October 2019 | VOL. 18, NO. 10| www.McGowan.pitt.edu
The State of Cell-Based Therapies for Arthritis and Osteoporosis

A new report highlights the latest advances in cell-based therapies for the treatment of disorders of the musculoskeletal system, such as arthritis and osteoporosis, and it identifies key unanswered questions that should be addressed through ongoing research. The report is published in the Journal of Bone and Mineral Research and concurrently in the Journal of Orthopaedic Research, and was issued by a joint Task Force of the American Society for Bone and Mineral Research and the Orthopaedic Research Society. McGowan Institute for Regenerative Medicine affiliated faculty member Rocky Tuan, PhD, Vice-Chancellor and President of The Chinese University of Hong Kong and the former Associate Director of the McGowan Institute, is the co-chair of the Task Force.
With cell-based therapies, cells are injected, grafted, or implanted into a patient. Due to the lack of rigorous clinical studies and randomized clinical trials, however, these treatments should be considered experimental. The Task Force provides specific recommendations and ethical considerations for preclinical and clinical investigations of cell-based therapies, and it highlights the importance of determining the direct and indirect effects of these therapies on disease.
“This is an area of enormous public interest and scientific importance,” said lead author and task force co-chair Regis O’Keefe, MD, PhD, of Washington University School of Medicine in St. Louis. “Musculoskeletal diseases such as osteoarthritis, intervertebral disc degeneration, and tendinopathies cause pain, impair function, and can lead to a sense of helplessness. Currently, a large number of unproven cell-based therapies are marketed to a vulnerable population of patients that suffer from musculoskeletal disease.”
“The goal of the task force was to provide, in an unbiased way, a balanced assessment of the current state of cell-based therapies and to define the scientific agenda needed to develop more proven and effective approaches to treatment in the future,” said Dr. Tuan.
RESOURCES AT THE MCGOWAN INSTITUTE
November Histology Special
Reticular fibers, or reticulin is a type of fiber in connective tissue, composed of type III collagen secreted by reticular cells. Reticular fibers crosslink to form a fine meshwork (reticulin). This network acts as a supporting mesh in soft tissues such as liver, bone marrow, and the tissues and organs of the lymphatic system.
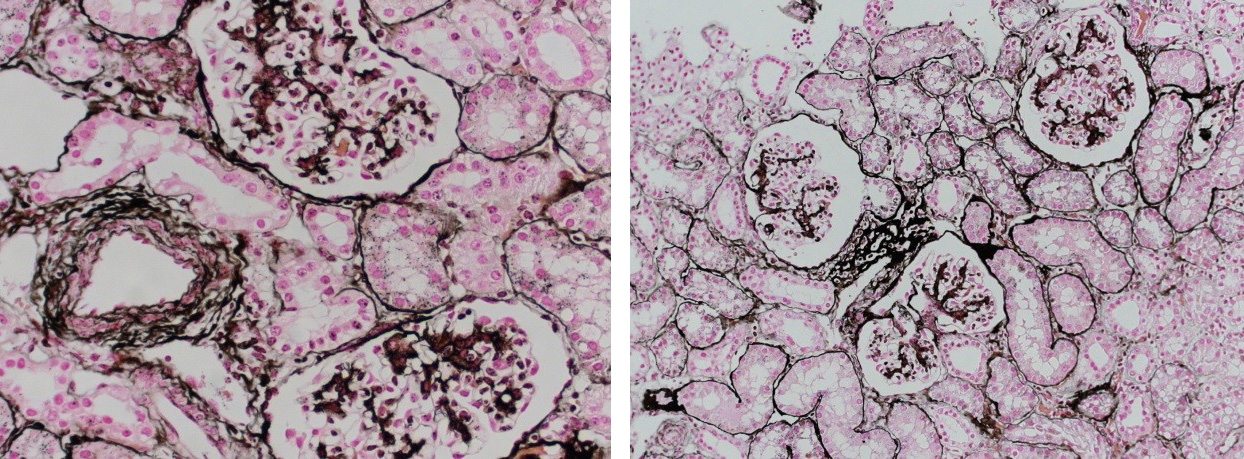
Jones Silver Stain is used to identify basement membrane and reticulin.
You’ll receive 25% off Jones Silver Stain in November when you mention this ad. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology work with the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
LifeX Office Hours
 The McGowan Institute is excited to announce a new initiative with LifeX. LifeX Labs is offering McGowan innovators and entrepreneurs the opportunity to consult with experienced and trusted service providers at no cost. These 30-minute meetings are open to all McGowan faculty and their research team and are being scheduled for the second Friday of each month at the McGowan Institute. To find more information or to schedule your session please click here.
The McGowan Institute is excited to announce a new initiative with LifeX. LifeX Labs is offering McGowan innovators and entrepreneurs the opportunity to consult with experienced and trusted service providers at no cost. These 30-minute meetings are open to all McGowan faculty and their research team and are being scheduled for the second Friday of each month at the McGowan Institute. To find more information or to schedule your session please click here.
Save the Date!
Save the dates of March 9 and 10, 2020 for the McGowan Institute Scientific Retreat. The program committee under the leadership of Julie Phillippi, PhD is developing an exciting program. Updates will follow soon.
SCIENTIFIC ADVANCES
McGowan Institute Teams Win Big at PInCH

The Pitt Innovation Challenge (PInCh®) 2019 focused on bold solutions to an important health problem. The final pitches from teams hoping to win this year’s competition, sponsored by the Clinical & Translational Science Institute, were heard on September 25, 2019, at the University Club. The following winning teams included the efforts of McGowan Institute for Regenerative Medicine affiliated faculty members.
These teams won $100,000:
- CyteSolutions Lens: Alexis Nolfi; Vishal Jhanji, MD; Mangesh Kulkarni, PhD; Bryan Brown, PhD. A silicone-hydrogel-based contact lens that has been coated with natural biopolymers containing an immune modifying drug for the treatment of dry eye disease.
- OneValve: Garrett Coyan, MD (Wagner Lab); Antonio D’Amore, PhD; William Wagner, PhD. Self-regenerating heart valve that uses the patient’s natural healing process to replace diseased heart valves, decreasing the risk of blood clots and improving durability over current therapy.
These teams won $25,000:
- Good Vibrations: Brianna Perry; Kevin Quinn; Goeran Fiedler, PhD; Mary-Ann Miknevich, MD; David Brienza, PhD. A novel vibration therapy device for treating residual/phantom limb pain and muscle atrophy in amputees.
- Push-to-Spin: Jeffrey Gusenoff, MD; Beth Gusenoff, DPM; Lauren Kokai, PhD; David Aliberti; Jason Morton. A novel device to make surgical fat grafting procedures more efficient. This device allows aspiration of fat, centrifugation, and reinjection of fat back into a patient in a single device without contamination.
Teams entered the challenge by submitting a two-minute video explaining their idea to improve human performance. Advancing teams submitted a three-page project proposal and budget for round two. Proposals were reviewed by a team of experienced reviewers with expertise and background in areas including clinical medicine, basic and clinical research, and innovation. Finalists selected for round three submitted a response to reviewer comments, made revisions, and prepared a pitch. The funds will be used for direct costs and project management support to help execute a 12-month project to take the team’s solution one step farther along the path of development.
View all of the 2019 PInCH winners and their corresponding project videos here.
Congratulations, all!
Illustration: Clinical & Translational Science Institute.
McGowan Institute’s Pediatric VAD Technology, PediaFlow, to One Day Help the Smallest of Heart Transplant Candidates
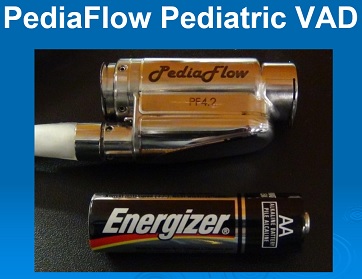
The recent Pittsburgh Post-Gazette newspaper article by Jill Daly highlights the significant challenges infants and children face if they require a heart transplant.
According to an article recently published in the American Journal of Transplantation, “pediatric heart transplant candidates face the highest waitlist mortality of all solid organ transplant candidates, in part due to limited organ availability.” “Although there has been an increase in the number of pediatric heart transplantations performed annually in the United States, this has not overcome the growing population of candidates.” Further, according to this journal article, “there has been no decrease in overall waitlist mortality, in spite of recent changes in US pediatric heart allocation policy.”
What this means for many infants and small children, like Dean in the Pittsburgh news article, for whom a donor heart was not readily available, is that a ventricular assist device (VAD) is used to help these patients survive the wait for a donor heart. In the Unites States, there is only one VAD approved by the Food and Drug Administration (FDA) for cardiac support of children, and that device is the Berlin Heart EXCOR … the VAD that was implanted in Dean. As described in the news article, the Berlin Heart EXCOR poses limitations for pediatric patients, which is not unexpected given that the Berlin Heart EXCOR is 30-year old technology.
To address this current limitation of pediatric VAD technology, pediatric cardiologists, pediatric cardiac surgeons, and bioengineers at the McGowan Institute for Regenerative Medicine have developed a miniaturized, magnetically levitated, rotary VAD that can provide the blood flow needs for infants and small children with congenital and/or acquired cardiac disease. This VAD, known as PediaFlow, is the size of a AA-cell battery; and as reported in The Journal of Thoracic and Cardiovascular Surgery, has demonstrated excellent biocompatibility in pre-clinical testing which is an essential condition for any VAD intended to support the failed circulation of these most special patients. The ultimate goal is to make the PediaFlow developed at the McGowan Institute available to patients like Dean so that their wait for a heart transplant is associated with a good quality-of-life for them and their loved ones.
Illustration: PediaFlow. McGowan Institute for Regenerative Medicine.
Revealed: How Tooth Enamel Is Strong Enough to Last a Lifetime
Break any bone in the human body and the body can repair the tissue and fix the damage. Yet tooth enamel — the strongest tissue in the human body — cannot repair itself. Still, our teeth last a lifetime.
“We apply huge pressure on tooth enamel every time we chew, hundreds of times a day,” says Pupa Gilbert, PhD, professor of physics at the University of Wisconsin (UW)–Madison. “Tooth enamel is unique in that it has to last our entire lifetime. How does it prevent catastrophic failure?”
In new research published in the journal Nature Communications, Dr. Gilbert and her collaborators—including MIT engineering Professor Markus Buehler, PhD, and McGowan Institute for Regenerative Medicine affiliated faculty member and University of Pittsburgh oral biology Professor Elia Beniash, PhD—used advanced imaging techniques to see a clearer picture of the organization of individual enamel crystals in human teeth. They found that these crystals are not perfectly aligned, as had been previously thought, and that this misorientation likely deflects cracks, leading to enamel’s lifelong strength.
Dr. Beniash uses synchrotron time to study enamel organization at different scales. The team applied this methodology to human dental enamel and found a number of previously unknown features which might explain to some extent the unusual mechanical properties of enamel.
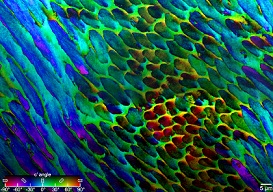
“Prior to this study, we just didn’t have the methods to look at the structure of enamel,” Dr. Gilbert says. “But with a technique that I previously invented, called polarization-dependent imaging contrast (PIC) mapping, you can measure and visualize in color the orientation of individual nanocrystals and see many millions of them at once. The architecture of complex biominerals, such as enamel, becomes immediately visible to the naked eye in a PIC map.”
Tooth enamel is organized in micron-length rods made up of long, skinny crystals of hydroxyapatite. Dr. Gilbert and her group at UW–Madison applied PIC mapping to several human tooth samples and measured the orientation of each crystal in tooth cross sections.
“By and large, we saw that there was not a single orientation in each rod, but a gradual change in crystal orientations between adjacent nanocrystals,” Dr. Gilbert says. “And then the question was, ‘Is this a useful observation?’”
To address that question, Dr. Gilbert collaborated with Dr. Buehler to perform computer simulations of chewing-like force to hydroxyapatite crystals. In the simulations, two blocks of crystals were placed together. Within each block, the individual crystals were aligned. But where they met — at the crystal interface — their orientation was rotated at different angles. The researchers then modeled the chewing force and watched how a crack propagated toward and through the interface.
When the two sides were perfectly aligned — crystals in both blocks had the same orientation — the crack propagated straight through the interface. When the blocks were rotated about 45 degrees from each other, the crack also went straight through the interface. But at a smaller angle, the crack was deflected by the interface.
“I started wondering, is there an ideal misorientation angle that is most effective at deflecting cracks?” Dr. Gilbert recalls. “The experiment to test this hypothesis could not be done at the nanoscale, nor by simulations, so I started thinking, okay, we trust evolution. If there is an ideal angle of misorientation, I bet it’s the one in our mouths.”
Cayla Stifler, a physics graduate student in Dr. Gilbert’s group and co-author of the study, went back to the PIC mapping data and measured the angular distance between every two adjacent pixels, generating millions of data points. She found that 1 degree was the most common misorientation angle, and that the angular distance never surpassed 30 degrees, consistent with the modeling result that a small misorientation angle is better than a larger one at deflecting cracks.
PIC mapping could be applied to teeth in the fossil record to observe trends in enamel evolution over time, or to compare enamel structures between animals to relate structure to function, such as how tooth structure differs between plant eaters and omnivores.
“Now we know that cracks are deflected at the nanoscale and thus can’t propagate very far,” says Dr. Gilbert. “That’s the reason our teeth can last a lifetime without being replaced.”
Illustration: PIC mapping, which measures biomineral crystal orientations and assigns different colors to different rotation angles, reveals that the crystals in tooth enamel are not perfectly aligned. Courtesy of Dr. Pupa Gilbert.
β-blockers Build Heart Muscle

Surgery can mend congenital heart defects shortly after birth, but those babies will carry a higher risk of heart failure throughout the rest of their lives. Yet, according to a Science Translational Medicine study published by UPMC Children’s Hospital of Pittsburgh researchers, β-blockers could supplement surgery to regenerate infant heart muscle and mitigate the lasting effects of congenital heart disease.
“The question is no longer ‘can we save this baby?’” said senior author Bernhard Kühn, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, director of the Pediatric Institute for Heart Regeneration and Therapeutics at UPMC Children’s Hospital, and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “The challenge for our young patients is that we want to enable them to have a long lifespan, ideally as long as a person without heart disease.”
For a relatively common congenital heart defect called Tetralogy of Fallot, treatment typically involves surgery at around 3-6 months of age, which is incidentally when heart muscle cells — cardiomyocytes — are at peak production. Decreased heart function during the first few months may be causing these infants to miss an essential opportunity to build heart muscle.
Dr. Kühn’s team collected heart tissue from 12 infants who underwent corrective surgery for Tetralogy of Fallot and found that more than half of the cardiomyocytes in these samples had started to divide but then got stalled midway through the process, like conjoined twins. The ultimate result was fewer cardiomyocytes overall, which makes the heart more vulnerable to damage later.
“By the time our surgeons operate on these patients, the horse is already out of the barn,” Dr. Kühn said. “Our data show that they have up to 30% fewer cardiomyocytes than a normal infant has at this age. That’s significant. To put that in context, an adult’s heart attack can destroy up to 30% of cardiomyocytes.”
Through a series of experiments in human and mouse tissue, the researchers traced this cell division failure back to β-adrenergic receptors.
The natural next step was to ask whether the β-adrenergic receptor blocker propranolol — a common blood pressure medication — could stimulate proper cell division in infants with congenital heart defects and improve heart function.
Indeed, in the heart tissue samples taken from infants with Tetralogy of Fallot, propranolol enabled dividing cells to separate properly.
And in mice, propranolol treatment during the first weeks of life allowed for better recovery from heart attacks in adulthood. Compared to untreated controls, mice who were given propranolol as pups retained 30% more cardiomyocytes and were able to eject 24% greater blood volume following a heart attack.
According to Dr. Kühn, having such promising results with a tried-and-true drug like propranolol means the pathway to clinical translation could be relatively quick.
“This all comes together in a very applicable way,” Dr. Kühn said. “Propranolol was synthesized nearly 60 years ago, so we’re able to bypass a lot of the ground work that would have to be done if we had identified a receptor that doesn’t have a drug for it.”
$1.5 Million to Study the Mechanism of Osteoarthritis Pain

McGowan Institute for Regenerative Medicine affiliated faculty members Hang Lin, PhD, and Douglas Weber, PhD, received $1.5 million from the NIH to study the mechanism of osteoarthritis pain and test drugs in a novel microphysiological tissue chip (microJoint). The team will use the microJoint to also assess the impact of opioids on the neural activity and tissue health in the knee joint.
The grant is entitled “Joint Pain-on-a-Chip: Mechanistic Analysis Therapeutic Targets and an Empirical Strategy for Personalized Pain Management” and will run for 2 years, 2019-2021. The research team includes:
- Multi-PI: Hang Lin, Assistant Professor in Department of Orthopaedic Surgery
- Multi-PI: Michael Gold, Professor in Department of Neurobiology
- Co-I: Douglas Weber, Associate Professor in Bioengineering and the Director of Rehab Neural Engineering Labs
The project abstract follows:
Life’s wear and tear can leave joints damaged. All too often, the result is the pain and disability of osteoarthritis (OA). Because pain is the most debilitating symptom of OA, it remains the primary target for therapeutic interventions that typically progress from non-steroidal anti-inflammatory drugs (NSAIDs) to weak opioids and then to stronger opioids. If pain can’t be controlled, total joint replacement surgery remains the only viable long-term treatment option. However, the associated risks and costs will necessarily keep surgery an option of last resort. Thus, there is a critical need for the development of safe and effective methods for the treatment of OA-associated pain.
Recently, the team successfully developed an in vitro, multi-component joint-on-a-chip (microJoint), in which engineered osteochondral complexes, synovium, and adipose tissues were integrated. OA-like pathology has also been successfully modeled in the microJoint. In this new grant application, they propose to introduce sensory innervation into the microJoint. The result will be a more “complete” joint that enables the dynamic interplay between the peripheral nervous system and joint tissues.
The researchers hypothesize that a distinct combination of factors released from different cellular/tissue compartments within the joint mediate pain, hypersensitivity, and hyperinnervation of OA. Furthermore, they hypothesize that opioids not only increase the rate of joint degeneration but potentiate the release of pain producing mediators.
- In aim 1, a neuron-containing microfluidic ally will be developed to innervate the current microJoint (Neu-microJoint). This new bioreactor will be 3D printed and allow for the non-invasive assessment of neural activity via multi electrode arrays embedded in the tissue chamber and high-speed optical recording. Human sensory neurons or induced pluripotent stem cell (iPSC)-derived sensory neuron progenitors, will be cultured in the new bioreactor chamber.
- In aim 2, the previously established OA-model will be created in the Neu-microJoint system. We will assess the activation and/or sensitization of nociceptive afferents with electrophysiology as well as neurite outgrowth.
- In aim 3, they will mechanically insult the Neu-microJoint and assess the emergence of “pain” in response to prolonged mechanical stress of the joint with the goal of creating a more natural OA-model.
- In aim 4, the researchers will assess the impact of drugs used clinically for the management of OA on our OA models in the Neu-microJoint as a means of validating this platform for the assessment of therapeutic efficacy and toxicity of novel compounds. We will then use “omic” approaches to identify new biomarkers and therapeutic targets. Results from this unbiased screen may not only reveal an injury specific pain signature, but suggest medications approved for use in people that could be re-purposed for the treatment of OA pain.
- In aim 5, they will assess the impact of opioids on neural activity in the presence and absence of joint injury triggered with different stimuli as well as the integrity of all joint elements.
The team’s Neu-microJoint will enable identification of mechanisms responsible for pain associated with joint injury and therefore novel therapeutic targets, screening of therapeutic interventions, and the implementation of personalized therapeutic strategies.
Potential Treatment of Metastatic Solid Tumors Receives Funding Boost

Oncorus, Inc., an oncolytic virus company focused on driving innovation to transform outcomes for cancer patients, announced recently the completion of an oversubscribed $79.5 million Series B financing. Oncorus is a University of Pittsburgh spin-out company based on the pioneering research of McGowan Institute for Regenerative Medicine affiliated faculty member Joseph Glorioso III, PhD, Professor in the Department of Microbiology and Molecular Genetics at the University of Pittsburgh School of Medicine with a secondary appointment in the Department of Human Genetics, University of Pittsburgh Graduate School of Public Health. Dr. Glorioso is a co-founder of Oncorus and heads its Scientific Advisory Board.
“Oncolytic virus therapies have the potential to transform outcomes for cancer patients,” said Theodore (Ted) Ashburn, MD, PhD, President and Chief Executive Officer of Oncorus. “We intend to use the proceeds from this oversubscribed financing to support our advancement of novel, proprietary intratumoral and intravenous approaches with the goal of addressing severe unmet medical needs in oncology.”
Oncorus plans to use the Series B proceeds to advance its lead candidate, ONCR-177, into the clinic in early 2020. ONCR-177 is an intratumorally administered oncolytic virus clinical candidate for multiple solid tumor indications. ONCR-177 is built on the company’s next-generation oncolytic herpes simplex virus (oHSV) platform, which enables the development of oncolytic viruses with unparalleled payload capacity for potent activation of multiple arms of the immune system. Oncorus’ oHSV platform also uniquely enables full replication competency and selective attenuation in normal tissues to cause not only direct tumor destruction, but also exposure of all neoantigens within a tumor to the immune system in a highly immune activating environment. Oncorus also intends to progress its innovative synthetic oncolytic virus platform, which employs a novel delivery methodology to enable repeat, intravenous (systemic) administration of oncolytic viruses for the treatment of cancers, particularly in indications such as lung cancer where repeat, intratumoral administration is not feasible.
About ONCR-177
ONCR-177, an intratumorally administered oncolytic virus therapy for the treatment of multiple solid tumor indications, is built on Oncorus’ proprietary, next-generation oncolytic herpes simplex virus HSV platform. ONCR-177 is armed with five transgenes, IL-12, CCL4, FLT3L, and CTLA-4 and PD-1 antagonists – representing the largest payload in the oncolytic virus therapy class – for the stimulation of multiple arms of the immune system. ONCR-177 is also a fully replication-competent virus in that it retains the ability to express g34.5, which allows the virus to replicate in the presence of host antiviral immune responses, a feature unique among oncolytic HSV therapies. The ability to develop a heavily armed virus with native replication competency is enabled by propriety safety strategies that involve micro-RNA (miR) attenuation and inactivation of neuronal retrograde transport to enable selective and unattenuated viral replication in tumor cells, while preventing replication in healthy tissues. Oncorus presented preclinical data supporting the clinical advancement of ONCR-177 at the American Association for Cancer Research (AACR) Annual Meeting earlier in 2019.
Manufacturing in Microgravity

Magnesium and magnesium alloys have the potential to become a revolutionary material for a variety of industries because of their lightweight structure and ability to quickly biodegrade in water or inside the human body. Researchers, however, are still struggling to process this very reactive metal to eliminate defects that accelerate corrosion.
McGowan Institute for Regenerative Medicine affiliated faculty member Prashant Kumta, PhD, the Edward R. Weidlein Chair Professor of Bioengineering at the University of Pittsburgh, believes that a microgravity environment may positively affect the solidification mechanisms of these alloys. He received grant funding from the International Space Station (ISS) U.S. National Laboratory to examine microgravity’s influence on his lab’s novel patented magnesium alloys.
The team is partnering with Tec-Masters, Inc., the commercial hardware facility partner that operates the high-temperature SUBSA furnace aboard the ISS National Lab. Once in the microgravity environment of the space station, the alloy composition will be melted in the SUBSA furnace, and then solidified for further analysis.
This is the first selected project in the new Biomedical Research Alliance – a multi-year collaboration between the ISS U.S. National Laboratory and the McGowan Institute for Regenerative Medicine – to push the limits of biomedical research and development aboard the orbiting laboratory.
“The alloy’s improved mechanical properties, ability to store charge, and lightweight structure will make it an attractive material for aerospace, energy storage, and automotive applications,” said Dr. Kumta.
He believes that this research will play a major role in the economical manufacturing of magnesium alloys, particularly in additive manufacturing and customized 3D printing of magnesium structures.
“Magnesium and magnesium alloys are extremely light, with a density similar to natural bone,” explained Dr. Kumta. “They are two-fold lighter than titanium alloys and five-fold lighter than stainless steel and cobalt-chrome alloys – all of which are materials typically used in today’s implants and frameworks. Thus, the development of these materials could open new International Space Station applications as a lightweight structural framework material.”
Because of their weight and earth abundance, the alloys may also prove to be beneficial for climate change and energy storage.
“Fixtures or accessories in the aerospace industry – such as seats and lighting – that are made from magnesium alloys will be lighter which will consequently reduce fuel consumption,” said Dr. Kumta. “These benefits will help reduce costs and decrease greenhouse gas emissions – an advantage that can be applied to the automotive industry which accounts for a large amount of emissions in the United States. The material could also be used as a rechargeable battery, similar to lithium-ion batteries.”
The magnesium alloys developed by Dr. Kumta’s team may also serve as a cheaper and improved bioresorbable material for implanted medical devices. This type of material, which can be broken down and absorbed by the body, has a variety of applications in regenerative medicine and tissue engineering, such as implanted scaffolds that help guide the growth of new tissue.
“Despite expensive post-processing steps to minimize defects, magnesium alloys processed on earth react in a physiological fluid environment and form large amounts of hydrogen gas, resulting in gas pockets that must be aspirated by a syringe,” said Dr. Kumta. “We believe that processing the material in microgravity will considerably minimize or perhaps even eliminate melting and casting defects. As a result, the alloys will likely exhibit improved corrosion resistance, resulting in soluble hydrogen and salt products with better bioresorption response when implanted as scaffolds. Further, expensive post-processing will likely be eliminated, thereby reducing costs by almost 50 percent.”
Dr. Kumta, who holds secondary appointments in chemical and petroleum engineering, mechanical engineering and materials science, and oral biology, will work with a team of researchers from his laboratory in the Swanson School of Engineering, including Bioengineering Research Assistant Professors Abhijit Roy, PhD; Moni Kanchan Datta, PhD; and Oleg Velikokhatnyi, PhD.
The research team hopes that this work will lead to the processing of better-quality magnesium alloys, which will be free of many of the defects that form in terrestrially processed alloys, ultimately enabling improved functionality on Earth with significantly reduced processing steps and costs.
“This work offers a tremendous opportunity for advancing the science and technology of microgravity metal casting, widening the translational potential of the versatile magnesium-based systems for biomedical, energy, and aerospace applications,” said Dr. Kumta. “Magnesium has not yet been studied in space, so this project gives us the chance to explore a new frontier in scalable manufacturing of high-quality magnesium and magnesium alloys in space.”
Illustration: This image features examples of novel magnesium alloy device sample (left) made from the alloy cast and solidified on earth (right). These alloys will be sent to ISS for microgravity casting. Credit: Prashant Kumta.
Improving Neural Implants

A microelectrode array (MEA) is an implantable device through which neural signals can be obtained or delivered. It is an invaluable tool in neuroscience research and is critical to advancements in brain-computer interface (BCI) research, which has progressed to allow humans to operate robotic devices with their minds.
McGowan Institute for Regenerative Medicine faculty member Xinyan Tracy Cui, PhD, professor of bioengineering at the University of Pittsburgh, developed a coating that improves the performance of MEA technology and received a $2,370,218 award from the National Institutes of Health BRAIN initiative to help bring it closer to commercialization and clinical translation.
“Researchers have yet to find a long-term and stable microelectrode array that provides high-yield and high-quality recordings,” explained Dr. Cui, “but our lab has developed a biomimetic coating that mitigates the inflammatory host tissue reaction and improves recording quality and longevity.
“Manufacturers and users have expressed strong interest in this technology, but the coating made of biological protein is fragile and may lose bioactivity during the harsh environment of shipping, storage, and sterilization,” she continued.
Dr. Cui leads the Neural Tissue/Electrode Interface and Neural Tissue Engineering Lab, where they develop new engineering tools to study and clinically control the interface between tissue and implanted neural devices. With this NIH award, Dr. Cui and her group plan to optimize the coating stability and develop a protocol to preserve, store, package, deliver, and sterilize the technology.
“The active ingredient of the biomimetic coating is a brain-derived neural adhesion molecule that promotes neurons and inhibits inflammatory cell attachment on the electrode,” said Dr. Cui. “This is a patented technology with proven efficacy to improve neural recording by establishing a healthy electrode-neuron interface. This new project will make the wide dissemination of the technology possible by overcoming the protein stability issues with nanotechnology.”
Once her lab has optimized the technology, they will deliver it to collaborators who will test and evaluate the device performance in rodents and non-primate animal subjects. Additionally, they will work with representatives from two manufacturing companies who will help guide them toward commercialization and ensure that the developed procedures are compatible with their devices.
Dr. Cui said, “In addition to improvements in BCI research, this technology may greatly improve our ability to perform long-term mapping of neural activity and ultimately give neuroscience researchers a more robust understanding of brain function in learning and memory, development and aging, or disease progression and wound healing.”
NIH Grant: $21.5 million to Establish the LB3P MRC
Primary investigators Gwendolyn Sowa, MD, PhD, of Pitt’s Department of Physical Medicine and Rehabilitation and the UPMC Rehabilitation Institute, and McGowan Institute for Regenerative Medicine affiliated faculty member Nam Vo, PhD, of the Department of Orthopaedic Surgery, were awarded $21.5 million to establish the Low Back Pain: Biological, Biomechanical, Behavioral Phenotypes Mechanistic Research Center (LB3P MRC), a multidisciplinary research center dedicated to categorizing patients into chronic low back pain subgroups with the goal of targeting treatments specific to individual patients’ pain and reducing the use of opioids.
LB3P MRC’s unique approach of integrating the biological, biomechanical and behavioral contributors to chronic low back pain with novel mathematical modeling will ultimately provide predictive tools to help personalize treatments for this common and debilitating condition.
As part of the National Institutes of Health Helping to End Addiction Long-term, or the NIH HEAL Initiative, researchers from UPMC and the University of Pittsburgh have been awarded nine grants totaling more than $32 million to improve prevention and treatment strategies for opioid misuse and addiction and to enhance pain management. The awards are part of 375 grant awards across 41 states made by the NIH in fiscal year 2019 to apply scientific solutions to reverse the national opioid crisis.
Illustration: Wikipedia.
Project Receives Pitt’s Center for Medical Innovation Award in Round-1 2019 Pilot Funding

The University of Pittsburgh’s Center for Medical Innovation (CMI) awarded grants totaling $70,000 to three research groups through its 2019 Round-1 Pilot Funding Program for Early Stage Medical Technology Research and Development. McGowan Institute for Regenerative Medicine affiliated faculty member Carl Snyderman, MD, MBA, is a co-investigator on one of the selected projects, along with Garrett Coyan, MD, a surgery resident in the lab of William Wagner, PhD:
“ET3: an endotracheal tube that prevents and monitors migration”
For the development of a novel endotracheal tube that reduces risk from associated clinical complications.
- Garrett Coyan, MD, MS: Resident, Department of Cardiothoracic Surgery, UPMC (Wagner Lab)
- Carl Snyderman, MD, MBA: Professor of Otolaryngology and Neurological Surgery at the University of Pittsburgh School of Medicine and Otolaryngology Director of the Center for Cranial Base Surgery at UPMC
- Jeffrey Vipperman, PhD: Professor, Department of Mechanical Engineering & Material Science
The overall aim of the ET3 project is to develop a novel endotracheal (ET) tube design that reduces luminal migration, monitors for unsafe movement, and limits aspiration/ventilator associated pneumonia (VAP). An aim of the project is to conduct a prospective clinical study to document migration rates and complications of ICU intubation at UPMC.
CMI, a University Center housed in Pitt’s Swanson School of Engineering, supports applied technology projects in the early stages of development with “kickstart” funding toward the goal of transitioning the research to clinical adoption. Proposals are evaluated based on scientific merit, technical and clinical relevance, potential health care impact and significance, experience of the investigators, and potential in obtaining further financial investment to translate the particular solution to healthcare.
“This is our eighth year of pilot funding, and our leadership team could not be more excited with the breadth and depth of this round’s awardees,” said McGowan Institute affiliated faculty member Alan D. Hirschman, PhD, CMI Executive Director. “This early-stage interdisciplinary research helps to develop highly specific biomedical technologies through a proven strategy of linking UPMC’s clinicians and surgeons with the Swanson School’s engineering faculty.”
Dr. John Pollock Designs App to Improve Mental Health

McGowan Institute for Regenerative Medicine affiliated faculty member and Duquesne University professor John Pollock, PhD, and his team aim to develop an app to improve mental health. As reported by Kellen Stepler, staff writer for The Duquesne Duke, the app’s objective is to help pre-teens develop coping skills to manage stress and anxiety. A 2019 report published in the Journal of the American Medical Association claims that the teen suicide rate has reached its highest level in nearly two decades.
Dr. Pollock is a professor of biological sciences and co-director of the Chronic Pain Research Consortium at Duquesne. An active neuroscientist researching the biology of pain, Dr. Pollock has created an Emmy award-winning television program and educational apps as the director of The Partnership of Education at Duquesne.
Dr. Pollock and his team use storytelling through 21st century technology that address both the biology of the nervous system and healthy management techniques for dealing with pain, anxiety and stress for school-age kids. The new digital media stories will explore common day-to-day stress experienced by children. With discovery and problem-solving, the app will let children learn research-based, non-pharmacological pain relief and coping strategies for dealing with stress and anxiety. Dr. Pollock notes that stress is something people of all ages experience.
“The world of a pre-teen has real-world stress and anxious situations, but the scale and scope are a different dimension compared to what adults carry as concerns,” Dr. Pollock said.
He also mentioned the lack of education in schools regarding coping skills to manage stress and anxiety.
“Rarely are any stress management and coping skills taught in school and if they are, it’s usually in a high school health class. High school stresses are very real and very real world,” Dr. Pollock said. Dr. Pollock said that it is important to teach pre-teens to develop coping skills to manage stress and anxiety, because developing these skills at a younger age can help kids be prepared for different challenges later in life.
The stories will be produced for iOS/Android devices with a similar format to Dr. Pollock’s award-winning Biblio-Tech series. The earlier stories titled “CITYHACKS: In Search of Sleep” and “REBOUND: Beating Concussion,” involve characters facing a challenge and then go about finding the facts and resources to solve the problem. Through a build-your-own-adventure style format, the narratives involve graphics, games, videos and other interactive features to keep kids engaged.
One of the features in CITYHACKS and REBOUND is called Adaptive Reader, which lets the user decide the reading level.
“The Adaptive Reader is important because it empowers the kids and makes the story accessible to a broad age range and broad range of reading proficiency,” Dr. Pollock said.
This current project is still in the works, just the beginning of five years of funding. Right now, Dr. Pollock’s team is gearing up to run surveys and focus groups with kids in late elementary and middle school, and then separately with parents and teachers. The survey to kids will focus on situations where they have experienced stressful situations, anxiety and pain.
“The survey results and the story lines that emerge from the kids as well as the parent’s perspective and the teacher’s insight will give us a starting point for developing a collection of narratives,” Dr. Pollock said.
The team also intends on asking experts in the field what they see as important, such as experts in mindfulness, critical evaluation, the health department, pediatricians and scientists.
“We’ll bring in their perspective and use that as guidance. We’ll also be adding in aspects of the underlying neurobiology and physiology of stress, anxiety and pain,” Dr. Pollock said.
When the research and surveys are completed, Dr. Pollock and his team will be ready to start developing narratives that will be relevant and interesting to the kids.
Dr. Pollock notes three outcomes for the project: that the kids read them and have fun with them, that the kids relate to some of the characters and learn more about the biology of their own body in response to the challenges of stress, anxiety and minor pain, and that the kids learn that there are a range of things that they can actually do to help them take control and manage how they feel.
The project is supported by a $1.3 million Science Education Partnership Award (SEPA) from the National Institute of General Medical Sciences, a component of the National Institutes of Health. The award is Dr. Pollock’s fourth SEPA since 2000 for developing health literacy multimedia and STEM educational tools, representing NIH grants totaling more than $6.4 million.
Applying Structural Monitoring Technology to the Human Spine

McGowan Institute for Regenerative Medicine affiliated faculty member Richard Debski, PhD, professor of bioengineering at the University of Pittsburgh and the co-director of the Orthopedic Robotics Laboratory, is a member of a team of researchers on a project which received $400K from the NIH to design and test a miniature, implantable, and battery-free sensor to monitor spinal fusion progress after surgery.
Amir Alavi, PhD, assistant professor of civil and environmental engineering at the University of Pittsburgh’s Swanson School of Engineering and the project PI; Shantanu Chakrabartty, PhD, Clifford Murphy professor of electrical and systems engineering at Washington University in St. Louis; and Dr. Debski, represent the team of the two-year grant which is entitled “Wireless, Self-Powered Sensors for Continuous and Long-Term Monitoring of Spinal Fusion Process.” The work began on September 1, 2019.
Spinal fusion is performed to treat a wide variety of spinal disorders. During the spinal fusion surgery, a special type of bone screw and symmetrical titanium or stainless-steel rods are implanted to stabilize vertebrae movement, which allow bone grafts to incorporate into the adjacent vertebra. Of the over 400,000 lumbar spinal fusion surgeries performed each year, approximately 30 percent of cases experience post-operative complications.
A clear understanding of the spinal fusion rate is essential for better surgical outcomes. Currently, spinal fusion progress is assessed using radiographic images, such as X-ray and CT scans, which are costly, expose the patients to significant radiation, and, more importantly, do not provide a continuous history of the spinal fusion process. To avoid relying on radiographic imaging, Dr. Alavi’s team is developing wireless sensors that will be attached to the spine fixation device to monitor the spinal fusion process and will completely rely on the energy harvested from the spine’s natural micromovements for operation.
“This implantable sensor has a major advantage over other existing spinal implants in that it does not rely on batteries, which are not really suitable for biomedical implants due to their limited lifetime, large size, and chemical risks. If there is spine movement, the sensor will self-power itself and track the progress of spinal fusion,” says Dr. Alavi. “Also, the data from the sensor can be wirelessly interrogated using a diagnostic ultrasound scanner, rather than the commonly-used RFID technology, which faces severe limitations inside the tissue.”
Clinicians can read the generated time-evolution curves using the ultrasound scanner to properly assess the bone fusion period, and for more accurate implant removal scheduling.
“Surgeons will be able to monitor the fusion process consistently over time simply with a portable scanner,” continues Dr. Alavi. “While CT scans and X-rays present only a ‘snapshot’ at the time where the measurements are taken, our sensor will give a clearer picture of the entire course of fusion.”
In addition to avoiding the costly imaging appointments, the sensor itself is expected to be inexpensive to produce—less than $5 in raw materials each.
AWARDS AND RECOGNITION
 Dr. Steven Little Named Program Chair of 2020 CRS Annual Meeting
Dr. Steven Little Named Program Chair of 2020 CRS Annual Meeting
The 47th Annual Meeting of the Controlled Release Society (CRS) will be held in the Paris Hotel June 27th – July 1st, 2020, in Las Vegas, Nevada. McGowan Institute for Regenerative Medicine faculty member Steve Little, PhD, a William Kepler Whiteford Endowed Professor of Chemical Engineering, Bioengineering, Pharmaceutical Sciences, Immunology, and Ophthalmology at the University of Pittsburgh, is the Program Chair.
The theme of the meeting is “2020 Vision for Global Impact,” and features a truly staggering set of speakers including Nobel Laureates, Academic thought leaders and Industry game changers that will cast a vision of the future of the CRS field in the areas of drug delivery, material science, pharmaceutics, bioengineering, consumer and diversified products, and veterinary science.
McGowan Institute affiliated faculty member Kathryn Whitehead, PhD, Associate Professor and Dean’s Career Fellow in the Departments of Chemical Engineering and Biomedical Engineering at Carnegie Mellon University, is also a member of the 2020 Program Committee. She will serve as an Ancillary Committee Chair.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#202 –– Dr. Robert Kellar discusses his research treating in infected or chronic wounds with novel biomaterial scaffolds.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: O’Keefe RJ, Tuan RS, Lane NE, Barry F, Bunnell BA, Colnot C, Drake MT, Drissi H, Fortier LA, Guldberg RE, Little DG, Marshall MF, Mao JJ, Nakamura N, Robey PG, Rosen V, Rowe DW, Schwarz EM
Title: American Society for Bone and Mineral Research‐Orthopaedic Research Society Joint Task Force Report on Cell‐Based Therapies
Summary: Cell-based therapies, defined here as the delivery of cells in vivo to treat disease, have recently gained increasing public attention as a potentially promising approach to restore structure and function to musculoskeletal tissues. Although cell-based therapy has the potential to improve the treatment of disorders of the musculoskeletal system, there is also the possibility of misuse and misrepresentation of the efficacy of such treatments. The medical literature contains anecdotal reports and research studies, along with web-based marketing and patient testimonials supporting cell-based therapy. Both the American Society for Bone and Mineral Research (ASBMR) and the Orthopaedic Research Society (ORS) are committed to ensuring that the potential of cell-based therapies is realized through rigorous, reproducible, and clinically meaningful scientific discovery. The two organizations convened a multidisciplinary and international Task Force composed of physicians, surgeons, and scientists who are recognized experts in the development and use of cell-based therapies. The Task Force was charged with defining the state-of-the art in cell-based therapies and identifying the gaps in knowledge and methodologies that should guide the research agenda. The efforts of this Task Force are designed to provide researchers and clinicians with a better understanding of the current state of the science and research needed to advance the study and use of cell-based therapies for skeletal tissues. The design and implementation of rigorous, thorough protocols will be critical to leveraging these innovative treatments and optimizing clinical and functional patient outcomes. In addition to providing specific recommendations and ethical considerations for preclinical and clinical investigations, this report concludes with an outline to address knowledge gaps in how to determine the cell autonomous and nonautonomous effects of a donor population used for bone regeneration.
Source: J Bone Miner Res. 2019 Sep 23. [Epub ahead of print]
GRANT OF THE MONTH
PI: Xinyan Tracy Cui
Title: Optimization and Delivery of Bioactive Coating for High Yield and Stable Neural Recording
Description: The ability to monitor activity of ensembles of neurons at single-cell resolution, chronically, over long time periods is greatly desired by neuroscientists. A variety of multi-electrode arrays (MEAs) have been developed for in vivo studies. These arrays are capable of revealing the activity of neuronal ensembles. Unfortunately, none of the devices on the market is fully capable of obtaining recordings that are simultaneously high-yield and high-quality, as well as stable and useful over months to years. This well-known challenge has greatly limited our ability to track the activity of populations of single neurons over a sufficient period of time to fully investigate circuit change during learning and memory, development and aging, or disease progression and wound healing. Additionally, the clinical use of brain machine interface (BMI), which utilize recorded neural activities to decode movement intent for controlling machine, has been hindered by the unstable and unreliable recording. We have developed a biomimetic coating composed of a brain-derived L1-cell adhesion molecule that mitigate the inflammatory host tissue reaction. In rodents, L1 coated NeuroNexas probes maintained high quality neural recording over the period of 16 weeks with significant higher single unit yield and signal to noise ratio than the uncoated control probes. Meanwhile, recordings in non-human primates (NHPs) with L1-coated Blackrock MEAs also demonstrated high quality performance in single unit yield and signal amplitude for at least 6 months. MEA manufacturers and users expressed strong interest in utilizing this technology. However, the coating made of biological protein is fragile and may lose bioactivity during the harsh environment of shipping, storage and sterilization. In order to make the L1 coating a technology that can be widely adopted by the neuroscience community, we propose to optimize the coating stability and develop practical protocols for coating preservation, storage, packaging, delivery and sterilization. The bioactivity of the coating prepared with different protocols will first be tested with cell cultures. Promising procedures will then be tested with implantation and recording in rodents at the University of Pittsburgh. Once optimum coating and procedures are determined, coated arrays will be delivered to users to evaluate the coating performance. Dr. Buzsaki (NYU) will test the L1 coated NeuroNexas arrays in freely moving rats. Dr. Schwartz (U. Pitt) and Dr. Chestek (U. Michigan) will test the L1 coated Blackrock arrays in NHPs for BMI studies. Users will work closely with us to define specific performance criteria in their recording applications, compare performance of coated and uncoated arrays, and provide user input for us to improve the packaging and delivery. Throughout the project, representatives from two MEA manufacturers, Blackrock Microsystems and NeuroNexus Technology, will serve as consultants to ensure compatibility of our procedures with their devices and guide us on the path to dissemination. The project will produce a coating technology that is both easy to adopt and generalizable to all types of state- of-art and emerging MEAs. Solving the practical issues of sterilization, packaging and delivery is a critical step toward commercial and clinical translation of the technology. High quality and stable of neural recording will greatly improve our ability to map brain activity in long-term experiments, and benefit brain-computer interfaces and other types of neural prostheses. In a broader sense, the protocols developed here for preserving immobilized protein during storage, delivery and sterilization should be applicable to other medical implants containing bioactive proteins, immunoassays, protein arrays, enzyme-based biosensors or any micro/nano devices that incorporate biological components.
Source: National Institute of Mental Health and National Institute of Neurological Disorders and Stroke
Term: September 30, 2019 – August 31, 2023
Amount: $521,345 (2019)
