March 2022 | VOL. 21, NO. 3| www.McGowan.pitt.edu
Dr. David Kaczorowski on Xenotransplantation

McGowan Institute for Regenerative Medicine affiliated faculty member David Kaczorowski, MD, Surgical Director of the Advanced Heart Failure Center of UPMC Heart and Vascular Institute and an Associate Professor of Cardiothoracic Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh, was a member of the surgical team on the historic first successful transplant of a porcine heart into an adult human with end-stage heart disease. As a follow-up to this extraordinary accomplishment by the surgical team, Dr. Kaczorowski published an article entitled, “Organs from genetically engineered pigs may help shorten the transplant wait list,” in The Conversation. The article focuses on the history of xenotransplantion, or transplanting animal organs into human beings. The article is repeated here:
Organs from genetically engineered pigs may help shorten the transplant wait list
Demand for life-saving organ transplantation is at an all-time high. In 2021, a record 41,000-plus organ transplants were performed in the U.S., with top numbers for kidney, liver, and heart transplants. But a limited supply of donor organs remains an ongoing problem. Currently over 100,000 people are on the transplant wait list in the U.S., and many more are unable to get on the list because of strict eligibility requirements and racial disparities in access.
As a cardiac transplant surgeon, I have personally witnessed the tragedy of this shortage of donor organs. But I have also seen the potential of one possible solution to this problem: xenotransplantation, or transplanting animal organs into human beings.
In September 2021, researchers successfully transplanted two genetically engineered pig kidneys into a brain-dead patient. And in January 2022, I was part of the surgical team that conducted the first pig-to-human heart transplant in a living patient. Recent news about the patient’s death two months after the procedure is sobering, but researchers like me remain optimistic. While much work still needs to be done, these successes point to how far science has come toward making animal-to-human transplants a viable treatment possibility.
Early attempts
While animal-to-human transplants have attracted considerable attention recently, many attempts have been made to transplant animal cells, tissues, and organs into humans over the past 60 years, with varying degrees of success.
In the 1960s, kidney transplantation was not broadly practiced because of a lack of donor organs. Ethical and legal concerns made it difficult to obtain live donors, and organs collected from deceased donors did not meet much success.
So, a surgeon named Keith Reemtsma performed a series of 12 kidney transplants using chimpanzees as donors. While most of the transplanted organs – and thus the human patients – survived for only a few weeks, one of the patients survived for nine months. Infection was the major issue in half of the patients, while irreversible organ rejection occurred in the other half.
Thomas Starzl is another surgeon who attempted animal-to-human organ transplants. He performed a similar series of kidney transplants around the same time as Reemtsma using baboons as donors, with the organs surviving up to two months. He’s most known for his liver transplants, with three attempts using chimpanzee livers from 1966 to 1974 that lasted from 24 hours to less than 14 days. In the early 1990s, his two baboon liver transplants lasted for 26 and 70 days. While one of the baboon livers functioned well, the patient ultimately died from overwhelming infection.
Doctors have also made attempts to transplant animal hearts, the first of which predated the first human-to-human heart transplant. In 1964, a chimpanzee heart transplanted by James Hardy survived for only a few hours. Len Bailey’s 1983 attempt at transplanting a baboon heart into an infant known as Baby Fae prolonged her life for 20 days, a record at the time.
Overcoming barriers
While these early results may seem poor at first glance, a number of these transplants actually lasted longer than many early human-to-human kidney transplants. The first patient to receive a donated kidney lasted for only four days in 1933, and later attempts in the 1940s and 1950s yielded similar results. Immunosuppressing drugs that prevent the immune system from attacking donor organs also weren’t available at the time of these early attempts at xenotransplantation, pointing to the promise of these procedures as science advanced.
But transplanting organs across species faces a number of obstacles, the most integral of which is evolution. As species grow apart, increasing differences in their molecular makeup can result in incompatibilities that make cross-species transplant difficult or impossible. Among the most problematic are differences in immunity, inflammation, and blood clotting that damage both the transplanted organs and the host’s body.
The similarity of nonhuman primates like chimpanzees and baboons to humans, both in anatomy and in their immune systems, made them appealing donors for early transplants. But their strong similarities to people also raised ethical concerns that dissuaded some physicians like Starzl from using them as donors.
On the other hand, pigs offer a potentially better source of donor organs. Compared with nonhuman primates, pigs mature much more quickly and produce more offspring. They are also a common source of food for people, and their tissues are already used for prosthetic heart valves and other medical treatments.
While pig-to-human transplants have also been attempted in the past, 80 million years of evolution stood in the way. Pigs have molecules on the surfaces of their cells that humans do not. If these molecules are introduced into a person’s body, their human immune system will register them as foreign and mount an attack. This process, called hyperacute rejection, is a central reason many transplanted animal organs fail.
A number of advances that reduce these incompatibilities have helped overcome the problem of hyperacute rejection. Genetically engineered pigs without the genes that produce the foreign molecules triggering rejection and with additional human genes that help the recipient’s body accept the new organ are one key improvement. The pig heart my team and I transplanted this year was genetically engineered, as were the pig kidneys from late 2021. There have also been improvements in medications that suppress the immune system of the recipient so it’s less likely to mount an attack against the organ.
Looking forward
Recent successes with genetically engineered pig transplants make clear that xenotransplantation is no longer a dream from a distant future but something becoming increasingly achievable by modern medicine.
But many questions still remain. What is the best way to suppress a recipient’s immune system so the transplanted organ survives but the risk of infection stays low? Can animal organs be tailored to individuals to minimize rejection? How can animal organs be better preserved and distributed?
Answering these and many other questions will be key to realizing the therapeutic potential of xenotransplantation and helping the hundreds of thousands of people waiting for an organ.
RESOURCES AT THE MCGOWAN INSTITUTE
April Histology Special – Toluidine Blue
Mast cells play a key role in the inflammatory process.
A mast cell is a myeloid derived cell and is part of the immune system. Mast cells contain distinctive granules rich in histamine and heparin. Although best known for their role in allergy and anaphylaxis, mast cells play an important protective role as well, being intimately involved in angiogenesis, defense against pathogens, and wound healing.
Toluidine blue is one of the most common stains for acid mucopolysaccharides and glycoaminoglycans, both of which are components of mast cells granules.
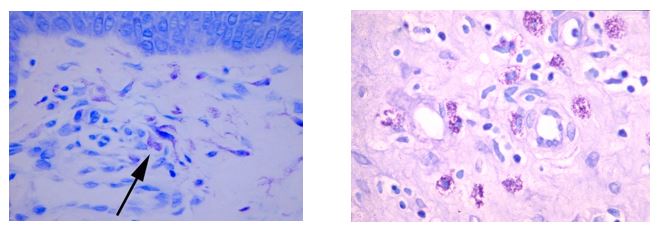
You’ll receive 25% off your Toluidine Blue staining for the entire month of April when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
March is Women’s History Month

Women’s History Month had its origins as a national celebration in 1981 when Congress passed Pub. L. 97-28 which authorized and requested the President to proclaim the week beginning March 7, 1982, as “Women’s History Week.” This continued throughout the next five years. In 1987 after being petitioned by the National Women’s History Project, Congress passed Pub. L. 100-9 which designated the month of March 1987 as “Women’s History Month.” Since 1988, presidents have issued a series of annual proclamations designating March as “Women’s History Month.” These proclamations celebrate the contributions women have made to the United States and recognize the specific achievements women have made over the course of American history in a variety of fields.
The 2022 Women’s History theme, “Providing Healing, Promoting Hope,” is both a tribute to the ceaseless work of caregivers and frontline workers during this ongoing pandemic and also a recognition of the thousands of ways that women of all cultures have provided both healing and hope throughout history.
Notable Pitt Women You Should Know This Women’s History Month

From top: Dr. Anna Balazs and Dr. Dan Ding
In celebration of the women of the University of Pittsburgh, Pittwire featured an article written by Acacia O’Connor entitled “Notable Pitt Women You Should Know This Women’s History Month.” Two of McGowan Institute for Regenerative Medicine affiliated faculty members were highlighted in the piece. They are:
- Anna Balazs, PhD, the John A. Swanson Chair in Engineering at the Swanson School of Engineering and a Distinguished Professor of Chemical Engineering in the Department of Chemical & Petroleum Engineering
- Dan Ding, PhD, Associate Professor in the Department of Rehabilitation Science and Technology with a secondary appointment in the Department of Bioengineering and a Research Scientist at the Human Engineering Research Laboratories in the VA Pittsburgh Healthcare System
Engineering from biology
Engineer and “biomimicry” scholar, Dr. Balazs is an internationally acclaimed expert, her many accolades also include being the first woman to receive the prestigious Polymer Physics Prize from the American Physical Society in 2016. Most recently, she was named as one of the newest members of the National Academy of Engineering — less than a year after being elected to the National Academy of Sciences. Read more about Professor Balazs work in “soft robotics” here.
Regenerating medical technology
Voice-activated technology has become a part of our everyday lives. For some, including those with disabilities and injuries, these can be life-giving technologies. This is where Dr. Ding comes in. Her research is changing the field of assistive robots and wearables to improve accessibility and promote wider wireless use. She is the principal investigator on a new Rehabilitation Engineering Research Center called Promoting Mainstream Wireless Inclusion through Technology Services. The project received a $4.6 million grant from the Department of Health and Human Services in 2021.

From top: Drs. Fatima Syed-Picard, Heather Szabo-Rogers, and Dobrawa Napierala
Celebrating Pitt Dental Medicine Women in Research During Women’s History Month: University of Pittsburgh Women in Craniofacial and Oral Research Committee
Started at the School of Dental Medicine, the University of Pittsburgh Women in Craniofacial and Oral Research Group serves to support the interests and development of women working in the areas of dental, oral, and craniofacial research. The Committee engages in activities to improve collaboration with other departments and programs at Pitt; highlights research accomplishments; cultivates communication, collaboration, and mentoring networks; emphasizes the value of diversity and inclusion in oral and craniofacial research; and advocates for the needs of women in the field to help reduce the world-wide dental research gender gap. Three McGowan Institute affiliated faculty members are on this committee:
- Fatima Syed-Picard, PhD, Assistant Professor in the Department of Oral Biology, University of Pittsburgh School of Dental Medicine, and a faculty member in the Center for Craniofacial Regeneration.
- Heather Szabo-Rogers, PhD, Assistant Professor in the Department of Oral Biology, University of Pittsburgh School of Dental Medicine, and a faculty member in the Center for Craniofacial Regeneration.
- Dobrawa Napierala, PhD, Associate Professor in the Department of Oral Biology, University of Pittsburgh School of Dental Medicine, and a faculty member in the Center for Craniofacial Regeneration.
Thank you to these and all of the women of the McGowan Institute for your continued contributions and successes!
Illustrations: National Women’s History Alliance (logo)/McGowan Institute for Regenerative Medicine (affiliated faculty members)
21st McGowan Institute for Regenerative Medicine Scientific Retreat

With over 200 in attendance, the McGowan Institute for Regenerative Medicine held its 2022 Scientific Retreat on March 7-8, 2022.
The participation and contributions of the guests and external collaborators – along with McGowan Institute for Regenerative Medicine affiliated faculty and trainees – provided for insightful topics for discussion. The program chair was Bryan Brown, PhD, associate professor Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute.
Program Highlights
The Director’s State of the Institute Address/Retreat Kickoff was presented by McGowan Institute Director William Wagner, PhD, Distinguished Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh.
The sessions addressed:
- Challenges of Biomedical Innovations and Entrepreneurship
- Synthetic Biology Approaches to Regenerative Medicine
- Regenerative Rehabilitation
- Cardiovascular Bioengineering
- Center for Military Medicine Research – Strategic Partnerships & Funding Opportunities
- Pediatric Device Trial Infrastructure: Clinical investigation of an implantable pediatric ventricular assist device–NHLBI PumpKIN (Pumps for Kids Infants & Neonates) Trial
Poster Session
The poster session was effective in introducing the focus of the Retreat and interests of the faculty and the guests. McGowan Institute faculty member Andrew Duncan, PhD, Associate Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. The winners of the poster session were:
*CELL AND GENE THERAPY*
Poster of Distinction
Raphael Crum
Immunomodulatory matrix-bound nanovesicles mitigate acute and chronic pristane-induced rheumatoid arthritis
Mentor: Stephen Badylak, DVM, PhD, MD
McGowan Institute for Regenerative Medicine
University of Pittsburgh
Merit Poster Award
Reiley Cotter
Role of microcalcifications in breast cancer bone mimicry
Mentor: Shilpa Sant, PhD
Dept. of Pharmaceutical Sciences
University of Pittsburgh
Merit Poster Award
Hannah Yankello
The impact of placental hypoxia on monocyte recruitment
Mentor: Elizabeth Wayne, PhD
Dept. of Chemical Engineering
Carnegie Mellon University
*COMPUTATION AND MODELING*
Poster of Distinction
Shaniel Bowen
Geometric characteristics of the pelvic floor muscles associated with anatomic recurrence after prolapse repair
Mentor: Steven Abramowitch, PhD
Dept. of Bioengineering
University of Pittsburgh
Merit Poster Award
Sommer Anjum
Using a cell-based model to investigate cellular mechanics during convergent extension and apoptotic cell extrusion in epithelial tissues
Mentor: Lance Davidson, PhD
Dept. of Bioengineering
University of Pittsburgh
*MEDICAL DEVICES*
Poster of Distinction
Tyler Meder
Assessing the macrophage-directed remodeling kinetics of a peripheral nerve matrix hydrogel
Mentor: Bryan Brown, PhD
Dept. of Bioengineering, McGowan Institute for Regenerative Medicine
University of Pittsburgh
Merit Poster Award
Fuat Baris Bengur
Isolated support with an extracorporeal perfusion system deters ischemia-related metabolic derangement of a rat fasciocutaneous free flap
Mentor: Mario Solari, MD
Dept. of Plastic Surgery
University of Pittsburgh
Merit Poster Award
Liyang Wang
Real-time nitric oxide monitoring for wound healing with flexible and multiplexed electrode array
Mentor: Tzahi Cohen-Karni, PhD
Dept. of Materials Science and Engineering
Carnegie Mellon University
*TISSUE ENGINEERING*
Poster of Distinction
Kai Wang
Engineering 3D skeletal muscle constructs to model age-related regenerative defects and responses to intervention
Mentor: Fabrisia Ambrosio, PhD, MPT
Dept. of Physical Medicine and Rehabilitation
University of Pittsburgh
Merit Poster Award
Piyumi Wijesekara
Engineering rotating apical-out airway organoids for assessing respiratory cilia motility
Mentor: Xi Ren, PhD
Dept. of Biomedical Engineering
Carnegie Mellon University
Merit Poster Award
Dorota Jazwinska
Study of ovarian cancer clearance dynamics utilizing a novel tumor-mesothelial assay
Mentor: Ioannis Zervantonakis, PhD
Dept. of Bioengineering
University of Pittsburgh
Merit Poster Award
Michelle Drewry
Scaffold-free conduits formed from dental pulp stem cell sheets provide neurotrophic and directional support for regenerating axons
Mentor: Fatima Syed-Picard, PhD
Dept. of Bioengineering
University of Pittsburgh
All poster session awards were sponsored by sciVelo. Poster of Distinction Award ($200) and Merit Poster Awards ($100). Congratulations to all!
See the full Retreat Program here.
A special thank you is extended to the retreat committee (Bryan Brown-Chair, Patrick Cantini, Andy Duncan, Rebecca Bauroth, Lainey Becker, Katy Wharton, Jamelle Price, John Murphy, and Stanley Stachelek) and to all who made this year’s Retreat a success!
Photo Credit: Jamelle Price.
Investigating the Effects of Critical Illness in Early Childhood on Neurocognitive Outcomes

Approximately 23,700 children in the U.S. undergo invasive mechanical ventilation for acute respiratory failure annually. Although most survive, little is known if they have worse long-term neurocognitive function than children who do not undergo such procedures. There are concerns about neurotoxic effects of critical illness and its treatment on the developing brain. Therefore, infants and young children may be uniquely susceptible to adverse neurocognitive outcomes after invasive mechanical ventilation.
A four-year sibling-matched cohort study, published in JAMA, conducted at 31 U.S. PICUs and associated neuropsychology testing centers sheds light on the subject. McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, executive vice president and chief innovation officer at UPMC, distinguished professor and the Mitchell P. Fink Endowed Chair in Critical Care Medicine at the University of Pittsburgh, and chair of the Department of Critical Care Medicine, is a co-author on the study.
Researchers found that children who survived PICU hospitalization for respiratory failure and were discharged without severe cognitive dysfunction had significantly lower subsequent IQ scores than their matched siblings.
“While the difference in IQ scores between patients and unexposed siblings was small, the data provide strong evidence of the existence and epidemiology of pediatric post-intensive care syndrome (PICS-p) after a single typical episode of acute respiratory failure necessitating invasive ventilation among generally healthy children,” says Martha A.Q. Curley, PhD, RN, FAAN, Professor of Nursing at the University of Pennsylvania School of Nursing (Penn Nursing) and the study’s lead researcher.
The study reaffirms the importance of assessing long-term outcomes as part of any trial evaluating acute interventions in pediatric critical care. It also underscores the importance of further study to understand which children may be at highest risk, what modifiable factors could cause it, and how it can be prevented.
Dr. Dan Ding: New Tech Making Fitness More Accessible to People of All Abilities
 Pittsburgh tech and education leaders at this year’s South by Southwest (SXSW) festival, held in Austin, Texas, March 6-20, 2022, were recognized by Sophie Burkholder, a Staff Writer for Technical.ly, a news organization that serves a community of technology professionals, including entrepreneurs, technologists, and their supporters. This is the first in-person SXSW since 2020.
Pittsburgh tech and education leaders at this year’s South by Southwest (SXSW) festival, held in Austin, Texas, March 6-20, 2022, were recognized by Sophie Burkholder, a Staff Writer for Technical.ly, a news organization that serves a community of technology professionals, including entrepreneurs, technologists, and their supporters. This is the first in-person SXSW since 2020.
McGowan Institute for Regenerative Medicine affiliated faculty member Dan Ding, PhD, an associate professor and researcher at the University of Pittsburgh’s Human Engineering Research Laboratories, participated in a SXSW panel discussion on “The Technology Making Fitness More Accessible.” Dr. Ding, who also has an appointment in Pitt’s Department of Bioengineering, discussed new tech making fitness more accessible to people of all abilities, including adaptive sports and wheelchairs designed for activities like hiking.
Dr. Ding has authored or co-authored over 140 peer-reviewed journal publications, conference papers and abstracts, and book chapters. She has been awarded grants from the VA, DOD, NIDRR, NSF, and NIH for her work. Dr. Ding has extensive experience in developing sensor-driven health applications for wheelchair users, and assistive robotic devices and systems. She was the Thrust Leader of the Home and Community Health and Wellness (HCHW) Testbed in the National Science Foundation’s Quality of Life Technology Engineering Research Center (a joint effort between Pitt and Carnegie Mellon), which focuses on creating and evaluating home and community-based solutions for assessing and informing health and functional status of people with disability and older adults.
Funding to Advance the Pittsburgh Center for Interdisciplinary Bone and Mineral Research
The multidisciplinary team consisting of seven McGowan Institute for Regenerative Medicine affiliated faculty members from four University of Pittsburgh Schools:
- Giuseppe Intini, DDS, PhD, Associate Professor of Periodontics and Preventive Dentistry at the School of Dental Medicine and an Associate Professor of Medicine at the School of Medicine
- Dobrawa Napierala, PhD, Associate Professor, School of Dental Medicine, Center for Craniofacial Regeneration
- Juan Taboas, PhD, Associate Professor with the Department of Oral and Craniofacial Sciences in the School of Dental Medicine and the Department of Biomedical Engineering in the Swanson School of Engineering
- Deborah Galson, PhD, Associate Professor of Medicine, Division of Hematology/Oncology and of Microbiology & Molecular Genetics, School of Medicine
- Peter Alexander, PhD, Assistant Director of the Center for Cellular and Molecular Engineering and an Assistant Professor in the Department of Orthopaedic Surgery
- Shilpa Sant, PhD, Associate Professor at University of Pittsburgh School of Pharmacy
- Partha Roy, PhD, Associate Professor of Bioengineering, Cell Biology, and Pathology
has been awarded $60,000 from the University of Pittsburgh’s Momentum Teaming grants program.
The award will be used to advance the Pittsburgh Center for Interdisciplinary Bone and Mineral Research (PCIBMR), a 73-member consortium of faculty whose research interests converge around musculoskeletal disorders, bone injuries, osteoporosis, cancer, and regenerative medicine.
The team will organize a multidisciplinary scientific retreat, a hands-on workshop on bioengineered models for bone research, and will develop a Bioengineered Models resource core and a Mechanosensing resource core.
Illustration: Pittsburgh Center for Interdisciplinary Bone and Mineral Research.
Newly Issued Patents for McGowan Institute Affiliated Faculty Members

During the month of February 2022, University of Pittsburgh innovators were issued eight U.S. patents. Five of those patents were based on research work conducted by McGowan Institute for Regenerative Medicine affiliated faculty members. Their newly issued patents include:
Treatment of Ocular Conditions Utilizing a Histone/Protein Deacetylase Inhibitor: Steven Little; Michelle Ratay (U.S. Patent No. 11,253,480)
Methods and Compositions for Diagnosis and Prognosis of Renal Injury and Renal Failure: Alexander Zarbock; Paul McPherson; John Kellum Jr. (U.S. Patent No. 11,243,202)
Management of Acute Kidney Injury Using Insulin-like Growth Factor-Binding Protein 7 and Tissue Inhibitor of Metalloproteinase 2: John Kellum Jr.; James Kampf; Paul McPherson (U.S. Patent No. 11,243,217)
Chemical Inhibition of the E3 Ligase Subunit FBX07 Confers Neuroprotection and Anti-Inflammatory Activity by Stabilizing Mitochondria: Yuan Liu; Beibei Chen; Rama Mallampalli; Charleen Chu (U.S. Patent No. 11,242,339)
Thermoresponsive Hydrogel Containing Polymer Microparticles for Noninvasive Ocular Drug Delivery: Morgan Fedorchak; Steven Little; Joel Schuman; Anthony Cugini (U.S. Patent No. 11,246,838)
During the month of January 2022, University of Pittsburgh innovators were issued 11 U.S. patents. Five of those patents were based on research work conducted by McGowan Institute for Regenerative Medicine affiliated faculty members. Their newly issued patents include:
ECM Hydrogel for Treating Esophageal Inflammation: Lindsey Saldin; Stephen Badylak; Juan Gutierrez (U.S. Patent No. 11,213,545).
SARS-COV-2 Subunit Vaccine and Microneedle Array Delivery System: Andrea Gambotto; Eun Kim; Louis Falo (U.S. Patent No. 11,213,482).
Self-Regulating Device for Modulating Inflammation: Yoram Vodovotz; Alexey Solovyev; David Okonkwo; Maxim Vyacheslavovich; Qi Mi; Jorg Gerlach; Gregory Constantine (U.S. Patent No. 11,224,685).
Methods and Compositions for Diagnosis and Prognosis of Renal Injury and Renal Failure: John Kellum Jr.; Paul McPherson (U.S. Patent No. 11,229,676).
Non-Noble Metal Based Electro-Catalyst Compositions for Proton Exchange Membrane Based Water Electrolysis and Methods of Making: Prashant Kumta; Karan Kadakia; Moni Datta; Oleg Velikokhatnyi; Prashanth Hanumantha (U.S. Patent No. 11,230,775).
Congratulations on these successful developments!
Illustration: U.S. Patent and Trademark Office logo.
Dr. Kurt Weiss Named MIB Agents’ 2021 OutSmarting Osteosarcoma Research Grant Winner

Congratulations to McGowan Institute for Regenerative Medicine affiliated faculty member Kurt Weiss, MD, Associate Professor, Vice Chair of Translational Research, University of Pittsburgh
Department of Orthopaedic Surgery, Division of Musculoskeletal Oncology, who was named the 2021 OutSmarting Osteosarcoma Research Grant Winner by the MIB (Make It Better) Agents, a leading pediatric osteosarcoma nonprofit.
The Outsmarting Osteosarcoma Research Award is a $100,000 grant over one year to Dr. Weiss who has a proven track record in osteosarcoma and a compelling study that will Make It Better for osteosarcoma patients. Details on his winning project follow:
Initiative Name: Exploring Aldehyde Dehydrogenase and Androgen Receptor Actions to OutSmart Osteosarcoma Metastases
Desired Impact: The desired impact of this research is to understand the importance of aldehyde dehydrogenase 1A1 (ALDH) and the androgen receptor (AR) as they relate to osteosarcoma metastatic potential. The ultimate translational goal of the project is to discover biologically intelligent targeted therapies for osteosarcoma metastases.
Congratulations, Dr. Weiss!
The Relationship Between Blood-Based Bioenergetics and Muscle Function, Mobility, and Aging
 Older adults experience declining mobility, which leads to diminished quality of life, high health care costs, and thus constitutes a major health care problem. It is unclear what factors lead to mobility decline, but energy production by the mitochondrion is thought to play a role. This longitudinal study co-lead by McGowan Institute for Regenerative Medicine affiliated faculty member Sruti Shiva, PhD, Professor of Pharmacology & Chemical Biology, University of Pittsburgh, utilizes new methodology to measure systemic mitochondrial function in blood cells of older people over time, to understand the role of mitochondrial function in age-associated physical function decline.
Older adults experience declining mobility, which leads to diminished quality of life, high health care costs, and thus constitutes a major health care problem. It is unclear what factors lead to mobility decline, but energy production by the mitochondrion is thought to play a role. This longitudinal study co-lead by McGowan Institute for Regenerative Medicine affiliated faculty member Sruti Shiva, PhD, Professor of Pharmacology & Chemical Biology, University of Pittsburgh, utilizes new methodology to measure systemic mitochondrial function in blood cells of older people over time, to understand the role of mitochondrial function in age-associated physical function decline.
Dr. Sruti’s project was funded by the NIH/National Institute on Aging and will run from February-2022 through November-2026. The abstract follows:
As people age, they experience declining physical performance, which is associated with diminished quality of life, augmented health care costs, and is a strong predictor of morbidity and mortality. Thus, uncovering mechanisms that underlie age-associated mobility decline and identifying reliable biomarkers to predict this decline is imperative for the development of interventions to maintain physical ability with age. Mitochondria generate chemical energy to support homeostatic function of most cells in the body, and mitochondrial dysfunction is linked to age-associated decline in physical performance. This has been studied predominantly in skeletal muscle mitochondria since muscle function is central to physical ability. However, it is recognized that muscle function is not the sole determinant of mobility, and that input from other organ systems (cardiovascular and central nervous system) is also required. While age associated mitochondrial dysfunction has been observed across all organ systems, the contribution of this systemic bioenergetic dysfunction to age-associated mobility decline has not been assessed.
The current study brings together two PIs with expertise in mitochondrial biology who have independently optimized and validated complementary assays (high resolution respirometry and Seahorse extracellular flux analysis) for the measurement of systemic bioenergetic function utilizing blood cells (platelets and peripheral blood mononuclear cells). Preliminary data using these assays show that blood cell mitochondrial function reflects bioenergetics of solid tissues (e.g., skeletal muscle, heart, lung, brain) and correlates with multiple measures of physical ability. However, it is unknown whether blood cell bioenergetics reflect skeletal muscle function or are predictive of mobility decline in older adults.
The Study of Muscle, Mobility and Aging (SOMMA) is a multi-site longitudinal study of older adults (≥70 years; n=875). SOMMA focuses on the relationship between skeletal muscle mitochondria and mobility decline and will obtain skeletal muscle biopsies to measure mitochondrial function in all participants. Physical performance measures will be assessed at baseline and three years follow-up.
The current proposal is an ancillary study that synergizes with SOMMA to add blood cell bioenergetic measurements in all SOMMA participants at baseline as well as at the three-year follow-up visit. Using these data, we will test whether blood cell bioenergetics are 1) reflective of skeletal muscle mass and function, 2) are associated with physical performance measures (400 m walk), and 3) are predictive of physical performance decline in older adults. Completion of this study will elucidate systemic mitochondrial changes that are associated with age-related physical decline, and potentially establish blood cell bioenergetics as a biomarker of systemic mitochondrial function that can be utilized as a surrogate for muscle biopsies, and as a predictor of mobility decline in the aging population.
Study Points to Missing Link Between Age-Related Fat Loss and Diseases of Aging

At Harvard Medical School during her first postdoctoral position, Aditi Gurkar, PhD, and her team were testing a cancer drug in young mice. The treatment worked, effectively shrinking the tumors, but when the researchers repeated the experiments in older rodents, the drug was no longer effective.
“This was a big wake up call for me,” said Dr. Gurkar, now an assistant professor of geriatric medicine at the University of Pittsburgh School of Medicine. “We weren’t addressing the question in the right way. The realization that age is the root cause of many diseases prompted me to switch fields from cancer biology to aging.”
Elderly people are highly prone to frailty, cardiovascular disease, cancer, arthritis, neurodegeneration, and other maladies. Exactly why age makes people more susceptible to these disorders is not yet clear, but recent research suggests that age-related diseases are often preceded by weight loss.
“People typically think that aging is linked with putting on fat, which is true, but there’s a tipping point when weight gain switches to weight loss: People seem to lose weight about nine to 10 years before they develop age-related diseases. However, there hasn’t been a mechanistic understanding of the connection between loss of fat and age-related diseases,” said Dr. Gurkar, who is a member of the University of Pittsburgh/UPMC’s Aging Institute. “We think we have stumbled upon this connection.”
In a new study, published in Science Advances, Dr. Gurkar and her team used a grain-of-sand-sized worm called Caenorhabditis elegans to show that DNA damage — a hallmark of aging — rewires metabolism, triggering breakdown of fat deposits and production of inflammatory compounds that drive age-related disorders. McGowan Institute for Regenerative Medicine affiliated faculty member Valerian Kagan, PhD, DSc, Professor and Vice-Chairman in the Department of Environmental and Occupational Health as well as a Professor in the Department of Pharmacology and Chemical Biology, the Department of Radiation Oncology, and the Department of Chemistry at the University of Pittsburgh, and the Director of the Center for Free Radical and Antioxidant Health, is a co-author of the study.
“DNA damage happens to all of us, all of the time,” said Dr. Gurkar. “Even sitting in the sun or eating that burger last night generates compounds that damage DNA. But with age, our DNA repair pathways become less efficient, and damage accumulates.”
To understand how persistent DNA damage drives aging, Dr. Gurkar and her team used C. elegans. The worm’s 20-day lifespan allows scientists to study aging without gaining too many grey hairs themselves. C. elegans shares many cellular features and molecular pathways with mammals, making insights potentially relevant to humans.
The researchers compared normal C. elegans to mutants that lacked key DNA repair genes. Unable to fix DNA lesions, the mutants accumulate damage faster than usual and experience premature aging and shortened lifespans.
By “middle age,” the mutant worms had elevated expression of genes involved in lipid breakdown and depleted fat stores compared with their normal peers. When the researchers did the same experiments in “young adults,” they didn’t observe these differences. These results indicate that accumulation of DNA damage with age rewires cellular metabolism to breakdown fat deposits.
Looking more closely, the researchers found that lipid breakdown led to elevated levels of omega-6 polyunsaturated fatty acids in the mutant C. elegans. These fatty acids are precursors for compounds called lipid mediators, which promote inflammation, a known driver of age-related diseases.
The findings could explain how shedding pounds with age can lead to development of geriatric diseases, said Dr. Gurkar. They also add weight to the idea that DNA damage is more than just a consequence of getting old: It drives aging.
“When cells recognize DNA damage, they sound an alarm, and if the damage isn’t resolved, the siren never shuts off. It’s not the damage itself, but the noise that seems to be driving aging,” she explained.
When the researchers reduced fatty acid oxidation in mutant worms, they no longer lost fat stores and normal lifespan was restored.
“By reducing fatty acid oxidation, we can inhibit inflammation and excessive fat loss,” said Dr. Gurkar. “It’s about calming the cell down so it’s no longer screaming S.O.S. all the time.”
Lipid metabolism pathways in C. elegans are shared by mice and humans, suggesting that the team’s findings could help identify therapeutic targets for age-related diseases and accelerated aging disorders. This research could also explain why pediatric cancer patients who were treated successfully with chemotherapy or radiotherapy as children often become frail and have accelerated aging by their mid-40s.
“We plan to partner with pediatric doctors to follow chemotherapy patients over time,” said Dr. Gurkar. “By measuring their DNA repair proteins, we hope to identify patients who might be more susceptible to frailty and age-related disorders later in life. This could inform tailored, or precision medicine, approaches to chemotherapy dose.”
Agreement to Bioengineer Innovative Approaches to Organ Transplantation

McGowan Institute for Regenerative Medicine affiliated faculty member Keith Cook, PhD, Professor and Chair of the Department of Biomedical Engineering at Carnegie Mellon University (CMU), is the CMU lead in a research agreement between the Mayo Clinic and CMU to transform organ transplantation. The institutions will bioengineer innovative approaches to address barriers in organ transplantation.
“This relationship with the esteemed CMU Biomedical Engineering team is a very important step in Mayo Clinic’s Transforming Transplant strategic initiative,” says Burcin Taner, MD, chair of the Transplant Center at Mayo Clinic in Florida. “Research and innovation breakthroughs resulting from this initiative will address challenges and limitations that have historically existed for transplantation and subsequently unmet patient needs.”
“Mayo Clinic’s Center for Regenerative Medicine is excited to collaborate with Transplant Center colleagues at Mayo Clinic to support the innovation being driven through our unique engagement with CMU,” says Guojun Bu, PhD, associate director of the Center for Regenerative Medicine at Mayo Clinic in Florida. “This initiative will accelerate our mission in transforming the practice of medicine through biotherapeutic technologies that make organ transplantation more accessible, affordable and available to a broader population.”
As part of the collaboration, Mayo Clinic biomedical researchers and CMU faculty will focus on four core areas:
- Biofabrication
- Organ repair.
- Organ monitoring using sensor systems.
- Artificial intelligence to optimize transplant processes.
“Mayo Clinic is the preeminent academic medical center and the largest organ transplant provider in the United States, and CMU is a leader in innovating and applying cutting-edge technologies to real-world problems,” says Dr. Cook. “We are excited to bring these leading institutions together to create real improvements in access to, and effectiveness of, organ transplantation.”
CMU’s commitment to organ bioengineering is ongoing through its Bioengineered Organs Initiative founded and directed by Dr. Cook. This initiative facilitates collaborative research focused on designing, creating, and testing a new generation of long-term replacement organs that are fully biological, artificial, or a combination of both.
Both institutions also will participate in ongoing seminars focused on the challenges facing organ transplantation and the development of new technologies to address them.
Mayo Clinic is committed to transplantation research and innovation. Mayo Clinic’s Transplant Center, with locations in Arizona, Florida, and Minnesota, has performed more than 27,000 organ transplants since 1963. More than 100,000 patients nationwide are awaiting organ transplants.
Podcasts: Drs. Fabrisia Ambrosio and George Hussey

McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, MPT, is the Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh. She holds secondary appointments in the Departments of Bioengineering, Physical Therapy, Orthopaedic Surgery, Microbiology & Molecular Genetics, and Environmental & Occupational Health. Dr. Ambrosio’s research has the long-term goal of developing Regenerative Rehabilitation approaches to enhance skeletal muscle function with increasing age and in the setting of disease. Her laboratory uses murine and human models to investigate the underlying mechanisms by which targeted and specific mechanotransductive signals can be used to enhance donor and/or host stem cell functionality. Dr. Ambrosio’s research has been supported by the NIH, the DOD, the Foundation for Physical Therapy, the Claude D. Pepper Older American’s Independence Center, and the University of Pittsburgh Institute on Aging, among others.
Dr. Ambrosio recently spoke with Regenerative Medicine Today host John Murphy, McGowan Institute Executive Director, about
- Regenerative Rehabilitation and the International Consortium of Regenerative Rehabilitation, both founded by Dr. Ambrosio, the latter includes 16 participating institutions representing North America, Europe, and Asia
- Klotho molecule and its effect on older muscle strength
- Extracellular vesicles-based therapeutics via injection to muscle or bloodstream
Listen to their conversation here.
 George Hussey, PhD, is an Assistant Professor in the University of Pittsburgh School of Medicine, Department of Pathology. Prior to joining the Department of Pathology, he was a Research Assistant Professor, McGowan Institute for Regenerative Medicine, University of Pittsburgh School of Medicine, Department of Surgery. He was also a McGowan Institute Postdoctoral Associate in the laboratory of Stephen Badylak, DVM, PhD, MD, and a Postdoctoral Fellow in the laboratory of Philip Howe, PhD, Hollings Cancer Center, Medical University of South Carolina, Charleston, Department of Biochemistry & Molecular Biology.
George Hussey, PhD, is an Assistant Professor in the University of Pittsburgh School of Medicine, Department of Pathology. Prior to joining the Department of Pathology, he was a Research Assistant Professor, McGowan Institute for Regenerative Medicine, University of Pittsburgh School of Medicine, Department of Surgery. He was also a McGowan Institute Postdoctoral Associate in the laboratory of Stephen Badylak, DVM, PhD, MD, and a Postdoctoral Fellow in the laboratory of Philip Howe, PhD, Hollings Cancer Center, Medical University of South Carolina, Charleston, Department of Biochemistry & Molecular Biology.
Dr. Hussey’s research interests focus upon the discovery, development, and clinical translation of extracellular matrix (ECM)-based materials for functional tissue reconstruction. These interests also extend into an in-depth understanding of the molecular mechanisms of ECM-induced constructive remodeling, specifically in examining the remodeling response and the effect of matrix components on immune modulation toward a constructive, anti-inflammatory phenotype, with the broad goal of identifying molecular targets to bias these responses. Cell:Matrix interactions, including the role of matrix bound nanovesicles (MBV), constitutive phospholipids and incorporated (extracellular) RNA on matrix biology and the host tissue response, are prime targets of his investigations. With a more thorough understanding of matrix composition, as well as the interplay between fibrillar and soluble components within matrix, Dr. Hussey and his team will be able to apply a more rational approach toward the design of transformative new regenerative medicine technologies.
Dr. Hussey, a McGowan Institute for Regenerative Medicine affiliated faculty member, recently spoke with Regenerative Medicine Today host John Murphy, McGowan Institute Executive Director, about
- tissue engineering and the interplay of substrates which promote growth
- new formulations for ECM, i.e., hydrogels, and the clinical translation of ECM-based materials for functional tissue reconstruction
- ECM Therapeutics, local start-up company
Listen to their conversation here.
AWARDS AND RECOGNITION
Dr. Cecelia Yates Receives Chancellor’s Distinguished Research Award

University of Pittsburgh Chancellor Patrick Gallagher recognized 15 faculty members — and one entire office — this year with Chancellor’s Distinguished Awards across three categories. McGowan Institute for Regenerative Medicine affiliated faculty member Cecelia Yates, PhD, associate professor in the Department of Health Promotion and Development, School of Nursing, was recognized in the Chancellor’s Distinguished Research Awards/Junior category for her research to understand the signaling networks that underlie pathologic fibrosis across multiple tissue and organ systems. Cited as a “rising star” in the field, Dr. Yates’ approach also includes developing therapeutics for diseases caused by this phenomenon.
Dr. Yates received a letter from Chancellor Gallagher along with a $2,000 cash prize and a $3,000 grant to support her work. She will be recognized at the Faculty Honors Convocation on April 1 at 3 p.m. in Soldiers and Sailors Memorial Hall.
Congratulations, Dr. Yates!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#231 –– Dr. Harvey Borovetz discusses the design and clinical utilization of cardiovascular organ replacements.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Michel Modo, Harmanvir Ghuman, Reem Azar, Ryan Krafty, Stephen F Badylak, T Kevin Hitchens
Title: Mapping the acute time course of immune cell infiltration into an ECM hydrogel in a rat model of stroke using 19 F MRI
Summary: Extracellular matrix (ECM) hydrogel implantation into a stroke-induced tissue cavity invokes a robust cellular immune response. However, the spatio-temporal dynamics of immune cell infiltration into peri-infarct brain tissues versus the ECM-bioscaffold remain poorly understood. We here tagged peripheral immune cells using perfluorocarbon (PFC) nanoemulsions that afford their visualization by 19F magnetic resonance imaging (MRI). Prior to ECM hydrogel implantation, only blood vessels could be detected using 19F MRI. Using “time-lapse” 19F MRI, we established the infiltration of immune cells into the peri-infarct area occurs 5-6 h post-ECM implantation. Immune cells also infiltrated through the stump of the MCA, as well as a hydrogel bridge that formed between the tissue cavity and the burr hole in the skull. Tissue-based migration into the bioscaffold was observed between 9 and 12 h with a peak signal measured between 12 and 18 h post-implantation. Fluorescence-activated cell sorting of circulating immune cells revealed that 9% of cells were labeled with PFC nanoemulsions, of which the vast majority were neutrophils (40%) or monocytes (48%). Histology at 24 h post-implantation, in contrast, indicated that macrophages (35%) were more numerous in the peri-infarct area than neutrophils (11%), whereas the vast majority of immune cells within the ECM hydrogel were neutrophils (66%). Only a small fraction (12%) of immune cells did not contain PFC nanoemulsions, indicating a low type II error for 19F MRI. 19F MRI hence provides a unique tool to improve our understanding of the spatio-temporal dynamics of immune cells invading bioscaffolds and effecting biodegradation.
Source: Biomaterials. 2022 Mar;282:121386. doi: 10.1016/j.biomaterials.2022.121386. Epub 2022 Jan 25.
GRANT OF THE MONTH
PI: Sruti Shiva
Co-PI: Anthony J. Molina
Title: The relationship between blood based bioenergetics and muscle function, mobility, and aging
Description: As people age, they experience declining physical performance, which is associated with diminished quality of life, augmented health care costs, and is a strong predictor of morbidity and mortality. Thus, uncovering mechanisms that underlie age-associated mobility decline and identifying reliable biomarkers to predict this decline is imperative for the development of interventions to maintain physical ability with age. Mitochondria generate chemical energy to support homeostatic function of most cells in the body, and mitochondrial dysfunction is linked to age-associated decline in physical performance. This has been studied predominantly in skeletal muscle mitochondria since muscle function is central to physical ability. However, it is recognized that muscle function is not the sole determinant of mobility, and that input from other organ systems (cardiovascular and central nervous system) is also required. While age associated mitochondrial dysfunction has been observed across all organ systems, the contribution of this systemic bioenergetic dysfunction to age-associated mobility decline has not been assessed. The current study brings together two PIs with expertise in mitochondrial biology who have independently optimized and validated complementary assays (high resolution respirometery and Seahorse extracellular flux analysis) for the measurement of systemic bioenergetic function utilizing blood cells (platelets and peripheral blood mononuclear cells). Preliminary data using these assays show that blood cell mitochondrial function reflects bioenergetics of solid tissues (e.g. skeletal muscle, heart, lung, brain) and correlates with multiple measures of physical ability. However, it is unknown whether blood cell bioenergetics reflect skeletal muscle function or are predictive of mobility decline in older adults. The Study of Muscle, Mobility and Aging (SOMMA) is a multi-site longitudinal study of older adults (≥70 years; n=875). SOMMA focuses on the relationship between skeletal muscle mitochondria and mobility decline and will obtain skeletal muscle biopsies to measure mitochondrial function in all participants. Physical performance measures will at baseline and three years follow-up. The current proposal is an ancillary study that synergizes with SOMMA to add blood cell bioenergetic measurements in all SOMMA participants at baseline as well as at the three year follow up visit. Using these data, we will test whether blood cell bioenergetics are 1) reflective of skeletal muscle mass and function, 2) are associated with physical performance measures (400 m walk), and 3) are predictive of physical performance decline in older adults. Completion of this study will elucidate systemic mitochondrial changes that are associated with age-related physical decline, and potentially establish blood cell bioenergetics as a biomarker of systemic mitochondrial function that can be utilized as a surrogate for muscle biopsies, and as a predictor of mobility decline in the aging population.
Source: National Institute on Aging
Term: February 15, 2022 – November 30, 2026
Amount: $689,439 (one year)
