vorp said. “In the past few years we have expanded and diversified our research portfolio, increased our public-private research partnerships through the creation of our Making Research Work initiative, and more. But there is so much more that we can do, and I’m excited to see up-close the best practices and novel programs developed by other research universities and learn from the best minds in the business.”
During his fellowship, Dr. Vorp intends to focus on working more closely with Pitt’s Office of Community and Governmental Relations; integrating research data and analytics into proactive planning and research portfolio management; and developing more sustainable revenue models for the Swanson School’s several research centers and institutes. He also plans to investigate how the Swanson School can play a greater role in regional economic development as well as develop stronger multidisciplinary and sponsored research programs.
Congratulations, Dr. Vorp!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#200 –– Dr. José-Alain Sahel discusses his research in optogenetic vision restoration.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Collin de l’Hortet A, Takeishi K, Guzman-Lepe J, Morita K, Achreja A, Popovic B, Wang Y, Handa K, Mittal A, Meurs N, Zhu Z, Weinberg F, Salomon M, Fox IJ, Deng CX, Nagrath D, Soto-Gutierrez A
Title: Generation of Human Fatty Livers Using Custom-Engineered Induced Pluripotent Stem Cells with Modifiable SIRT1 Metabolism
Summary: The mechanisms by which steatosis of the liver progresses to non-alcoholic steatohepatitis and end-stage liver disease remain elusive. Metabolic derangements in hepatocytes controlled by SIRT1 play a role in the development of fatty liver in inbred animals. The ability to perform similar studies using human tissue has been limited by the genetic variability in man. We generated human induced pluripotent stem cells (iPSCs) with controllable expression of SIRT1. By differentiating edited iPSCs into hepatocytes and knocking down SIRT1, we found increased fatty acid biosynthesis that exacerbates fat accumulation. To model human fatty livers, we repopulated decellularized rat livers with human mesenchymal cells, fibroblasts, macrophages, and human SIRT1 knockdown iPSC-derived hepatocytes and found that the human iPSC-derived liver tissue developed macrosteatosis, acquired proinflammatory phenotype, and shared a similar lipid and metabolic profiling to human fatty livers. Biofabrication of genetically edited human liver tissue may become an important tool for investigating human liver biology and disease.
Source: Cell Metabolism. 2019 Aug 6;30(2):385-401.e9.
GRANT OF THE MONTH
PI: Fabrisia Ambrosio
Co-PI: Philip LeDuc
Co-Investigators: Antonio D’Amore, Aaron Barchowsky, and Claudette St. Croix
Title: Role of Extracellular Matrix in Age-Related Declines of Muscle Regeneration
Description: Skeletal muscle trauma resulting from an injury or surgery often results in significant functional declines in older adults. These declines are at least partially attributed to failed muscle healing. Muscle regeneration is predominantly dictated by the action of muscle stem, or “satellite”, cells (MuSCs), a reserve cell population that typically demonstrates considerable dysfunction with increasing age. According to the stem cell “niche” concept, stem cell responses are largely determined by biophysical and biochemical cues that emanate from the surrounding microenvironment. Indeed, expanding recognition of the influence of the microenvironment on stem cell behavior has led to a recent surge in the development of bioinspired and engineered extracellular matrix (ECM) approaches for the treatment of skeletal muscle injuries. Still lacking, however, is an in-depth knowledge of whether and how pathogenic instructional characteristics of the native ECM disrupt MuSC function and skeletal muscle regeneration. While it is evident that MuSC activation, self-renewal, proliferation and differentiation are influenced by physical and dynamic niche interactions, a mechanistic understanding of the direct impact of age-related ECM alterations on skeletal muscle regenerative capacity is unknown.
The over-arching goal of this project is to test the central hypothesis that age-related biophysical alterations in the skeletal muscle ECM promotes a fibrogenic conversion in MuSCs, ultimately driving impaired skeletal muscle regeneration. Further, the team hypothesizes that these pathogenic biophysical changes may be reverted, at least partially, by mechanical stimulation. To achieve this goal, they will employ an integrated approach that encompasses cutting-edge super-resolution imaging and 3-D tissue engineering methods to address two specific aims.
Aim 1 studies will measure, manipulate, and mimic the biophysical properties of young and aged skeletal muscle ECM in order to dissect the effect of age-related architectural and elastic ECM modifications on MuSC fate. Aim 2 studies will identify mechanisms by which mechanical stimulation modulates biophysical properties of the aged ECM to promote MuSC myogenicity and muscle regeneration. Successful achievement of these aims will further our understanding of 1) the instructional capabilities of the native ECM on MuSC lineage specification, 2) how these instructional capabilities change over time, and 3) the molecular mechanisms controlling age-related declines in skeletal muscle regenerative potential. Taken together, successful completion of these studies may provide a foundation for the identification of novel ECM targets in the treatment of skeletal muscle injuries for a geriatric population. More broadly, an improved insight into how age-associated alterations in biomechanical, architectural and dynamic ECM properties direct MuSC function will expand the fundamental understanding of aging and stem cell biology.
Source: National Institute on Aging
Term: August 1, 2019 – April 30, 2020
Amount: $474,362
