September 2020 | VOL. 19, NO. 9| www.McGowan.pitt.edu
$31 Million Received to Study Regenerative Therapy Solutions for Dental, Oral, and Craniofacial Medicine

A multi-institute collaboration including the University of Pittsburgh received more than $31 million by the National Institute of Dental and Craniofacial Research (NIDCR) to study regenerative therapies and to improve patient care by providing solutions for the unmet clinical problems in dental, oral, and craniofacial medicine.
The Michigan-Pittsburgh-Wyss Regenerative Medicine (MPWRM) Resource Center is a multi-institute collaboration between the University of Michigan School of Dentistry, Pitt’s School of Dental Medicine, McGowan Institute for Regenerative Medicine, sciVelo, and the Harvard University/Wyss Institute.
The MPWRM Resource Center is led by a multidisciplinary Operating Committee that includes leaders of academic and industry research in tissue engineering and regenerative medicine with expertise in commercial translation and program management. At the University of Pittsburgh, the Operating Committee includes McGowan Institute for Regenerative Medicine affiliated faculty members
- Charles Sfeir, DDS, PhD, Associate Dean for Research at the School of Dental Medicine, Founding Director of the Center for Craniofacial Regeneration (CCR), Associate Professor of Periodontics and Preventive Dentistry, Oral Biology and Bioengineering, University of Pittsburgh,
- Donald Taylor, PhD, MBA, CLP, Executive Director of sciVelo and Assistant Vice Chancellor for Health Sciences Translation, University of Pittsburgh, and
- William Wagner, PhD, Director of the McGowan Institute for Regenerative Medicine and Distinguished Professor of Surgery, Bioengineering and Chemical Engineering, University of Pittsburgh,
who each contribute to the center by leveraging their expertise in translational and clinical research in addition to academic commercialization.
“The Resource Center reflects a key part of the McGowan Institute’s mission to develop technologies that address tissue and organ failure. We are very focused on not just the invention of solutions, but on moving these concepts forward to the patients in need. The Resource Center allows us to focus on the non-technical barriers to translation, such as regulatory guidance, appropriate pre-clinical testing, or transition to regulated manufacturing. Our success in this partnership provides a model for translational approaches in other tissue areas as well,” said Dr. Wagner.
“The strength of the CCR lies in the synergistic approach to translational research by clinicians, engineers and basic scientists. The environment at CCR fosters multidisciplinary approaches to regenerate mineralized and soft tissues of craniofacial structures and employ tissue engineered strategies such as cellular therapies, biomaterials and mechanobiology. Our innovation has resulted in new and unique technologies developed by the CCR members,” said Dr. Sfeir.
Funded by the NIDCR, the mission of the MPWRM Resource Center is to support translation of pre-clinical regenerative therapies for dental, oral, and craniofacial (DOC) tissues. Since its founding in 2017, the MPWRM Resource Center has been providing financial and advisory resources to translational projects in the field through its interdisciplinary network of clinical, engineering, regulatory and commercialization experts. The center has supported 19 interdisciplinary translational research projects that focus on development of regenerative therapies to address unmet needs in DOC tissues, including biologics, drug delivery, cellular therapies, and medical devices. All 19 of these project teams have issued patents or filed patent applications, and 57% of the teams have had formal interactions with the FDA to refine strategies for regulatory clearance or approval for clinical applications.
Illustration, pictured left to right: Patrick Cantini, William Wagner, Dave Kohn (University of Michigan, Dental School), Will Giannobile (Harvard, Dental School), Dave Mooney (Harvard, Wyss Institute), Charles Sfeir, and Mutsumi Yoshida (MPWRM Managing Director, University of Michigan (site)). sciVelo.
RESOURCES AT THE MCGOWAN INSTITUTE
October Histology Special
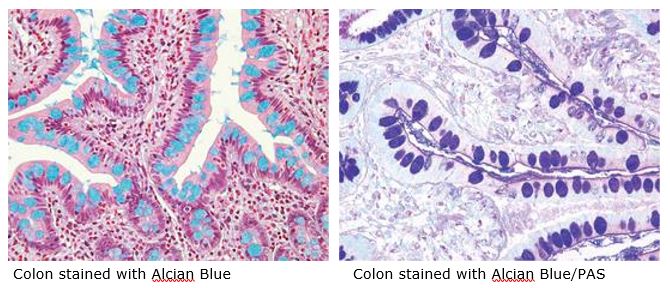
The combination of the Alcian blue and PAS stains can be used to distinguish neutral mucins from acid mucins. Mucins are a key component in lubrication, cell signaling, and forming chemical barriers. Using Alcian blue (pH 2.5), acid mucins will be stained a deep blue. The subsequent application of the PAS technique will stain the neutral mucins a bright magenta. Tissues or cells that contain both neutral and acidic mucins will be stained a dark blue or purple color.
The combined Alcian blue/PAS technique is the most sensitive and comprehensive method for detection of mucins, since all mucins should react regardless of their charge nature.
The McGowan Histology Core offers TUNEL staining on paraffin embedded or frozen tissue.
You’ll receive 30% off your Alcian Blue, PAS, or combination Alcian Blue/PAS staining in September when you mention this ad. Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
Organ Regeneration Technology Is Focus of Four Peer-Reviewed Publications

LyGenesis, Inc., a biotechnology company focused on regenerative medicine founded by McGowan Institute for Regenerative Medicine faculty member Eric Lagasse, PharmD, PhD, recently announced the publication of four peer-reviewed papers on its organ regeneration technology. Dr. Lagasse is LyGenesis’s Chief Scientific Officer, Associate Professor in the Department of Pathology at the University of Pittsburgh, and Director of the Cancer Stem Cell Center at the McGowan Institute. Dr. Lagasse is a co-author on each of the publications.
“For a decade and supported by multiple NIH grants, my laboratory has been laying the scientific foundation for the advancement of our organ regeneration technology. Having previously published work showing that we can use the lymph node as a bioreactor to produce functioning ectopic organs including the liver, pancreas, thymus and kidney, we are now rapidly advancing those technologies, while simultaneously expanding our understanding of the molecular mechanisms that support organogenesis,” said Dr. Lagasse.
LyGenesis’ lead development asset is liver regeneration, which was the focus of the first two papers. “Development of Ectopic Livers by Hepatocyte Transplantation into Swine Lymph Nodes” by Laasse et al. was published in Liver Transplantation, demonstrating that functional mini livers could be grown using the lymph node as a bioreactor. In this swine model using surgically induced liver injuries, liver cells were isolated from the animals and then transplanted into lymph nodes in the abdominal region of the animal. In 100% of the animals, the transplanted liver cells engrafted into the lymph nodes, proliferated, and formed ectopic mini livers, which had the same structure and function as native liver tissue. The cell transplant procedure was safe, well tolerated, and as expected, the amount of liver mass generated by the ectopic livers was proportional to the degree of the native liver’s damage. Paulo Fontes, MD, LyGenesis’s Chief Medical Officer and former Director of the Liver Transplant Program, Starzl Transplant Institute at the University of Pittsburgh Medical Center, is an affiliated faculty member of the McGowan Institute. He is currently a Professor of Surgery and Director, Surgical Innovation & Research, Department of Surgery, West Virginia University Medicine, Morgantown, WV.
“The development and FDA approval of novel therapies for life-threatening diseases requires a rigorous approach to preclinical studies and our ability to grow ectopic organs to support failing organs in patients is no exception,” said Dr. Fontes. “Showing that our cellular therapy was able to safely and effectively induce organogenesis – the forming of a novel, well-vascularized organ within the body – in multiple models of liver disease in large animals was a crucial step toward beginning our forthcoming clinical trial for patients with end stage liver disease who are currently ineligible for standard liver transplantation.”
The second paper, “Ex Vivo Cell Therapy by Ectopic Hepatocyte Transplantation Treats Porcine Tyrosinemia Model of Acute Liver Failure,” by Nicolas and colleagues was published in Molecular Therapy: Methods & Clinical Developments. In this study, a human liver disease (tyrosinemia Type I) was modeled in swine, and liver cells transplanted into lymph nodes were capable of forming ectopic livers that cured all of the animals of otherwise fatal liver disease. The engraftments were again shown to be safe and also structurally and functionally similar to native liver tissue. Maria Giovanna Francipane, PhD, is a co-author on this publication and is Principal Investigator in Regenerative Medicine at Fondazione RiMED in Palermo, an international partnership between the Italian Government, the Region of Sicily, the Italian National Research Council (CNR), the University of Pittsburgh, and UPMC. Also, Dr. Francipane is an affiliated faculty member of the McGowan Institute.
The other two published peer-reviewed papers focused on kidney regeneration. “Kidney–in–a–Lymph Node: A Novel Organogenesis Assay to Model Human Renal Development and Test Nephron Progenitor Cell Fates” published in The Journal of Tissue Engineering and Regenerative Medicine by Francipane and colleagues replicated and extended previous findings in mice showing that human ectopic kidney tissues could also be grown using the lymph node as a bioreactor. The paper also demonstrated the lymph node’s remarkable ability to promote vascularization, a key rate-limiting issue for many transplant technologies, which brings blood flow and effective oxygenation to ectopic organs including kidney tissues engrafted into lymph nodes. In the paper, “Host Lymphotoxin-ß Receptor Signaling is Crucial for Angiogenesis of Metanephric Tissue Transplanted into Lymphoid Sites,” published in The American Journal of Pathology, Francipane and colleagues have begun to unravel some of the molecular signaling – focusing on the lymphotoxin-ß receptor (LTßR) pathway in particular – that appears to be crucial for engrafted kidney tissue to grow and function properly.
Mesoscale Diffusion MRI of the Ex Vivo Human Hippocampus
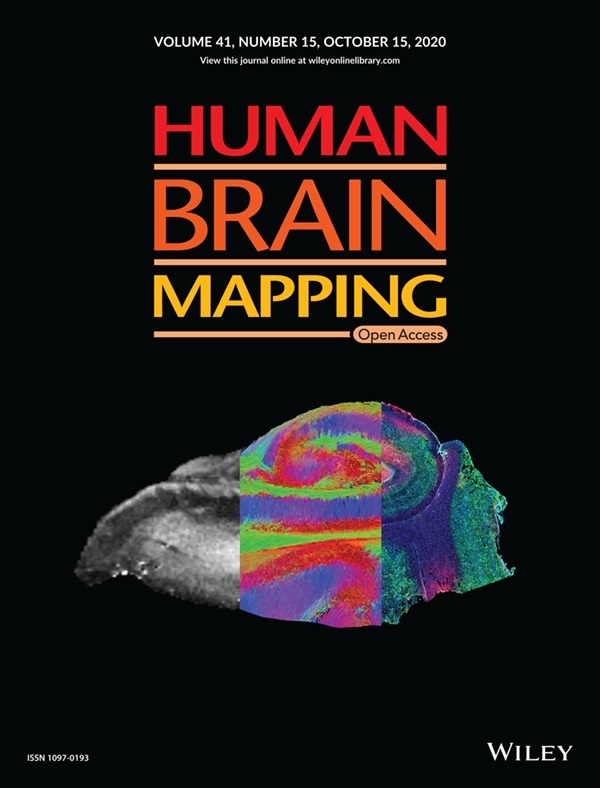
McGowan Institute for Regenerative Medicine affiliated faculty member Michel Modo, PhD, Professor in the Department of Radiology at the University of Pittsburgh with secondary appointments in the Department of Bioengineering and the Center for Neural Basis of Cognition, and colleagues recently published a paper, titled “Mesoscale diffusion magnetic resonance imaging of the ex vivo human hippocampus,” in Human Brain Mapping where the authors anticipate that ex vivo mesoscale imaging will yield novel insights into human hippocampal connectivity. The paper was also the featured cover of the journal. The abstract of the paper reads:
Mesoscale diffusion magnetic resonance imaging (MRI) endeavors to bridge the gap between macroscopic white matter tractography and microscopic studies investigating the cytoarchitecture of human brain tissue. To ensure a robust measurement of diffusion at the mesoscale, acquisition parameters were arrayed to investigate their effects on scalar indices (mean, radial, axial diffusivity, and fractional anisotropy) and streamlines (i.e., graphical representation of axonal tracts) in hippocampal layers. A mesoscale resolution afforded segmentation of the pyramidal cell layer (CA1‐4), the dentate gyrus, as well as stratum moleculare, radiatum, and oriens. Using ex vivo samples, surgically excised from patients with intractable epilepsy (n = 3), we found that shorter diffusion times (23.7 ms) with a b‐value of 4,000 s/mm2 were advantageous at the mesoscale, providing a compromise between mean diffusivity and fractional anisotropy measurements. Spatial resolution and sample orientation exerted a major effect on tractography, whereas the number of diffusion gradient encoding directions minimally affected scalar indices and streamline density. A sample temperature of 15°C provided a compromise between increasing signal‐to‐noise ratio and increasing the diffusion properties of the tissue. Optimization of the acquisition afforded a system’s view of intra‐ and extra‐hippocampal connections. Tractography reflected histological boundaries of hippocampal layers. Individual layer connectivity was visualized, as well as streamlines emanating from individual sub‐fields. The perforant path, subiculum and angular bundle demonstrated extra‐hippocampal connections. Histology of the samples confirmed individual cell layers corresponding to ROIs defined on MR images. We anticipate that this ex vivo mesoscale imaging will yield novel insights into human hippocampal connectivity.
Illustration: Human Brain Mapping cover illustration, 09/20/20. Mesoscale diffusion MR‐based tractography bridges the knowledge gap between anatomical MRI and histology. Maria Ly, Lesley Foley, Ashwinee Manivannan, T. Kevin Hitchens, R. Mark Richardson, Michel Modo.
Dr. Stephen Badylak’s Work Featured on Life 2.0: Body Shop

TV network Xploration Station’s new series Life 2.0 recently covered how the clinical trial efforts in the laboratory of McGowan Institute for Regenerative Medicine Deputy Director Stephen Badylak, DVM, PhD, MD, are changing lives with breakthroughs in regenerative medicine. The series, entitled “Body Shop,” highlighted Dr. Badylak’s patient, Keith Kaufman, and his muscle recovery following a motorcycle accident.
The accident occurred in 2002. Mr. Kaufman underwent more than a dozen surgeries to “put back together again” his right leg. However, even with all the surgeries, Mr. Kaufman had little use of the remaining muscle in his leg. Primarily his leg was used for stability and balance, not much more. When more than 20% of a muscle is damaged, the tissue can’t regenerate and a stiff scar forms in place of the missing muscle, which often leads to significant disability.
Learning about the clinical trial being conducted through the McGowan Institute for Regenerative Medicine, he contacted Dr. Badylak to see if he would be a candidate for the regenerative medicine therapy being tested. He was. His old injuries were surgically implanted with extracellular matrix (ECM) derived from pig bladder. “If you put the matrix at the site of the injury, the body’s own stem cells get recruited into that matrix and they say, ‘okay, I know where I’m at, I know what I’m supposed to do,’ and when they move into that structure, they begin to remodel it, and turn it into a structure that they want,” says Dr. Badylak. Mr. Kaufman also underwent pre- and post-operative physical therapy.
Mr. Kaufman is now back to his younger self, keeping up with his very active daughter. “I can say I went through a lot of hell to get to this point, and now, it’s blue skies again,” says Mr. Kaufman.
The series is carried on the Fox TV network and will be available on Amazon Prime.
Steroids Improve Survival in Very Ill COVID-19 Patients

In a tremendous demonstration of global collaboration, an international team led by clinician-scientists at UPMC and the University of Pittsburgh School of Medicine have pooled data from 121 hospitals in eight countries to find that inexpensive, widely available steroids improve the odds that very sick COVID-19 patients will survive the illness.
The findings were made through the “Randomized Embedded Multifactorial Adaptive Platform-Community Acquired Pneumonia” (REMAP-CAP) trial and are reported in JAMA as part of a four-article package. The World Health Organization is updating its COVID-19 treatment guidance as a result.
REMAP-CAP includes the UPMC-REMAP-COVID19 trial, the only U.S.-based trial to test corticosteroids — a class of drug that lowers inflammation and modulates immune system activity — for treating critically ill COVID-19 patients. An analysis combining the REMAP-CAP data with that from six other randomized controlled trials to test corticosteroids reinforces the results of the UK RECOVERY trial reported in June, which found the steroid dexamethasone reduced deaths by 29% in ventilated COVID-19 patients.
“It is relatively rare in medicine that you find drugs where the evidence of their effectiveness in saving lives is so consistent,” said lead author Derek Angus, MD, MPH, chief health care innovation officer at UPMC, professor and chair of the Department of Critical Care Medicine at Pitt, and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “This is, in many respects, the single clearest answer we’ve had so far on how to manage terribly ill COVID-19 patients. People on ventilators or oxygen and under intensive care should definitely be given corticosteroids.”
Between March and June, the REMAP-CAP corticosteroid trial randomized 403 adult COVID-19 patients admitted to an intensive care unit to receive the steroid hydrocortisone or no steroids at all. The trial found a 93% probability that giving patients a seven-day intravenous course of hydrocortisone would result in better outcomes than not giving the steroid. The results were consistent across age, race, and sex.
“This gives physicians like me, who treat the sickest of the sick, hope,” said co-author Bryan McVerry, MD, UPMC pulmonologist and associate professor of pulmonary, allergy and critical care medicine at Pitt. “We are beginning to get a handle on the deadly side of this disease.”
REMAP-CAP and the other corticosteroid trials did not test the drugs in non-hospitalized patients with COVID-19 who did not need respiratory support. Steroids currently are not recommended for these patients because they can dampen the immune system and have serious side effects. In addition, the REMAP-CAP corticosteroid trial was mostly conducted in resource-rich countries across Europe, North America, and Australasia, so the findings may not translate to low- and middle-income countries.
Because it is designed to simultaneously test multiple combinations of potential therapies — as opposed to the traditional, slow clinical trial process that tests one therapy at a time — REMAP-CAP is particularly well-suited for rapidly identifying effective treatments during the COVID-19 pandemic. It currently is testing thousands of different treatment regimens, including various doses and combinations of vitamin C, convalescent plasma, blood thinners, antivirals, and immune modulators.
“REMAP-CAP and our findings on corticosteroids are possible because of a global community of clinicians and scientists coordinating and sharing data across different languages and countries,” said co-author Christopher Seymour, MD, UPMC intensivist and director of the Translational and Clinical Science Program at the Clinical Research, Investigation and Systems Modeling of Acute Illness (CRISMA) Center in Pitt’s School of Medicine. “This is how we get definitive answers as fast as possible on how to best treat patients. Outcomes in Amsterdam are helping patients at UPMC Altoona.”
Timothy Girard, MD, Christopher Horvat, MD, David Huang, MD, Kelsey Linstrum, MS, and Stephanie Montgomery, MS, all of Pitt’s CRISMA Center, and research staff from Pitt’s Multidisciplinary Acute Care Research Organization (MACRO) also contributed to this research.
Additional authors on the JAMA publication are from the Raymond Poincaré Hospital – AP-HP (Greater Paris University Hospitals), University of Versailles and University Paris Saclay, all in France; King Saud Bin Abdulaziz University for Health Sciences in Saudi Arabia; Imperial College London, Imperial College Healthcare NHS Trust, University of Oxford, Bristol Royal Informatory, University of Bristol, NHS Blood and Transplant, Queen’s University Belfast, and Intensive Care National Audit & Research Centre, all in the UK; Berry Consultants, LLC, the Global Coalition for Adaptive Research, University of California at Los Angeles and Harbor-UCLA Medical Center, all in the US; St. Michael’s Hospital of Unity Health Toronto, Université de Sherbrooke, University of Toronto, University Health Network, University of British Columbia and University of Manitoba, all in Canada; Jena University Hospital in Germany; Monash University, Alfred Health, Princess Alexandra Hospital University of West Australia, The George Institute for Global Health and St. John of God Hospital, all in Australia; University Medical Center Utrecht, University of Amsterdam and Radboud University Medical Center, all in the Netherlands; Antwerp University Hospital in Belgium; Network for Improving Critical Care Systems and Training in Sri Lanka; Mahidol Oxford Tropical Medicine Research Unit in Thailand; Auckland City Hospital, The Health Research Council of New Zealand and University of Auckland, all in New Zealand; and St. Vincent’s University Hospital and University College Dublin, both in Ireland.
This research was funded by The Platform for European Preparedness Against (Re-) emerging Epidemics (PREPARE) consortium FP7-HEALTH-2013-INNOVATION-1 (#602525), the Australian National Health and Medical Research Council (#APP1101719 and #1116530), the New Zealand Health Research Council (#16/631), the Canadian Institute of Health Research Strategy for Patient-Oriented Research Innovative Clinical Trials Program Grant (#158584), the UK National Institute for Health Research (NIHR) and the NIHR Imperial Biomedical Research Centre, the Health Research Board of Ireland (CTN 2014-012), the UPMC Office of Healthcare Innovation, the Breast Cancer Research Foundation, the French Ministry of Health (PHRC-20-0147), and the Minderoo Foundation.
Editing the Immune Response to Boost Gene Therapy

Gene therapy generally relies on viruses, such as adeno-associated virus (AAV), to deliver genes into a cell. In case of CRISPR-based gene therapies, molecular scissors can then snip out a defective gene, add in a missing sequence or enact a temporary change in its expression, but the body’s immune response to AAV can thwart the whole endeavor.
To overcome that obstacle, researchers at the University of Pittsburgh School of Medicine—including McGowan Institute for Regenerative Medicine affiliated faculty members Samira Kiani, MD, and Mo Ebrahimkhani, MD—created a system that uses CRISPR in a different way. Their system briefly suppresses genes that are related to AAV antibody production so the virus can deliver its cargo unimpeded. These results are published in Nature Cell Biology.
“Many clinical trials fail because of the immune response against AAV gene therapy,” said study co-senior author Dr. Kiani, associate professor of pathology at Pitt and member of the Pittsburgh Liver Research Center (PLRC). “And then you can’t re-administer the shot because people have developed immunity.”
So, Dr. Kiani and her long-time collaborator Dr. Ebrahimkhani, associate professor of pathology at Pitt and member of PLRC, set out to modify gene expression related to the body’s immune response to AAV. But this gene is important for normal immune function, so the researchers didn’t want to shut it down forever, just tamp it down momentarily.
Since CRISPR is such a convenient system for editing the genome, the pair figured they would put it to use for altering the master switches that orchestrate genes involved in immune response.
“We’re hitting two birds with one stone,” said Dr. Ebrahimkhani. “You can use CRISPR to do your gene therapy, and you also can use CRISPR to control the immune response.”
When the researchers treated mice with their CRISPR-controlled immune suppression system and then exposed them to AAV again, the animals didn’t make more antibodies against the virus. These animals were more receptive to subsequent AAV-delivered gene therapy compared to controls.
Beyond gene therapy, the study also shows that CRISPR-based immune suppression can prevent or treat sepsis in mice, highlighting the potential for this tool to be broadly useful for a range of inflammatory conditions, including cytokine storm and acute respiratory distress syndrome, both of which can crop up with COVID-19, though more studies are needed to engineer safety features.
“The main goal of this study was to develop CRISPR-based tools for inflammatory conditions,” said study lead author Farzaneh Moghadam, a PhD student in Dr. Kiani’s lab. “But when we looked at bone marrow samples, we saw that the group treated with our tool showed a lower immune response to AAV compared to the control group. That was very interesting, so we started exploring how this tool contributes to antibody formation against AAV and could potentially address safety and efficacy concerns with gene therapy trials.”
Dr. Kiani cofounded SafeGen Therapeutics with the goal of bringing this technology to the clinic.
Plexiglass Alone Can’t Protect Against Aerosolized Virus
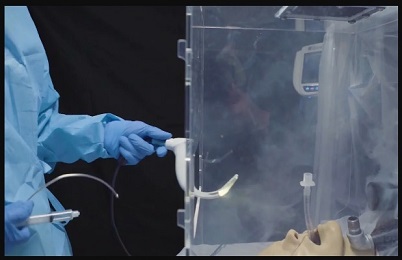
Especially in settings where personal protective equipment, or PPE, is in short supply, intubation — inserting a breathing tube down a patient’s throat — poses a major risk of SARS-CoV-2 exposure for doctors and nurses as viral particles are released into the air.
Researchers from UPMC—including McGowan Institute for Regenerative Medicine faculty member Peter Rubin, MD, FACS, Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh—and the U.S. Army Combat Capabilities Development Command’s Army Research Laboratory (CCDC-ARL) created an individual biocontainment unit, or IBU, to keep front line health care workers safe while they provide life-saving care. The device is described in a study published today in the Annals of Emergency Medicine.
Earlier attempts to minimize exposure to health care workers involved placing a plexiglass intubation box over a patient’s head and shoulders. Clinicians place their hands through two large holes in the box to intubate the patient inside. While such a device may contain the worst of the splatter, it can’t keep aerosols from leaking out.
The IBU is designed to suck contaminated air out of the box with a vacuum and trap infectious particles in a filter before they seep into the room.
Simulating a COVID-19 patient, the researchers placed a mannequin inside the IBU as well as in a commercially available intubation box. Near its mouth, they piped in an oil-based aerosol which formed tiny droplets in the air, similar in size to the SARS-CoV-2 particles in breath that spread COVID-19.
The IBU trapped more than 99.99% of the simulated virus-sized aerosols and prevented them from escaping into the environment. In contrast, outside of the passive intubation box, maximum aerosol concentrations were observed to be more than three times higher than inside the box.
“Having a form of protection that doesn’t work is more dangerous than not having anything, because it could create a false sense of security,” said study co-lead author David Turer, MD, MS, a plastic surgeon who recently completed his residency at UPMC.
Because of concerns about the potential of airborne viruses to leak from the plexiglass boxes, the Food and Drug Administration (FDA) recently revoked their Emergency Use Authorization (EUA) for these enclosures.
Several months ago, Dr. Turer and colleagues submitted an EUA application for the IBU and are preparing to manufacture the devices for distribution.
“It intentionally incorporates parts from outside the medical world,” said Dr. Turer, who now is at the University of Texas Southwestern Medical Center. “So, unlike other forms of PPE, demand is unlikely to outstrip supply during COVID-19 surge periods.”
Besides protecting providers during intubation, the IBU also can provide negative pressure isolation of awake COVID-19 patients, supplying an alternative to scarce negative pressure hospital isolation rooms, as well as helping isolate patients on military vessels.
“The ability to isolate COVID-19 patients at the bedside is key to stopping viral spread in medical facilities and onboard military ships and aircraft,” said study co-lead author Cameron Good, PhD, a research scientist at the CCDC-ARL.
Devices similar to IBUs were first used in practice by military personnel in the Javits Center field hospital in New York City when local hospitals were overrun with COVID-19 patients during the first wave of the pandemic.
Once the EUA is granted, hospitals and military units will be able to use the IBU to protect health care workers caring for COVID-19 patients.
Additional authors on the study include Benjamin Schilling, MS, and Heng Ban, PhD, of the University of Pittsburgh; Robert Turer, MD, MSE, of Vanderbilt University Medical Center; Nicholas Karlowsky, of Filtech; Lucas Dvoracek, MD, of UPMC; and Jason Chang, MD, of UPMC and Pitt.
This work is supported by the University of Pittsburgh Center for Medical Innovation (grant F_309-2020-Turer).
Illustration: Individual Biocontainment Unit: A vacuum and filtration system pulls aerosols away from the provider and traps contaminants. UPMC.
Predictive Placentas: Using Artificial Intelligence to Protect Mothers’ Future Pregnancies
After a baby is born, doctors sometimes examine the placenta—the organ that links the mother to the baby—for features that indicate health risks in any future pregnancies. Unfortunately, this is a time-consuming process that must be performed by a specialist, so most placentas go unexamined after the birth. A team of researchers from Carnegie Mellon University (CMU) and the University of Pittsburgh Medical Center (UPMC)—including McGowan Institute for Regenerative Medicine affiliated faculty member Philip Leduc, PhD, William J. Brown Professor of Mechanical Engineering with appointments in Biological Sciences, Computational Biology, and Biomedical Engineering at CMU—report the development of a machine learning approach to examine placenta slides in The American Journal of Pathology, published by Elsevier, so more women can be informed of their health risks.
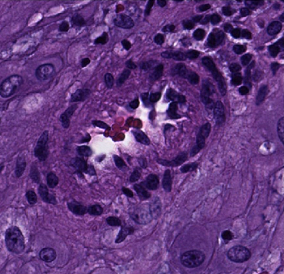
One reason placentas are examined is to look for a type of blood vessel lesions called decidual vasculopathy (DV). These indicate the mother is at risk for preeclampsia—a complication that can be fatal to the mother and baby—in any future pregnancies. Once detected, preeclampsia can be treated, so there is considerable benefit from identifying at-risk mothers before symptoms appear. However, although there are hundreds of blood vessels in a single slide, only one diseased vessel is needed to indicate risk.
“Pathologists train for years to be able to find disease in these images, but there are so many pregnancies going through the hospital system that they don’t have time to inspect every placenta,” said Daniel Clymer, PhD, alumnus, Department of Mechanical Engineering, CMU. “Our algorithm helps pathologists know which images they should focus on by scanning an image, locating blood vessels, and finding patterns of the blood vessels that identify DV.”
Machine learning works by “training” the computer to recognize certain features in data files. In this case, the data file is an image of a thin slice of a placenta sample. Researchers show the computer various images and indicate whether the placenta is diseased or healthy. After sufficient training, the computer is able to identify diseased lesions on its own.
It is quite difficult for a computer to simply look at a large picture and classify it, so the team introduced a novel approach through which the computer follows a series of steps to make the task more manageable. First, the computer detects all blood vessels in an image. Each blood vessel can then be considered individually, creating smaller data packets for analysis. The computer will then access each blood vessel and determine if it should be deemed diseased or healthy. At this stage, the algorithm also considers features of the pregnancy, such as gestational age, birth weight, and any conditions the mother might have. If there are any diseased blood vessels, then the picture—and therefore the placenta—is marked as diseased. The UPMC team provided the de-identified placenta images for training the algorithm.
“This algorithm isn’t going to replace a pathologist anytime soon,” Dr. Clymer explained. “The goal here is that this type of algorithm might be able to help speed up the process by flagging regions of the image where the pathologist should take a closer look.”
“This is a beautiful collaboration between engineering and medicine as each brings expertise to the table that, when combined, creates novel findings that can help so many individuals,” added lead CMU investigators Jonathan Cagan, PhD, and Dr. LeDuc.
“As healthcare increasingly embraces the role of artificial intelligence, it is important that doctors partner early on with computer scientists and engineers so that we can design and develop the right tools for the job to positively impact patient outcomes,” noted co-author Liron Pantanowitz, MBBCh, formerly vice chair for pathology informatics at UPMC. “This partnership between CMU and UPMC is a perfect example of what can be accomplished when this happens.”
Illustration: Machine learning can provide feedback about which cases would benefit from in-depth pathological inspection, like this sample revealing a decidual arteriole affected by early stage decidual vasculopathy. Source: Carnegie Mellon Engineering.
Abbott Lab Student Helps Build Silk Scaffolding for Tissue

Carnegie Mellon University (CMU) undergraduate Tahlia Altgold makes biomedical research using silk run more smoothly.
“Silk is a really incredible biomedical material that’s been used for a long time in things like sutures,” said Ms. Altgold, a junior majoring in materials science and biomedical engineering.
McGowan Institute for Regenerative Medicine affiliated faculty member Rosalyn Abbott, PhD, Assistant Professor in the Departments of Biomedical Engineering and Materials Science and Engineering at CMU, focuses on using silk in tissue engineering. In order for cells to grow, they need a support structure. Silk can provide this scaffold.
But first the raw material has to be processed. That’s where Ms. Altgold comes in. She dices discarded silkworm cocoons to boil in a sodium bicarbonate solution and separates out certain proteins for use.
“Tahlia’s very thorough,” Dr. Abbott said, noting how important silkworms are to multiple projects in her research lab. But it’s what happens to the boiled silk solution next that really makes Dr. Abbott smile. Ms. Altgold is aiding the development of a new method of 3D printing silk proteins to create personalized new tissues for patients needing regenerative medicine.
“Silk is very biocompatible,” she said. Producing silk scaffolds in a lab is nothing new but finding a way to 3D print the material without blending it with potentially problematic materials will be a breakthrough for regenerative medicine.
Silk is hard to work with at the cellular level, Dr. Abbot said. Researchers need to coerce the proteins to hold the shape they need to print, say, a new ear drum.
“We’re trying to find the exact right pretreatment method for pushing the solution through the nozzle in the 3D printer so it will hold its shape after printing,” Ms. Altgold said. Printing material as a 3D gel is a technique developed in the laboratory of McGowan Institute affiliated faculty member Adam Feinberg, PhD, Professor in the Departments of Biomedical Engineering and Materials Science and Engineering at CMU. “Combining this method with Dr. Abbott’s expertise in biomaterials has made the whole project feel in-house, totally CMU,” she said.
While the Abbott Lab was closed as part of CMU’s COVID precautions, Ms. Altgold worked remotely, honing her skills reading published literature and helping to prepare research articles for publication. The Abbott Lab is back up and running with modifications as part of CMU’s phased re-opening strategy.
“The work now is to prove our concept and refine the methodology we’re working on,” said Ms. Altgold, whose participation in the lab is funded by a Summer Undergraduate Research Fellowship (SURF) from CMU’s Undergraduate Research Office. She is mentored by Claude King III, a PhD candidate in biomechanical engineering. “Dr. Abbott’s lab does exactly the kind of work I want to pursue. I’m so interested in tissue engineering and using different cells through a materials science lens.”
Dr. Abbott, likewise, said Ms. Altgold is exactly the kind of student she needs for her research.
“She’s so inquisitive. The work she’s doing is really difficult and she’s helping us make exciting progress. I’m happy to be part of her journey,” Dr. Abbot said.
Illustration: Carnegie Mellon University (Altgold) and McGowan Institute for Regenerative Medicine (Abbott)
Calcium Helps Build Strong Cells

Every time you flex your bicep or stretch your calf muscle, you put your cells under stress. Every move we make throughout the day causes our cells to stretch and deform. But this cellular deformation can be dangerous and could potentially lead to permanent damage to the DNA in our cells, and even cancer. So how is it that we’re able to keep our bodies moving without constantly destroying our cells? Thanks to a new study by McGowan Institute for Regenerative Medicine affiliated faculty member and Carnegie Mellon University (CMU) Chemical Engineering Professor Kris Dahl, PhD, and Associate Professor Sara Wickström, MD, PhD, of the University of Helsinki, we now know that the answer lies in a humble mineral we consume every day.
“Basically, every time we flex a muscle, we’re risking DNA damage that could lead to cancer,” says Dr. Dahl. “Or we would be, that is, if it weren’t for the calcium in our cells.”
Their recent paper published in Cell marks the first time that researchers have definitively shown how cells maintain their structural integrity despite the strain of mechanical forces.
“As cells stretch and compress through the course of our daily activities,” says Dr. Dahl, “they have to rearrange their internal structures to compensate. Our study found that they are able to do this through the use of calcium. It’s kind of like when you’re tying a bow in a ribbon. When you have to shift your hands, you ask someone to put their finger on the knot to hold it in place and make sure it doesn’t come apart. For our cells, that ‘finger’ is calcium.”
In particular, calcium is essential in protecting the nucleus of the cell and the DNA it contains. When mechanical stretch acts on the cell, it deforms the nucleus, putting the DNA inside at risk. Healthy cells are able to counteract this deformation using a calcium-dependent nuclear softening, which allows the nucleus to stretch without breaking. But failure to mount this response can result in DNA damage, which can lead to cell death, loss of proper cell function, or in extreme cases, cancer.
“Our colleagues who research materials science are often trying to find materials that are force-responsive,” says Dr. Dahl. “But here we’ve found actively responding materials inside of living cells. Not only is there the quick response using calcium, but there’s also the longer-term response that cells use to withstand persistent, high amplitude stretch, by changing the epigenetics of the cell. This has exciting implications for how cells respond genetically, as well as how tissues respond mechanically.”
“This entire research project is truly a testament to the collaborative spirit here at Carnegie Mellon,” says Dr. Dahl. “The project was conceived during a conference in Singapore, where Professor Wickström and I met. We gathered the data using a microscope in Finland and analyzed it here at Carnegie Mellon using algorithms we developed in Pittsburgh. This is a truly global collaboration, the kind that the culture of CMU really encourages.”
Next, the researchers will use this new understanding of how cells respond to stretch to consider what happens to cells during the aging process. As we age, our cells and tissues don’t deform as well as they used to, and the consequences of this reduced deformation can lead to an increased risk of cell damage. The question is, do cells not deform as well because they are stiffer, resulting in cellular dysfunction? Or does cell dysfunction lead to reduced deformation? The next step will involve studying cellular deformation at different points throughout the aging process, to determine if it’s possible to interrupt this cellular stiffening and improve cell function during as we age.
npj Regenerative Medicine: Meeting Report of 8th Annual International Symposium on Regenerative Rehabilitation Now Available

Regenerative Rehabilitation seeks to optimize patient outcomes through an integration of two fields: regenerative medicine and rehabilitation science. The former focuses on tissue repair or replacement due to loss from injury, disease, or age. This is achieved primarily through the enhancement of endogenous stem cell function or the transplantation of exogenous stem cells. The latter focuses on the use of mechanical and other stimuli to promote functional recovery. This synergy between biological and bioengineering advances is critical to developing novel and impactful translational therapies. However, there currently are few opportunities for regenerative scientists to be exposed to the methodologies commonly employed in the clinic by rehabilitation professionals. Conversely, most rehabilitation scientists and clinicians are not exposed to the many advances of regenerative medicine. This disconnect has impeded the pace of progress in the field. To this end, the International Consortium for Regenerative Rehabilitation—comprised of 16 institutions—aims to increase interdisciplinary interaction. Thus, as technologies are developed and our understanding of regenerative biology progresses, advances may be efficiently translated to the clinic.
To address this need, the annual international symposium on Regenerative Rehabilitation is designed to propel the translation of regenerative technologies into functionally relevant treatment interventions that have the potential to transform rehabilitative healthcare. The Eighth Annual International Symposium on Regenerative Rehabilitation brought together basic scientists, engineers, and rehabilitation clinicians in order to create a fertile ground for discussion, interaction, and networking across disciplines. The symposium took place on 24–26 October 2019, in Charlottesville, Virginia, and was co-hosted by the University of Virginia and the Uniformed Services University of the Health Sciences. This symposium featured world-renowned researchers and clinicians, focusing on the emerging field of Regenerative Rehabilitation; it succeeded in creating a platform for bridging several areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking. A meeting report entitled “Progressing the field of Regenerative Rehabilitation through novel interdisciplinary interaction” is now available in npj Regenerative Medicine.
Due to COVID-19, the AR3T Executive Leadership recently made the difficult decision to postpone the 2020 symposium. The Ninth Annual International Symposium on Regenerative Rehabilitation will be held November 4-6, 2021, in Austin, Texas.
Illustration: The Alliance for Regenerative Rehabilitation Research and Training.
AWARDS AND RECOGNITION
Dr. Derek Angus Assumes JAMA Senior Editor Role

One of the world’s most prestigious academic medical journals announced recently that it is strengthening its editorial team with expertise from UPMC and the University of Pittsburgh School of Medicine.
Derek Angus, MD, MPH, chief health care innovation officer at UPMC and professor and chair of the Department of Critical Care Medicine at Pitt, will become a senior editor at the Journal of the American Medical Association (JAMA). Dr. Angus is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
“Derek is among the most prominent ICU physicians in the world and has helped JAMA recruit the best papers in this specialty,” said Howard Bauchner, MD, JAMA’s editor-in-chief.
Dr. Angus joined UPMC and Pitt in the early 1990s after earning his medical degree and completing residency training in internal medicine at the University of Glasgow, UK. His specialties include epidemiologic, economic and health services research aspects of critical illness, with a particular focus on improving randomized control trials to better serve the sickest of the sick. He has authored or co-authored hundreds of papers and abstracts and received several awards and honors nationally and internationally for his research.
“Serving as an associate editor at JAMA has been a professional honor and delight,” said Dr. Angus. “It has introduced me to the physicians and scientists who are transforming the way we care for patients and prevent disease. I look forward to my new role as senior editor.”
The Transplantation Society Honors Dr. Anthony Demetris with Recognition Award

The Transplantation Society (TTS) honored professionals in the field of transplantation during its 28th Annual TTS 2020 Virtual Congress. These awards recognize individuals who have made a major impact in the field of transplantation. This year, McGowan Institute for Regenerative Medicine affiliated faculty member Anthony “Jake” Demetris, MD, received the TTS Outstanding Achievement (Clinical) Award.
Dr. Demetris is the Endowed Chair and Starzl Professor of Liver and Transplant Pathology and currently directs the Division of Hepatic and Transplantation Pathology along with the Shared Research Histology Laboratory facilities located at the University of Pittsburgh Medical Center.
Dr. Demetris’ contributions have been noteworthy in all aspects of liver allograft pathology and clinical transplantation immunobiology, including drug development, acute and chronic antibody-mediated rejection, tolerance monitoring and multiple labeling and automated image analysis. He completed more than 480 peer-reviewed publications and more than 250 invited speaker engagements.
Congratulations, Dr. Demetris!
Cook Lab Student Awarded 2020 Mara H. Wasburn Early Engineering Educator Grant

Carnegie Mellon University Biomedical Engineering (BME) PhD student Erica Comber, a student in the laboratory of McGowan Institute for Regenerative Medicine affiliated faculty member Keith Cook, PhD, Professor and Associate Department Head for Graduate Education at Carnegie Mellon University (CMU) in its Department of Biomedical Engineering and the Director of the Bioengineered Organs Initiative, has been awarded the 2020 Mara H. Wasburn Early Engineering Educator Grant by the Women in Engineering Division (WIED) of the American Society of Engineering Education (ASEE). The award covers expenses associated with Ms. Comber’s presenting her research at the 2020 ASEE conference. Ms. Comber has served as a teaching assistant for BME senior design for two years, working under CMU’s Conrad Zapanta, PhD. Her education research at CMU aims to provide students with a capstone design course that mimics the work dynamic between Biomedical Engineers and Industrial Designers in the medical device industry.
Illustration: Carnegie Mellon University (Comber)/McGowan Institute for Regenerative Medicine (Cook)
Congratulations, Ms. Comber!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#213 –– Dr. Alejandro Nieponice discusses his research utilizing ECM-derived scaffolds to regenerate esophageal tissue with the aim of decreasing the morbidity and mortality of current surgical procedures.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Paulo Fontes, Junji Komori, Roberto Lopez, Wallis Marsh, Eric Lagasse
Title: Development of Ectopic Livers by Hepatocyte Transplantation into Swine Lymph Nodes
Summary: Orthotopic liver transplantation continues to be the only effective therapy for patients with end‐stage liver disease. Unfortunately, many of these patients are not considered transplant candidates, lacking effective therapeutic options that would address both the irreversible progression of their hepatic failure and the control of their portal hypertension. In this prospective study, a swine model was exploited to induce sub‐acute liver failure. Autologous hepatocytes, isolated from the left hepatic lobe, were transplanted into the mesenteric lymph nodes by direct cell injection. 30 to 60 days after transplantation, hepatocyte engraftment in lymph nodes was successfully identified in all transplanted animals with the degree of ectopic liver mass detected being proportional to the induced native liver injury. These ectopic livers developed within the lymph nodes showed remarkable histologic features of swine hepatic lobules, including the formation of sinusoids and bile ducts. Based on our previous tyrosinemic mouse model and the present pig models of induced sub‐acute liver failure, the generation of auxiliary liver tissue using the lymph nodes as hepatocyte engraftment sites represents a potential therapeutic approach to supplement declining hepatic function in the treatment of liver disease.
Source: Liver Transplantation. 2020 Aug 18.
GRANT OF THE MONTH
PIs: David H. Kohn, William V. Giannobile, David J. Mooney, Charles Sfeir, and William R. Wagner
Title: Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center: Advancing Dental, Oral, and Craniofacial Regeneration to Clinical Trial Initiation
Description: Reconstruction of dental, oral, and craniofacial (DOC) tissues through tissue engineering and regenerative medicine (RM) is a major goal in dentistry. Despite the continuing success and commercial viability of RM approaches in selected areas of medicine, the application of RM concepts to DOC problems has not been well- implemented in clinical practice. To meet this need, the Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center (MPWRM RC) was established as an interdisciplinary partnership amongst the University of Michigan, the University of Pittsburgh, and the Wyss Institute, along with technology, clinical, regulatory, marketing, and commercial translation experts from private practice and industry. Through this collaborative effort, we will accelerate clinical adoption of promising technologies by providing the interdisciplinary expertise and resources to guide technologies towards FDA submissions. The vision of this RC is to safely and effectively regenerate, reconstruct, and restore functional DOC tissues. To achieve this vision and fulfill the goals of DOC Tissue Regeneration Consortium (DOCTRC), we will continue this cooperative agreement with NIDCR for Stage 3 to: 1) implement the RC structure and processes developed in Stage 2 to catalyze Interdisciplinary Translational Projects (ITPs) towards clinical adoption; 2) direct clinical translation and commercialization of ITP technologies; and 3) broaden DOCTRC impact and build sustainability. Each of these endeavors will be done in partnership with the Center for Dental Oral and Craniofacial Tissue and Organ Regeneration (C-DOCTOR), our companion RC. Under a cooperative agreement and partnership with NIDCR, we have forged strategic partnerships, built infrastructure, and integrated assessment and advising processes, to support 19 ITPs in Stage 2 of the DOCTRC initiative. All projects are beyond basic discovery with demonstrable preclinical proof-of-concept, rapidly maturing product development and commercial translation roadmaps, and strong clinical impact potential. From this set, 7 projects have been identified as candidates for the initial cohort of the Stage 3 ITP program with a subset of other projects entering Stage 3 as they mature. These projects represent a balanced portfolio with respect to clinical needs addressed and technology types, with corresponding product development pathways. Upon completion of Stage 3 of the DOCTRC initiative, we will have advanced a portfolio of technologies with a spectrum of associated risk to FDA submissions and commercialization, towards eventual clinical adoption. We will work to secure leveraged and follow-on funding for ITPs so they may progress to human clinical trials. Finally, the RC will share our approach with the RM communities beyond solely the projects in our portfolio. The impact of these efforts will be a catalysis of translation in the DOC arena never previously achieved in an NIH extramural program, resulting in the transformation of dental, oral, and craniofacial medicine.
Source: National Institute of Dental & Craniofacial Research
Term: May 1, 2020 – April 30, 2025
Amount: $6,314,301 (2020)
