October 2022 | VOL. 21, NO. 10| www.McGowan.pitt.edu
Using Artificial Intelligence to Build a Better Organoid

The next breakthrough for artificial intelligence (AI) may help scientists design and print smarter cell structures that mimic human organs – thereby reducing trial-and-error and costs while contributing to new methods to fight disease and improve human health.
These three-dimensional cell structures are called organoids – they can be designed to exhibit unique organ function and are used to conduct complex research on human tissue physiology, genetic diseases, organ-specific infectious diseases, and cancer. Organoids can replicate development of native tissue in the architecture, function, cellular composition, and transcriptional profile of almost any organ.
But there are limitations. Current methods of manufacturing organoids have yet to demonstrate consistent and robust extraction of mature organoids from renewable cells. This is where AI comes into the picture – designing and testing organoids utilizing computers rather than traditional lab methods.
McGowan Institute for Regenerative Medicine affiliated faculty member Ipsita Banerjee, PhD (pictured top), Professor in the Department of Chemical and Petroleum Engineering at the University of Pittsburgh, together with a multi-disciplinary team of several co-principal investigators, recently received a $500,000 award from the National Science Foundation to realize their vision of scalable production of high-quality organoids. McGowan Institute for Regenerative Medicine affiliated faculty member Prashant Kumta, PhD (pictured bottom), Edward R. Weidlein Endowed Chair Professor at the University of Pittsburgh Swanson School of Engineering and School of Dental Medicine and is a professor in the Departments of Bioengineering, Chemical and Petroleum Engineering, Mechanical Engineering and Materials Science, and Oral Biology, is also a project co-principal investigator.
“Our vision is to enable organoid technology to finally realize its potential in clinical research and drug development,” said Dr. Banerjee. “This breakthrough research will benefit society and the healthcare system at large by creating a more efficient, effective, and sustainable way to design these structures.”
Most organoid production relies on chemical experiments, but Dr. Banerjee’s approach involves engaging mechano-transduction pathways, or the process in which cells respond to mechanical stimuli, to regulate manufacturing while also exploiting cytoskeletal rearrangements that are part of the organoid phenotype. The mechano-transduction will be controlled by bioprinting the organoid phenotype, while machine learning models will identify signature cytoskeletal states associated with the phenotype.
The team predicts using AI models will also enhance accuracy in predictions of organoid behavior for further research.
“Central to our goal of organoid manufacturing is the integration of bioprinting and artificial intelligence to enable automated and non-invasive learning of different types of organoids,” Dr. Banerjee explained. “Bioprinting also will enable us to scale up the production of these structures in quantity and quality over time, without the restrictions we currently face using traditional methods.”
This will be the first attempt to create a roadmap to producing high quality organoids, as well as integrate quality control in the manufacturing pipeline.
“This will be a novel application of artificial intelligence techniques in the organoid research field,” said Shandong Wu, PhD, a co-principal investigator for the project and artificial intelligence expert in the Department of Radiology at the University of Pittsburgh. “In recent years, artificial intelligence, especially machine learning, has shown transformative power in analyzing medical imaging data. The adoption of machine learning into biological image processing will create an innovative avenue for robust organoid function assessment.”
Congratulations, all!
RESOURCES AT THE MCGOWAN INSTITUTE
November Histology Special – Bone Staining
The Histology lab offers decalcification, processing and embedding, cutting and staining of those difficult bone samples. Our staff has years of experience with bone samples that include special staining and IHC on large and small bone samples that can be difficult to work with. We have even developed a technique to ensure your sample will not lift from the slide during aggressive antigen retrieval protocols.
Below are just some of the special stains we offer at the McGowan Histology Core:
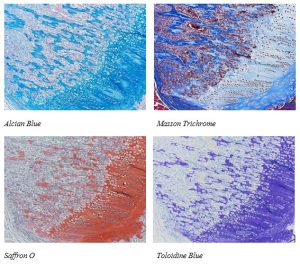
You’ll receive 25% off your bone staining November when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
UPCOMING EVENTS
Save the Date! 2023 McGowan Institute Scientific Retreat

The venue for the 2023 Retreat is the University Club, and the dates are March 6 & 7, 2023.
The program committee for the 2023 McGowan Institute Scientific Retreat, under the leadership of Bryan Brown, PhD, has begun to formulate the program and is requesting your suggestions for session topics.
The committee is also seeking to learn of possible interest by individuals in organizing a session. Please submit your program suggestions here.
SCIENTIFIC ADVANCES
Dr. Alejandro Almarza Receives $5.9M Grant to Study Pain

Joint pain can be difficult to manage. Learning more and having a better understanding of how pain works will improve how clinicians can help manage and reduce joint pain.
McGowan Institute for Regenerative Medicine affiliated faculty member Alejandro Almarza, PhD (pictured), Associate Professor in Oral Biology in the School of Dental Medicine at the University of Pittsburgh with a secondary appointment in the Department of Bioengineering, was awarded a $5.9M Restoring Joint Health and Function to Reduce Pain (RE-JOIN) Consortium Project (1UC2AR082196-01) from the National Institutes of Health. The project entitled, “Innervation of the Knee and the Temporomandibular Joint (TMJ),” is part of the Helping to End Addiction Long-term (HEAL) Initiative. This multi-principal investigator award with Dr. Almarza at Pitt and Dr. Kyle Allen at the University of Florida is accepted for three years with potential additional funding for two more years.
Nerve innervation to a joint is a complex network of different neural subtypes that vary between each different anatomical site, such as the knee or the TMJ and also vary with different biological factors (i.e., age, sex, disease) that the patient has. Moreover, to have clinical relevance, a shift in innervation must relate to a shift in symptoms. Our research proposal looks at joint pain from this perspective, generating pathology-pain relationships that are important to clinicians working with patients facing TMJ and knee osteoarthritis (OA) pain.
Teams at Pitt and the University of Florida will work to identify cell bodies in the dorsal root ganglia and trigeminal ganglia that project to the muscle, bone, or intra-articular joint tissues. These neurons will then be evaluated for their function using electrophysiologic tests and their transcriptome using single cell RNA-sequencing. By overlapping neural function with gene expression, they will identify promoter targets and design adeno-associated virus (AAV) vectors to produce fluorescent labels alongside the expression of these targets. Importantly, this approach will allow them to develop AAV-based tracers for specific functional neural subtypes, as well as combine traditional markers of functional subtypes with any newly identified markers that describe how the neuron changes with age, sex, and OA severity. Moreover, these tracers will be used to evaluate how joint innervation adapts following the application of two neural ablation techniques for pain relief in the knee and TMJ. To evaluate the clinical significance of these preclinical studies, innervation changes will be assessed in tissues collected from patients undergoing total joint replacement of the knee or TMJ. In all of these studies, joint innervation will be paired with detailed analyses of joint pain and disability. These analyses will include detailed behavioral characterizations; in patients, these analyses will include quantitative sensory tests and other assessments of joint function. Combined, this approach will allow them to evaluate pathology-pain relationships related to joint innervation from the preclinical model to the clinic.
The consortium project comprises 5 national teams bringing synergistic expertise about the types and distribution of neurons in joint tissues to identify key receptors and mediators that induce pain by activating specific sensory neurons. The Pitt team—under the direction of Dr. Almarza—will focus on the TMJ, and Dr. Allen will lead the work on the knee.
Congratulations, Dr. Almarza!
Abbott’s Heartmate 3™ Heart Pump Extends Life Beyond 5 Years for Advanced Heart Failure Patients

There is new data that show the HeartMate 3™ heart pump extends survival of advanced heart failure patients by at least five years, providing a clear life-saving option for people battling later stage heart disease. The HeartMate 3 heart pump Full MagLev™ technology was developed by researchers (James Antaki, PhD; Harvey Borovetz, PhD) at the McGowan Institute for Regenerative Medicine.
When McGowan Institute affiliated faculty member Robert Kormos, MD (pictured), a cardiothoracic surgeon, began his career 30 years ago, he would dream of extending the lives of his patients who were often battling advanced heart failure. Now, serving as division vice president of medical device company Abbott, that is no longer a dream.
“For me as a surgeon, this dream has now been met,” Dr. Kormos, a former McGowan Institute deputy director and the past director of UPMC’s Artificial Heart Program, said. He has been part of the education of over 100 residents and 60 Cardiothoracic Transplant Fellows.
More than 6.2 million Americans have heart failure, with diagnoses projected to double by 2030. Abbott’s HeartMate 3 heart pump is an implantable device that pumps blood through the body in people whose heart is too weak to do so on its own. It is the only commercially approved heart pump with Full MagLev technology, which allows the device’s rotor to be “suspended” by magnetic forces, a unique design that has been proven to reduce trauma to blood passing through the pump, improving patient survival and quality of life. These factors along with its ability to produce an artificial pulse, results in the lowest rate of pump-related complications of any other blood pump.
The data presented are from the MOMENTUM 3 trial, the world’s largest randomized clinical trial to assess long-term outcomes in people receiving a left ventricular assist device (known as an LVAD, or heart pump) to treat advanced heart failure. The data were communicated during a late-breaking session at the 2022 European Society of Cardiology Congress in Barcelona, Spain.
The MOMENTUM 3 trial studied more than 1000 patients and for the first time in a clinical trial setting found that people with advanced heart failure who received the HeartMate 3 heart pump lived beyond five years. The study showcases the significant benefits of Abbott’s heart pump technology, particularly in a patient population who – without a heart pump or transplant – would have limited therapy options or would require living with inotropic medication to help strengthen their heart function, limiting their median survival to less than a year.
“It’s important to understand that the reason this device is so successful is because it reduced the mortality from complications that typically end people’s lives with heart pumps,” Dr. Kormos said. “That was greatly reduced in this trial compared to other trials and in the HeartMate II, and that includes blood clots forming in the pump, thrombosis, it includes stroke and bleeding.”
“The MOMENTUM 3 study proves that the HeartMate 3 heart pump has significantly moved the needle in terms of options for increasing life expectancy for our most advanced heart failure patients,” said Divya Gupta, MD, medical director of Advanced Heart Failure and Heart Transplantation at Emory Healthcare. “This research shows strong consideration should be given for this life-extending therapy for the thousands of people who are in advanced heart failure and meet the indications for the HeartMate 3.”
Heart pumps: a life-extending option for thousands
Historically, many advanced heart failure patients who don’t qualify for a heart transplant rely on medication or are referred to palliative care to manage symptoms, but newer technological advancements like HeartMate 3 can provide this population another life-prolonging option. The benefits of heart pumps are especially true for the estimated 15,000 advanced heart failure patients whose median lifespan is under a year because they are on inotropic medication alone. While some of these patients await a donor heart, due to a limited number of organs available, heart pumps like the HeartMate 3 can improve survival while offering immediate, significant, and sustained quality of life.
The latest MOMENTUM 3 data also demonstrate that the five-year survival for HeartMate 3 patients (nearly 60%) is approaching the five-year survival rates of heart transplant recipients who have a similar risk profile.
Armed with these results, the company will work to educate physicians, patients, and hospital administrators about the benefits of the HeartMate 3. For hospital administrators, the message will be about cost management, Dr. Kormos said. Reimbursement of the device is dependent on local market factors and a patient’s insurance plan.
“We need to educate and make sure that hospital administrators understand that this is a cost-effective therapy,” he said. “It keeps people out of the hospital. If you fix their heart failure and you make them mobile, which this does, then that reduces the chronic rehospitalization from heart failure.”
He added that while the study shows promising results for HeartMate 3, work needs to be done to make utilization equitable across all populations.
“What’s concerning to me is that regionally across the United States, the use of this therapy is not uniform,” Dr. Kormos said. “In other words, look at the population of patients that get these devices: only about 30% of them are African American or Black. We know that the utilization of this type of therapy in the South is not as great as it is in, say, the Northeast … So, we have some work to do in terms of the equity of the utilization of these therapies.”
“The finding that the HeartMate 3 device can reliably add years to one’s life is compelling evidence for all cardiologists to evaluate their patients with progressive heart failure for this therapy,” said Daniel J. Goldstein, MD, surgical director, Department of Cardiothoracic and Vascular Surgery Cardiac Transplantation at Montefiore Medical Center. “Earlier referral and intervention are critical for this population where it can be challenging to make an accurate diagnosis from physical symptoms alone. The latest MOMENTUM trial data help contextualize the benefits of heart pumps and will help more physicians work with their patients to explore this life-enhancing and life-prolonging alternative as their disease moves into the territory of advanced heart failure.”
First Gear Teams Shine in SHRS Innovation Challenge

A new wheelchair and ramp designed to fit into standard vehicles without costly modifications and a system for keeping elderly people safe in their homes were both awarded $10,000 in the School of Rehabilitation Sciences Innovation Challenge.
The challenge was conducted in conjunction with the Innovation Institute’s First Gear innovation commercialization program, where Pitt innovators can discover the commercial potential of their discoveries.
Each First Gear team is composed of a faculty principal investigator (PI), an entrepreneurial lead (EL), which is typically a student, and a business mentor (M). Two of the nine projects in the most recent cohort were the winners and included McGowan Institute for Regenerative Medicine affiliated faculty members as principal investigators:
Saver — A wheelchair designed to fit into standard vehicles.
PI: Rory Cooper, PhD (pictured top)
EL: Brandon Daveler; Chang Dae Lee
M: Robert Huemmrich
Mobius — Improving senior’s ability to stay in their homes safely.
PI: David Brienza, PhD (pictured bottom)
EL: Paulina Villacreces; Alexandra Delazio; Jennifer McCartney
M: Ian Magazine
First Gear provides participants with $3,000 to assist in market research and customer discovery activities, along with one-on-one mentoring through the eight-week program. For the third time, the School of Rehabilitation Sciences has sponsored a competition as part of First Gear in order to encourage faculty and students translate their research from the lab to the market where it can have an impact outside the university.
Congratulations, all!
Multi-Center Study Sheds Light on Understudied Breast Cancer Type

A multi-center analysis of patients with invasive lobular carcinoma, or ILC — the second most common histological subtype of invasive breast cancer in the U.S. — showed that, despite its prevalence, ILC is detected later and has worse outcomes than the predominant subtype of invasive breast cancer, known as invasive ductal carcinoma (IDC), or no special type.
Published in the Journal of the National Cancer Institute, the study of more than 33,000 patient records from three large cancer centers — UPMC Hillman Cancer Center, Cleveland Clinic Cancer Center and The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (OSUCCC -– James) — shows that ILC and IDC are biologically distinct, highlighting important differences between the two diseases and the need for specific detection and treatment options for the lobular subtype.
“Lobular breast cancer makes up about 10% to 15% of breast cancer cases, but it has historically been neglected by the research community, so we really don’t know that much about it,” said co-lead author Steffi Oesterreich, PhD (pictured), co-leader of the Cancer Biology Program at UPMC Hillman, professor at the University of Pittsburgh School of Medicine’s Department of Pharmacology & Chemical Biology, and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “There has been increasing awareness that ILC and IDC are distinct, but this large multi-center study provides compelling evidence that these are two different diseases that require different management.”
Co-senior authors Megan Kruse, MD, a breast medical oncology specialist at Cleveland Clinic, and Nicole Williams, MD, a breast medical oncologist at OSUCCC – James, worked with Dr. Oesterreich to analyze records from patients treated at the three cancer centers for ILC or IDC between 1990 and 2017.
“These findings likely indicate that detection of lobular breast cancer is delayed,” said Dr. Kruse. “When these tumors are finally detected, they’re larger and they’ve already moved to the lymph nodes, indicating the cancer is spreading. We need to put more effort into improving early detection of ILC by developing new imaging technologies or other methodologies.”
ILC’s key feature is loss of a gene called E-cadherin that helps cells stick together. As a result, lobular cancer cells grow in lines, producing tumors that look more like spider webs than the familiar round lumps of IDC, explained Dr. Oesterreich. These web-like tendrils make ILC difficult to spot on mammograms until the cancer has grown and often advanced.
The analysis found that ILC cells were lower grade than IDC, meaning that they looked more similar to normal cells. However, ILC tumors were diagnosed twice as often at stage III or IV— advanced stages in which cancer cells have spread beyond breast tissue to the lymph nodes or metastasized to other parts of the body. Lobular tumors were also larger in size than their ductal counterparts.
The researchers restricted the next part of their analysis to patients with tumors bearing estrogen receptors and lacking the HER2 receptor. They found that patients with lobular cancer had worse disease-free survival and overall survival. ILC patients also had more disease recurrence than those with IDC, and recurrences tended to occur later.
“In other words, more tumors are coming back, and they’re coming back later for patients with ILC,” explained Dr. Oesterreich, who also holds the Shear Family Endowed Chair in Breast Cancer Research and is co-director of the Women’s Cancer Research Center, a partnership between UPMC Hillman and Magee-Womens Research Institute. “This suggests that tumor cells hibernate somewhere in the body until they are reawakened. We need to find where these cells hang out and why they reawaken.”
A commercially available advanced genomic test called Oncotype DX was used to predict risk of recurrence and response to chemotherapy for patients with early-stage estrogen-receptor-positive, HER2-negative breast cancer.
The analysis found that there was a significant association between the Oncotype DX score and cancer recurrence for patients with IDC. Very few ILC cases were classified as high-risk, despite more late recurrences, highlighting the need for specific molecular tests that improve predictions for lobular breast cancer.
“Lobular breast cancer and ductal breast cancer are two distinct diseases. Our study shows that lobular breast cancers are diagnosed at a more advanced stage and have increased chance of recurrence. However, invasive lobular cancer was less likely to be classified as high-risk by a commonly used genomic test,” said Dr. Williams. “Despite their differences, these cancers are often treated the same. We hope these findings will spark research aimed at developing new diagnostic tools and drugs to improve outcomes for patients with lobular breast cancer.”
The “Cellular” Network

The complexity of life on earth was derived from simplicity: from the first protocells to the growth of any organism, individual cells aggregate into basic clumps and then form more complex structures. The earliest cells lacked complicated biochemical machinery; to evolve into multicellular organisms, simple mechanisms were necessary to produce chemical signals that prompted the cells to both move and form colonies.
Replicating this behavior in synthetic systems is necessary to advance fields such as soft robotics. Chemical engineering researchers at the University of Pittsburgh Swanson School of Engineering have established this feat in their latest advancement in biomimicry.
The research, “Lifelike behavior of chemically oscillating mobile capsules,” was published in the Elsevier journal Matter. Lead author is Oleg Shklyaev, post-doctoral associate with McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD (pictured), Distinguished Professor of Chemical and Petroleum Engineering and the John A. Swanson Chair of Engineering.
“We utilized a computer model involving red, blue, and green capsules. With addition of appropriate reactants, each capsule triggers one of three interconnected reactions that convert the reactants into products. If the volume of the reactants is different from the products (as is frequently the case in bio-catalytic reactions), the fluid will encompass density gradients, which spontaneously generate buoyancy forces. The forces drive the flow of the surrounding solution and propel the immersed capsules.
Because of this dynamic behavior, the capsules are always experiencing new chemical environments and neighbors. If the moving capsules lie too far apart, the “networking” amounts to an exchange of constant chemical signals, allowing the capsules to “know” about the presence of others,” Dr. Shklyaev explains. “If, however, the flow brings the three different types of capsules sufficiently close to each other, their chemical “communications” become more involved, leading the “triad” to undergo spatial and temporal chemo-mechanical oscillations.
Namely, the simple system that initially featured time-independent exchange of chemical signals, self-organizes into a colony that displays chemo-mechanical oscillations, similar to the oscillations of the chemoattractant cAMP in colonies of ameba or even the periodic beating of a living heart. The system shows life-like autonomy because the “fuel” for the capsules’ motion is self-generated and in turn, the spontaneous motion of the fluid prompts the capsules’ communications and biomimetic, collective oscillations. With reactants to instigate the catalysis, the rest of the processes are accomplished by the system itself.
The specific interlinked reactions acting on the model capsules form a bio-inspired negative feedback loop (the “repressilator”), where each capsule suppresses chemical production by the next one in the loop. The repressilator model was used to successfully simulate and further understand communication (quorum sensing) in colonies of bacteria. In the “dormant” state, when capsules are sufficiently far from each other, the capsules coupled through the feedback loop do not exhibit oscillations, but rather produce constant chemical output and translational motion through the fluid. Eventually, the moving capsules come into contact with new neighbors and form a colony that exhibits a biomimetic collective response: an oscillatory chemical signal accompanied by the mechanical oscillations of the constituent parts.
Dr. Balazs notes that while their microcapsule system does not encompass any motives, it appears to replicate fundamental biological functions due to the simple rules imposed on the system and the introduction of reactants (nutrients) into the solution. In other words, the seemingly complex chemo-mechanical oscillations can result from simple mechanisms that occur inherently in chemical solutions.
“When developing remote systems and tiny machines, you want the systems to be as autonomous as possible, operating without the need for complex programming and hardware,” she said. “We have shown that simple chemical processes coupled to buoyancy forces, which arise naturally in chemical solutions, provide the instructions for particles to form complex systems and movements, potentially, just as with the earliest forms of life.”
Novel Gene Therapy Delivery System to the Retina

Gene therapy is a highly promising approach for the treatment of retinal blinding diseases, however, safe, efficient, and controlled gene delivery to the retina, particularly in the macula, remains a significant challenge. McGowan Institute for Regenerative Medicine affiliated faculty member José-Alain Sahel, MD (pictured), Chair and Distinguished Professor of the Department of Ophthalmology at the University of Pittsburgh School of Medicine, Director of the UPMC Eye Center, and the Eye and Ear Foundation Endowed Chair of Ophthalmology, is the co-principal investigator on the newly funded NIH/National Eye Institute project entitled, “Retinal-adhesive thermoresponsive gel for AAV-mediated gene delivery to the outer retina.” The project began on September 30, 2022, running for almost 4 years.
This project will develop an innovative biocompatible retinal-adhesive gel loaded with next-generation viral vectors as a new approach for safe and efficient targeting of photoreceptors and RPE via focal epiretinal gene delivery. As proof of concept, this novel gene therapy delivery system will be used to treat two well-characterized models of human retinal degeneration diseases: NPHP5-LCA and RPE65-LCA2.
The abstract of this project follows:
Until recently, there have been no effective treatments for retinal degenerations. FDA approval of Luxturna, the first gene therapy for bi-allelic mutations in RPE65, has opened the field for application to a broader range of retinal diseases. The rapidly growing number of clinical trials and emerging companies reflect the impact of this success and indicate the high expectations for retinal gene therapy. However, there is a significant need to develop new approaches for the many remaining forms of retinal degenerations, as RPE65-related dystrophies affect a very small population of patients. Moreover, the combination of the vector and surgical approach used for this disease is suboptimal for targeting the fovea in retinas with significant structural alterations. The major obstacles that must be addressed to improve clinical outcomes and extend the application of gene therapies to numerous retinopathies at various disease stages are: 1) efficient vector delivery to the central retina without damaging remaining photoreceptors, a significant, documented concern with sub-retinal injections in conditions where the retina is structurally compromised, 2) efficiently targeting gene delivery to affected cells, especially photoreceptors and RPE across the retina, and 3) limiting the inflammatory/immune responses associated with intravitreal injections. These issues are relevant to all current and future retinal gene therapy programs. Here, we address each of these obstacles through development of an innovative new epiretinal gene therapy approach for NPHP5-LCA and RPE65-LCA2, in which a novel, biocompatible, retinal adhesive gel developed by our team releases high efficiency AAVs directly to the retina. We have created a comprehensive and efficient development plan that allows for rapid translation, drawing on the complementary skill sets of a team of experts with a track record of successful translational development. We will further develop the tunable, biocompatible gel and injection system to deliver these vectors directly to the retina, and we will incorporate a backing layer into the implant that allows for directionality of vector release for increased efficiency. We will determine the most efficient implant-compatible photoreceptor and RPE-targeting AAV vectors by utilizing our recently developed single cell RNA-Seq paradigm. We will fully validate this new gene therapy platform in two well-studied naturally occurring models of LCA that affect primarily the photoreceptors (NPHP5-LCA) and RPE (RPE65-LCA2), and we will characterize immune response and toxicity. The innovative approach developed herein will result in a new platform for direct, non-invasive, and efficient AAV delivery to the retina, reducing diffusion and required dosage as well as the related immune response. This novel gene delivery platform has direct applicability to all outer retinal disease targets, paving the way forward for a safer, more efficient, and targeted approach to treat a wide spectrum of disorders.
Congratulations, Dr. Sahel!
Parkinson’s-Related Gene Also Impacts Brain Cell Branching and Function

As reported by Asher Jones, Manager/Science Writing for UPMC, a protein called PINK1 promotes complex branching of neurons in mice, according to a new study led by McGowan Institute for Regenerative Medicine affiliated faculty member Charleen Chu, MD, PhD (pictured), that could explain why Parkinson’s disease patients with mutations in the PINK1 gene often have cognitive impairment.
The findings by University of Pittsburgh researchers, published in The Journal of Neuroscience, may also help explain how neurodegeneration progresses in other diseases such as Alzheimer’s and could eventually point to new treatment approaches.
“We’re really excited about these findings because we show that PINK1 has an impact specifically on neuron structure and function, which we think could lead to a better understanding of why neurons degenerate and how we can prevent or reverse that process,” said senior author Dr. Chu who holds the A. Julio Martinez chair in neuropathology and is a professor of pathology in the Pitt School of Medicine.
Parkinson’s disease is driven by loss of neurons in the substantia nigra, a brain region that regulates movement, leading to trembling or shaking, stiffness and problems with balance and coordination. The genetic and environmental causes of Parkinson’s are complex and not fully understood, but several different genes have been linked with development of the disease.
Beyond motor symptoms of Parkinson’s, some patients also experience cognitive issues related to memory and attention, develop psychiatric or mood disorders, and have increased risk of developing dementia. These symptoms occur in patients with two mutated copies of the PINK1 gene and also in family members with a mutation in only one copy of the gene, suggesting that this protein plays a key role in brain function.
To learn more about PINK1 in neurons, Dr. Chu and her team studied mice that lack expression of the PINK1 gene. Using a microscope, they looked at the tree-like branches called dendrites that extend out from the neuron to receive signals.
Each neuron in the brain may be connected to thousands of other neurons. The more dense and intricate a neuron’s branches, the more connections called synapses it makes with other neurons, allowing better flow of information through the brain via electrical impulses. Simplification of this branching and loss of synaptic connections are associated with cognitive decline.
In normal mice, dendrites were intricately branched like a bushy, overgrown shrub. But in the mice that didn’t produce PINK1, the branches were less complex, as though the shrub had been pruned by an overzealous gardener.
The researchers also zoomed in to look at projections called dendritic spines, the structures that form a synapse with the end of other neurons. Like an unripe bud that unfurls into a leaf, dendritic spines begin as slender, hair-shaped structures called filopodia before swelling and finally maturing into toadstool-shaped mushroom spines.
“In neurons from the cortex of animals that lacked PINK1, we saw a decrease in the number of spines overall as well as altered distribution in the type of spines,” explained Dr. Chu. “We saw a shift from mushroom spines – which support strong neuronal connections and are important for memory — to filopodia, which are not yet functional.”
As a result of altered spine density and distribution, the electrical firing of these neurons was decreased, pointing to diminished connections between brain cells in the absence of PINK1.
When the researchers reintroduced human PINK1 to mouse neurons that lacked the protein, dendritic branching, spine density and neuron firing were restored to levels seen in normal neurons, confirming the importance of this protein for healthy neuron structure and function in both rodents and humans.
The next step for Dr. Chu and her team is to learn more about how PINK1 regulates dendritic branching and spine development. This research could eventually lead to new treatments to prevent or treat neurodegeneration in Parkinson’s and other diseases associated with reduced PINK1 expression such as some forms of Alzheimer’s.
Illustration: UPMC.
The Mysterious Mechanics of Morphogenesis

Tissue engineering—creating a “seed” of tissue that could grow into a functional, life-saving organ—sounds like magic. But it could become technology, thanks in part to the work led by McGowan Institute for Regenerative Medicine affiliated faculty member Lance Davidson, PhD (pictured), Professor and Wellington C. Carl Faculty Fellow of Bioengineering in the University of Pittsburgh’s Swanson School of Engineering.
Dr. Davidson’s MechMorpho lab in the Swanson School of Engineering works at the interface of physics and biology to understand both biological and physical principles of morphogenesis: the development of embryonic form in the frog embryo. The work not only lays the groundwork to better understand human cell and tissue development but also has implications for furthering tissue engineering, preventing birth defects, and understanding the effect of tissue mechanics on cancer cell growth and proliferation. The National Institutes of Health recently awarded Dr. Davidson a MERIT (Method to Extend Research in Time) Award of $2.2 million to carry out this work.
“We’re trying to understand, from a physical perspective, how organisms and their organs form,” said Dr. Davidson. “Development is commonly viewed as a cascade of biochemical reactions that “magically” generate the body and organs of the embryo. Our group seeks to open up that magic black box and understand how physical processes convert those reactions into work and living structures.”
The MERIT award is a highly prestigious award provided by the NIH to only the most outstanding scientists. The five-year grant — with an opportunity to renew for another five years, based on progress made in the first five years — allows recipients to focus more on their research and less on the need to continually seek renewed funding.
“Lance’s research on how cellular biomechanics contributes to the development of organisms at the cellular and tissue levels has wide-ranging implications for human health,” said Mark Redfern, PhD, professor and interim department chair of bioengineering. “This MERIT award is recognition by NIH of the importance of this work and the potential for new breakthroughs in the future. The very competitive award is well-deserved.”
The MechMorpho Lab’s long-term goal is to reverse-engineer the embryo and organ formation.
“There are a diverse set of chemical and physical pathways that regulate morphogenesis and that interact with the environment. In this project we want to understand how passive mechanics and active forces shape tissues in an early vertebrate embryo,” said Dr. Davidson. “We aim to understand the coupling between cell biological and physical mechanisms that drive cell shape changes, control cell behaviors, generate forces, and create tissue properties like stiffness.”
Embryonic engineering
Convergent extension — the process by which an embryo elongates from a simple clump of cells and begins to take on the shape of its eventual body — is crucial to a vertebrate’s development. If the convergent extension process goes wrong, it often leads to developmental defects in organs and overall anatomy.
Understanding the mechanical basis of this process is a central question in the field of developmental biology. It gives fresh insights into the fundamental principles of organ development and how a tissue assembles itself as it grows and regenerates.
To study this process, Dr. Davidson’s lab relies on frog embryos before they are recognizable as tadpoles. Frogs share a surprising amount of DNA with humans but develop much more quickly. In previous work, Dr. Davidson and his team were able to characterize the dynamics of key mechanical properties and the proteins involved in morphogenesis. They used confocal microscopy to observe protein complexes within living cells in an embryo on a sub-micron scale, finding that events happening on this microscopic level have a major impact on large-scale events.
Mechanical memory
Organ growth can follow mechanical clues from their environment, but researchers don’t yet know how those clues are stored or how they dissipate. At the intersection of developmental biology and bioengineering, Dr. Davidson is in a unique position to understand these important processes.
In this project, the team will dig deeper and understand how tissues are storing mechanical information.
“If genes are interacting with mechanics, the mechanics have to be able to hang around in a way that can be sensed by genetic pathways,” explained Dr. Davidson. “If mechanical stresses dissipate quickly, it is like a mechanical form of amnesia with cells forgetting their past and possibly resetting their biological processes to an earlier state.”
Reverse engineering organ and tissue development will be a great advance in tissue engineering, but it can also lead to medical interventions to better diagnosis and treatment.
“A great number of human diseases are associated with defects in mechanical signaling pathways,” explained Dr. Davidson. “Cardiovascular disease, for example, is thought to be triggered by defects in how cells interact with blood flow.”
The spread of cancer is also clearly dependent on mechanics, Dr. Davidson said, as cells are triggered to migrate out of a tumor by the mechanical microenvironment that surrounds them. “If you can modulate the environment around a tumor, and keep it in a softer state, the cells might just stay put,” he said.
This knowledge will give researchers a better basis to understand birth defects and their risk factors. For example, in spina bifida there are genetic risk factors but the mechanisms that cause them to manifest are unknown. If there is a clear mechanical element to the risk, future research could identify ways to offset this risk with medication.
“Our work opens the door for researchers to develop new hypotheses of morphogenesis and bioengineering tools to test them,” said Dr. Davidson. “With a better understanding of the role of mechanics in development, we can understand so much more about the human body and how we can overcome some of its most significant ailments.”
Congratulations, Dr. Davidson!
Development of Specific Immunotherapies for Allergic Contact Dermatitis

Allergic contact dermatitis (ACD) is a substantial health problem that with a prevalence of 18% is the second highest cause of work-related diseases in the USA. There is an unmet need for more specific immunotherapies to treat ACD, which is caused by intricate interactions among neuro-mediators, skin resident cells, and migratory leukocytes. The NIH National Institute of Allergy and Infectious Diseases funded a 5-year project to investigate relevant mechanisms of these cellular and neuroimmune networks to provide fundamental physiopathologic insight for the development of specific immunotherapies for ACD.
McGowan Institute for Regenerative Medicine affiliated faculty member Adriana Larregina, MD, PhD (pictured), Professor of Dermatology and Immunology at the University of Pittsburgh School of Medicine, is a co-principal investigator on this project entitled, “Role of Neurokinin 1 Receptor Signaling in Keratinocytes in Allergic Contact Dermatitis.” Work began on September 19, 2022.
The project abstract follows:
Allergic contact dermatitis (ACD) is a highly common chronic inflammatory skin disease initiated by skin exposure to a hapten, which triggers local neuroinflammatory responses and promotes the activation of pathogenic Type 1 biased effector T cells that sustain the chronicity of the disease. There is an unmet need for specific immunotherapies to treat ACD, and development of these treatments requires a thorough understanding of the cellular and molecular mechanisms of the disease. During hapten penetration of the skin, signaling the neurokinin-1 receptor (NK1R) by the proinflammatory neuropeptides substance P and hemokinin-1 promotes cutaneous inflammation. This proinflammatory skin environment is required for the T cell-stimulatory and Type 1 biasing function of resident dendritic cells (DCs). Because lacking NK1R in mice impairs the development of ACD it has been proposed that blockade of the receptor could be beneficial to treat the disease. Understanding the impact of NK1R-signaling in the immune response to haptens is of fundamental relevance for therapies attempting to inhibit the function of the receptor to treat ACD. Nonetheless, the cellular and molecular mechanisms and the pathogenic consequences of hapten-mediated NK1R activation in the skin remain to be elucidated. Keratinocytes constitute the first line of defense affected by contact sensitizers and they have per se a relevant role in innate and adaptive components of skin-initiated immune responses. Keratinocytes are the main cell subset that expresses constitutively the NK1R. Using NK1Rfl/fl mice, we published that specific deletion of the NK1R in keratinocytes impairs the synthesis and secretion of IL-1β, a cytokine necessary for the activation of T-cell stimulatory DCs and inhibits the innate and adaptive immune responses of ACD. Therefore, we hypothesize that: “Keratinocytes are early cell targets of hapten-mediated NK1R-signaling, and that they are required to generate the proinflammatory skin environment that supports the innate and effector immune responses of ACD.” We will address this hypothesis in the following specific aims. Specific aim 1 will analyze the mechanisms of the proinflammatory effects caused by NK1R-signaling in keratinocytes exposed to haptens. Specific aim 2 will analyze the mechanisms of intercellular communication by which keratinocytes interact with skin resident DCs to promote their T cell stimulatory functions during hapten initiated skin inflammation. Specific aim 3 will analyze the role of the NK1R-signaling exclusively in keratinocytes in the generation of the effector and memory T cells of ACD. Our studies include in-vitro and in-vivo mouse models and ex-vivo human skin models to test the translational relevance of our experiments. If successful, the data generated through this application will provide highly relevant missing information for the development of efficient specific therapies for the prevention and treatment of ACD.
Congratulations, Dr. Larregina!
Deep Learning Models to Predict the Intensity of Myofascial Pain

McGowan Institute for Regenerative Medicine affiliated faculty member Kang Kim, PhD (pictured), Associate Professor of Medicine and of Bioengineering at the University of Pittsburgh and the Heart and Vascular Institute at UPMC, is a co-principal investigator on the project entitled, “Development and Validation of a Multimodal Ultrasound- Based Biomarker for Myofascial Pain.” The NIH National Center for Complementary & Integrative Health funded this 3-year project which began on September 19, 2022. It is a two-phase study to develop and validate a biomarker for lumbar myofascial pain based on ultrasound obtained measurements of the lumbar muscles and fascia. Dr. Kang and the researchers will use advanced machine learning approaches and validation in a randomized controlled trial.
The project abstract follows:
Myofascial pain can affect many regions of the body and it is a key component of chronic low back pain in particular. Patients with chronic low back pain have a range of musculoskeletal pathologies perpetuating their pain syndrome in addition to the myofascial components, such as facet arthritis or stenosis. Hence, there is a significant clinical need to identify the components of chronic low back pain related to myofascial pain beyond use of the physical exam only. Such a biomarker would have immediate clinical diagnostic uses, as well as being important as a phenotyping tool and outcome measure in clinical trials. Advances in ultrasound technology have resulted in identification of several abnormalities in myofascial tissues related to myofascial pain, beyond identifying trigger points. In addition to echogenicity changes, these include shear wave elastography of muscles and fascia, and dynamic fascia tissue deformation capturing abnormalities in movement/glide of fascia tissue during lumbar flexion. Despite the clinical need and the available technology, no comprehensive study has integrated these ultrasound measures to validate a biomarker for the myofascial component of chronic low back pain. First, we propose to perform two detailed ultrasound assessments and standardized physical exams (including pressure algometry for painful trigger points) on 160 subjects each with and without chronic low back pain, divided into 4 phenotypic groups with and without painful trigger points. We will correlate the ultrasound measures to the clinical phenotype. Second, we will then use deep learning approaches to construct explainable machine learning models which integrate these measures to classify and predict the myofascial components of chronic low back pain, with latent and/or active trigger points. As performance metrics, we will report on area under the curve (AUC), sensitivity, and specificity. Third, in the R33 phase we will perform a single blinded, randomized controlled trial of dry needling versus sham needling in 80 patients with chronic low back pain and active trigger points. We will collect the ultrasound measures and perform a standardized examination for myofascial pain prior to the intervention and at a one-week follow-up. We will test the ability and performance metrics of the deep learning models to predict the intensity of myofascial pain prior to injection and changes in myofascial pain post-needling. We anticipate that this work will lead to a software module which can be incorporated into existing clinical ultrasound machines for assessment of the myofascial components of musculoskeletal pain.
Congratulations, Dr. Kim!
FLASH Registry: Mechanical Thrombectomy Confers Good Short-Term Outcomes in PE

Erik Swain, editor for Healio, reported on the research presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2022 Conference in Boston. He recounted that in patients with intermediate- and high-risk pulmonary embolism (PE), mechanical thrombectomy was safe and linked with good short-term hemodynamic and clinical outcomes, according to data from the FLASH registry.
The cohort included 800 U.S. patients with PE (mean age, 61 years; 54% men; 76.7% intermediate-risk), of whom 32.1% had contraindications for thrombolysis. All underwent mechanical thrombectomy with the FlowTriever System, Inari Medical, that extracts PE thrombus by aspiration or mechanical modes without need for thrombolytics. McGowan Institute for Regenerative Medicine affiliated faculty member Catalin Toma, MD (pictured), assistant professor of medicine at the University of Pittsburgh School of Medicine and director of interventional cardiology at the UPMC Heart and Vascular Institute, reported new data from the FLASH registry at a press conference at TCT 2022. The results were simultaneously published in Eurointervention.
“Mortality remains high in the PE space, up to 10% at 30 days in recent registries,” Dr. Toma said at the press conference. “Perhaps what’s less appreciated is the comorbidity these patients have with readmission rates, and up to 50% are still having symptoms at 6 months after their acute PE. The current guidelines don’t put a lot of emphasis on advanced therapies; they are reserved for patients who … have a contraindication to lytics or failed therapy. The problem with the lytic approach upfront is the risk of bleeding and the requirement for ICU stay. The mechanical thrombectomy approach can provide rapid resolution of the clot and improvement in hemodynamics without the risk of bleeding.”
The primary major adverse event endpoint — device-related death at 48 hours, major bleeding at 48 hours, and intraprocedural device- or procedure-related events — occurred in 1.8% of patients, Dr. Toma said, noting there were no deaths, 11 major bleeds, and three intraprocedural device- or procedure-related events.
The rates of all-cause mortality were 0.3% at 48 hours, 0.8% at 30 days, and 5% at 6 months, he said.
The 30-day mortality rate was far lower than that observed in the PERT Consortium Quality Database and in a meta-analysis of catheter-directed thrombolysis studies, Dr. Toma said.
He added the rates of 30-day readmission were 6.2% for any cause, 1.4% for something related to the PE treatment, and 4.8% for something unrelated to the PE treatment. This was far lower than that observed in the PERT Consortium, he said.
During the procedure, pulmonary artery pressure dropped a mean of 7.6 mm Hg, or 23% (P < .0001), while cardiac index increased by a mean of 0.3 L/min/m2, or 18.9% (P < .0001), according to the researchers.
More than half (62.6%) of patients required no overnight ICU stay.
At 48 hours, the right ventricle/left ventricle ratio on echocardiography declined from 1.23 to 0.98 (P < .0001), and the improvement continued through mean latest follow-up of 105.2 to 116.3 days, according to the researchers.
The percentage of patients with severe dyspnea dropped from 66.5% at baseline to 15.6% at 48 hours, Dr. Toma and colleagues found.
“The FLASH registry demonstrates the excellent safety profile of the FlowTriever System in a real-world PE population,” Dr. Toma said at a press conference. “These are patients who based on their baseline characteristics have high risk. Nevertheless, mortality remained less than 1% at the 30-day visit. Most importantly, hemodynamics improved rapidly during the procedure and clinical recovery continued for 6 months.”
Randomized controlled trials, including PEERLESS, are planned, he said.
Mr. Swain is a medical journalist and editor. He received his AB in Politics from Princeton University in 1993. Mr. Swain has been in medical publishing since 2013. He is currently Executive Editor of Healio Cardiology and Cardiology Today. Over the course of his career, he has covered, in addition to the clinical side, the business, manufacturing, packaging, R&D and regulatory aspects of health care.
Identifying New Therapeutic Targets to Treat Intervertebral Disc Degeneration

Research to investigate the role of a new pathway in intervertebral disc degeneration and low back pain was funded by the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases. The proposed studies entitled “Metabolic Symbiosis: Lactate as an Epigenetic Regulator and a Biofuel in Age-dependent Intervertebral Disc Degeneration,” aim to confirm the significant roles of lactate as an important source for disc nutrition and gene regulation. This is a new research direction to identify novel therapeutic targets for this common and costly condition.
The co-principal investigator on the almost 5-year project is McGowan Institute for Regenerative Medicine affiliated faculty member Nam Vo, PhD (pictured), Associate Professor in the University of Pittsburgh’s Department of Orthopaedic Surgery with a secondary appointment in the Department of Pathology. The project started on September 15, 2022.
The abstract for this project follows:
Intervertebral disc degeneration (IDD) underlies many spinal disorders and results in debilitating back pain, disability, and tremendous socioeconomic burden. The intervertebral disc (IVD) is the largest avascular organ comprised of a hypoxic nucleus pulposus (NP) center surrounded by an outer, more oxygenated annulus fibrosus (AF). The IVD contains copious amounts of lactic acid, which has long been viewed as a harmful waste byproduct of anaerobic glycolysis in the NP. However, we recently made two major advances that challenge this longstanding dogma. We demonstrated that AF cells can take up and utilize lactate as a carbon source via oxidative phosphorylation (OXPHOS), thus unveiling lactate-dependent metabolic symbiosis between NP and AF whereby the hypoxic NP cells make lactate to be used by the more aerobic AF cells as a carbon biofuel via OXPHOS. We also discovered high levels of IVD histone lactylation, a newly characterized type of histone post-translational modification (PTM) that uses lactate as a substrate precursor. Histone PTMs are critical to the dynamic modulation of chromatin structure and gene expression, and dysregulation of histone PTMs is closely linked with the development of many diseases. Based on our preliminary data, we hypothesize that disc lactate is not as a waste byproduct but rather serves as an important biofuel for the nutrient-poor disc and as a vital metabolic regulator of disc gene expression programming via histone lactylation. We propose three specific aims to test this hypothesis: (1) Determine whether lactate functions as an important metabolic regulator of disc gene expression through histone lactylation using rat disc cell culture models treated with chemical inhibitors of enzymes responsible for histone lactylation; (2) Determine whether disc histone lactylation and lactate-dependent metabolic symbiosis malfunction with age contributing to age-related IDD using young and old Fischer 344 rats; and (3) Determine whether interrupted disc lactate-dependent metabolic symbiosis disrupts disc histone lactylation pattern and promotes IDD using transgenic mouse models with AF-targeted genetic depletion to disrupt AF lactate uptake. Completion of the proposed studies will establish whether histone lactylation exerts epigenetic transcription regulation that controls disc matrix homeostasis and lactate-dependent metabolic symbiosis. Demonstrating the influences of lactate metabolism on age-related IDD through the mechanisms of epigenetic gene regulation and lactate-dependent metabolic symbiosis will be both novel and significant to identify new therapeutic targets to treat IDD with greater likelihood of success in the nutrient poor environment. This will be an innovative approach and significant advance over prior regenerative efforts which have had limited success in this unique tissue.
Congratulations, Dr. Vo!
Hydrogels Pave Way for Future of Soft Robotics
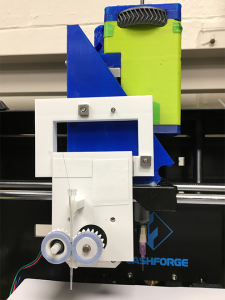
As their name suggests, hydrogels begin in liquid form as monomers. This viscous liquid, which can be made of synthetic or natural materials from polyester to sodium alginate, can be used as ink for 3D printing. The ink is first loaded into a syringe, then pumped through the needle as a thin filament and solidified following 3D printing to form a multidimensional structure, in the same way that Jell-O is mixed up first as a liquid before turning into a soft, bendable dessert. When hydrogels are placed in the right environment, the monomers in the liquid crosslink to form polymers, which gives shape to the hydrogel and lets it trap water.
You might imagine that these supple materials are also delicate—and that’s one drawback of working with hydrogels for robotic applications. To solve this problem and allow hydrogels to be used in a greater variety of tasks and harsh environments, researchers from Carnegie Mellon University–Wenhuan Sun, a PhD student in mechanical engineering, co-advised by McGowan Institute for Regenerative Medicine affiliated faculty members Victoria Webster-Wood, PhD, and Adam Feinberg, PhD–designed a continuous fiber extruder, a device that reinforces the hydrogels, so they don’t easily break apart or lose their shape when loaded. Dr. Feinberg, a professor of biomedical engineering and materials science and engineering, previously created the 3D printer that the fiber extruders were first tested on.
Embedding fibers into hydrogels during the printing process reinforces their mechanical properties so they’re not as fragile. Creating an open-source, commercially available fiber extruder will benefit future research with hydrogels. Not only is the team’s extruder design relatively cheap at about $53, but it’s also compatible with many at-home 3D printing devices and has been tested successfully in hydrogels embedded with both synthetic and natural fibers, including silk and collagen. The team’s paper, published in HardwareX, serves almost as a formula for other researchers who want to experiment with fiber embedded hydrogel 3D printing.
“This paper describes the entire process of how we built the fiber print hub so that other people can just reference our work and then build their own without additional instruction,” Mr. Sun says of how their research serves the robotics community.
When hydrogels maintain their structural integrity, they can be applied to a wider variety of situations. Their unique attributes like flexibility and softness make them ideal tools for drug delivery and tissue engineering, but physical sturdiness opens the door to broader tasks in soft robotics. Since the continuous fiber extruders work well with natural materials like collagen and alginate, reinforced hydrogels are poised to become an adaptable material for soft robots, and they are an environmentally friendly one, too.
“We’re really interested in how we can use biodegradable materials in robots,” says Dr. Webster-Wood, an assistant professor of mechanical engineering who founded the Biohybrid and Organic Robotics Group. “These plant-based hydrogels are a really interesting direction, because we can basically farm the materials for the robots and make them renewable.”
Illustration: 3D printing devices are crucial for enabling the fiber extruder to reinforce the hydrogel. Biohybrid and Organic Robotics Group, Carnegie Mellon University.
A Smart Pill

There are approximately 25 million Americans that suffer from Type 2 diabetes. Furthermore, about 35 million Americans >20 years old are diagnosed with prediabetes. More than one-third of Americans are also obese, an incidence that has nearly tripled from 1960 to 2010. Recent studies have shown that the gut-brain axis places a critical role in digestive and metabolic diseases. Further, direct communication between the sensory neurons in the gut and neural reward circuits in the brain plays a crucial role in detecting chemical cues such as hormones, satiety signals, or small molecule metabolites produced by bacteria in the gut.
With almost 3 years of funding from the NIH’s National Institute of Biomedical Imaging and Bioengineering, McGowan Institute for Regenerative Medicine affiliated faculty member Christopher Bettinger, PhD (pictured), Professor in the Departments of Biomedical Engineering and of Materials Science and Engineering at Carnegie Mellon University, will serve as the principal investigator on the project entitled, “Ingestible electronic devices for non-invasive vagal stimulation.” The project began on September 1, 2022. If successful, ingestible devices that modulate the gut-brain axis can serve as a platform technology to better understand and treat digestive and metabolic diseases including obesity and diabetes.
The abstract for this project reads:
There are approximately 25 million Americans that suffer from Type 2 diabetes. Furthermore, about 35 million Americans >20 years old are diagnosed with prediabetes. More than one-third of Americans are also obese, an incidence that has nearly tripled from 1960 to 2010. Recent studies have shown that the gut-brain axis places a critical role in digestive and metabolic diseases. Further, direct communication between the sensory neurons in the gut and neural reward circuits in the brain plays a crucial role in detecting chemical cues such as hormones, satiety signals, or small molecule metabolites produced by bacteria in the gut. Chemicals in the small intestine are detected by specialized epithelial cells that transduce chemical signals into neuronal activity that can be interpreted by the central nervous system. An ingestible device that supersedes chemical cues and stimulates sensory neurons directly using electronic pulses could mimic the chemical milieu in the gut that is associated with healthy metabolic and digestive states. Spatiotemporal control of intraluminal sensory nerve stimulation in the gut could with help us understand the link between chemical signaling in the digestive system and neural circuits in the brain that are modulated by the gut-brain axis. Here we propose an ingestible electronic device with flexible electrodes that can pace sensory nerve activity in the gut of a pig model. Physiological responses will be measured, and brain activity will be monitored using fMRI. Initially, this project will validate this proof-of-concept using tethered electrodes in porcine subjects. Specifically, sensory neurons in the gut will be paced using biomimetic waveforms that will simulate fed or fasted states in porcine subjects while brain activity will be measured simultaneously using fMRI. These results will help us understand the connection between gustatory signaling, sensory nerve activity, and neural reward circuits in the brain such as satiety. In the future, we will design a fully autonomous smart pill with an on-board power supply and circuitry that selectively and non-invasively stimulate or block the activity of sensory neurons in the gut of human subjects. If successful, this smart pill could serve as a low-risk device-based approach to help understand and potentially treat digestive and metabolic disorders of the gut-brain axis such as obesity and diabetes.
Congratulations, Dr. Bettinger!
AWARDS AND RECOGNITION
The American Board of Oral and Maxillofacial Surgery Names New President

The American Board of Oral and Maxillofacial Surgery (ABOMS) named McGowan Institute for Regenerative Medicine affiliated faculty member Bernard Costello DMD, MD, FACS (pictured), as its new president during its September 2022 Annual Meeting in New Orleans. Dr. Costello is an accomplished oral and maxillofacial surgeon, professor, and leader in the field; he will serve a one-year term.
“For me, the chance to work with the Board of Directors of the American Board of Oral and Maxillofacial Surgery as president is a career pinnacle. Our organization does important work stringently vetting oral and maxillofacial surgeons and, ultimately, protecting the public,” said Dr. Costello.
ABOMS conducts a robust certification process in which oral and maxillofacial surgeons submit application materials and undergo rigorous examinations. Once board-certified, Diplomates are required to annually submit evidence of certification maintenance.
This will not be Dr. Costello’s first role on the ABOMS Board of Directors; he served as Vice President from 2021-2022 under Past President Vincent J. Perciaccante, DDS, FACS. Of the board’s work, Dr. Costello says, “We work together with those in our specialty to cultivate excellence and continuously take our process to the next level. It is an honor and privilege to do this work.”
In the past, Dr. Costello has served as President of the American Academy of Craniomaxillofacial Surgeons and the American Cleft Palate-Craniofacial Association. A pediatric craniofacial specialist, Dr. Costello is also highly accomplished in the academic and hospital settings. He is the Associate Vice Chancellor for Health Science Integration and works with the senior administration in the Health Sciences and UPMC. He has been a full-time faculty member at Pitt since 2001 and is the former dean of the School of Dental Medicine. Dr. Costello is Professor of Oral and Maxillofacial Surgery, and Chief of Pediatric Maxillofacial Surgery at UPMC Children’s Hospital where he the longest serving surgeon on the cleft-craniofacial team. He has pioneered research in the use of regenerative medicine for craniofacial deformities and authored guidelines for responsible pain management that focuses on a non-opioid approach, advocating for measures to fight the opioid addiction crisis.
Congratulations, Dr. Costello!
Dr. Kyle Orwig Receives the ASRM Distinguished Researcher Award
McGowan Institute for Regenerative Medicine affiliated faculty member Kyle Orwig, PhD (pictured), professor in the Department of Obstetrics, Gynecology and Reproductive Sciences in the University of Pittsburgh School of Medicine, has been chosen as the recipient of the Distinguished Researcher Award from the American Society for Reproductive Medicine (ASRM). This award recognizes an ASRM member who has made outstanding contributions to clinical or basic research in reproduction published during the previous 10 years.
He will be recognized for this achievement during the president’s gala and opening ceremony of the ASRM 2022 Scientific Congress on Monday, Oct. 24, in Anaheim, California, and present during the 2023 Annual Meeting of the Society for the Study of Reproduction.
Dr. Orwig was recruited to join Magee-Womens Research Institute and the University of Pittsburgh as a tenure track professor in 2003 following his postdoctoral and junior faculty position at the University of Pennsylvania, where he studied stem cells and spermatogenesis, or sperm cell development.
He currently directs the UPMC Fertility Preservation Program and the UPMC Magee Center for Reproduction and Transplantation. His research lab focuses on germ lineage development, stem cells, and fertility and infertility. Located in the Magee-Women Research Institute and Magee-Womens Hospital, the Orwig Lab has received funding from the National Institutes of Health, the Sylvia Bernassoli Fund, the Richard King Mellon Foundation, and others.
Dr. Orwig serves as an ASRM Research Institute Advisory Committee Chair and has had work published more than 100 times in a leading biomedical database.
Congratulations, Dr. Orwig!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#238 –– Dr. Mo Ebrahimkhani discusses his research in systems and synthetic biology-based approaches to program development of induced pluripotent stem cells.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: P. Anthony Otero, Gabriella Fricklas, Aparna Nigam, Britney N. Lizama, Zachary P. Wills, Jon W. Johnson and Charleen T. Chu
Title: Endogenous PTEN-Induced Kinase 1 Regulates Dendritic Architecture and Spinogenesis
Summary: Mutations in PTEN-induced kinase 1 (PINK1) contribute to autosomal recessive Parkinson’s disease with cognitive and neuropsychiatric comorbidities. Disturbances in dendritic and spine architecture are hallmarks of neurodegenerative and neuropsychiatric conditions, but little is known of the impact of PINK1 on these structures. We used Pink1−/− mice to study the role of endogenous PINK1 in regulating dendritic architecture, spine density, and spine maturation. Pink1−/− cortical neurons of unknown sex showed decreased dendritic arborization, affecting both apical and basal arbors. Dendritic simplification in Pink1−/− neurons was primarily driven by diminished branching with smaller effects on branch lengths. Pink1−/− neurons showed reduced spine density with a shift in morphology to favor filopodia at the expense of mushroom spines. Electrophysiology revealed significant reductions in miniature EPSC (mEPSC) frequency in Pink1−/− neurons, consistent with the observation of decreased spine numbers. Transfecting with human PINK1 rescued changes in dendritic architecture, in thin, stubby, and mushroom spine densities, and in mEPSC frequency. Diminished spine density was also observed in Golgi–Cox stained adult male Pink1−/− brains. Western blot study of Pink1−/− brains of either sex revealed reduced phosphorylation of NSFL1 cofactor p47, an indirect target of PINK1. Transfection of Pink1−/− neurons with a phosphomimetic p47 plasmid rescued dendritic branching and thin/stubby spine density with a partial rescue of mushroom spines, implicating a role for PINK1-regulated p47 phosphorylation in dendrite and spine development. These findings suggest that PINK1-dependent synaptodendritic alterations may contribute to the risk of cognitive and/or neuropsychiatric pathologies observed in PINK1-mutated families.
Source: Journal of Neuroscience. 12 October 2022, 42 (41) 7848-7860.
GRANT OF THE MONTH
PI: Ipsita Banerjee and Prashant Kumta
Title: Integrating Artificial Intelligence with Bioprinting for Future Manufacturing of Organoids
Description: Human pluripotent stem cell (hPSC) derived organoids are highly valuable for disease treatment and therapeutic development. Translating organoids to practical use will require scalable and reproducible methods to produce high-quality organoids. This project will devise strategies exploiting three-dimensional (3D) bioprinting for scalable organoid manufacturing. Machine Learning (ML)/Artificial Intelligence (AI) techniques will be developed to rapidly assess organoid quality from microscopy images. The goal is to offer a pathway for large-scale manufacturing of tailored and patient-specific organoids by augmenting ML/AI techniques for 3D bioprinting. The research will provide trainees with multidisciplinary skill sets, encompassing bioprinting, organoid engineering, imaging, and AI methods. In addition, the project will integrate global and social outreach aspects into engineering curriculum, offering a holistic educational pathway to train the future, globally aware U.S. biomanufacturing workforce to lead the world in revolutionary manufacturing concepts.
This proof-of-concept Future Manufacturing Seed Grant (FMSG) project aims to integrate beneficial mechano-signaling pathways for organoid manufacturing via ML/AI aided 3D bioprinting. In executing this approach, the goal is to develop non-invasive assays to measure organoid phenotype to meet the critical quality attributes of the manufacturing pipeline. The central hypothesis is that imposing cell confinement via encapsulation will induce favorable mechano-transduction pathways, which will enhance organoid differentiation by deactivating actin filaments. The resulting cytoskeletal modification from actin remodeling can be exploited to extract information about cell characteristics to predict phenotype. This hypothesis will be tested by developing techniques for (i) cell-confinement driven organoid derivation using 3D bioprinting; and (ii) predicting cell fate from cytoskeletal modifications using Artificial Intelligence (AI) models to identify features of organoid development in bright-field images. This FMSG project demonstrate the feasibility of integrating AI with 3D bioprinting in the organoid generation pipeline, with the long-term goal of enabling scalable manufacturing of organoids with stringent in-line quality control.
Source: National Science Foundation
Term: January 1, 2023 – December 31, 2024
Amount: $500,000
