June 2020 | VOL. 19, NO. 6| www.McGowan.pitt.edu
Lab-Grown Miniature Human Livers Successfully Transplanted in Rats

Using skin cells from human volunteers, researchers at the University of Pittsburgh School of Medicine have created fully functional mini livers, which they then transplanted into rats.
In this proof-of-concept experiment, the lab-made organs survived for four days inside their animal hosts. These results were published in Cell Reports.
“Seeing that little human organ there inside the animal – brown, looking like a liver – that was pretty cool. This thing that looks like a liver and functions like a liver came from somebody’s skin cells,” said senior author Alejandro Soto-Gutierrez, MD, PhD, associate professor of pathology at Pitt and faculty member of both the McGowan Institute for Regenerative Medicine and the Pittsburgh Liver Research Center. Other McGowan Institute faculty member authors include Andrew Duncan, PhD, assistant professor in the Department of Pathology, and Ira Fox, MD, professor of surgery.
These mini livers secrete bile acids and urea, just like a normal liver, except they’re made-to-order in the lab using patient cells. And, although liver maturation takes up to two years in a natural environment, Dr. Soto-Gutierrez and colleagues did it in under a month.
The researchers created their mini livers by reprogramming human skin cells into stem cells, coaxing those stem cells to become various types of liver cells and, then, seeding those human liver cells into a rat liver with all of its own cells stripped out.
As an ultimate test, the researchers transplanted their lab-grown mini livers into five rats, who were bred to resist organ rejection. Four days after the transplant, researchers investigated how well the implanted organs were faring.
In all cases, blood flow problems had developed within and around the graft, but the transplanted mini livers worked – the rats had human liver proteins in their blood serum.
Dr. Soto-Gutierrez is optimistic that this research is not merely a stepping-stone on the path toward growing replacement organs in a lab, but also a useful tool in its own right.
“The long-term goal is to create organs that can replace organ donation, but in the near future, I see this as a bridge to transplant,” Dr. Soto-Gutierrez said. “For instance, in acute liver failure, you might just need hepatic boost for a while instead of a whole new liver.”
But there are significant challenges to overcome, he noted, including long-term survival and safety issues.
RESOURCES AT THE MCGOWAN INSTITUTE
July Histology Special
Reticular fibers, or reticulin is a type of fiber in connective tissue, composed of type III collagen secreted by reticular cells. Reticular fibers crosslink to form a fine meshwork (reticulin). This network acts as a supporting mesh in soft tissues such as liver, bone marrow, and the tissues and organs of the lymphatic system.
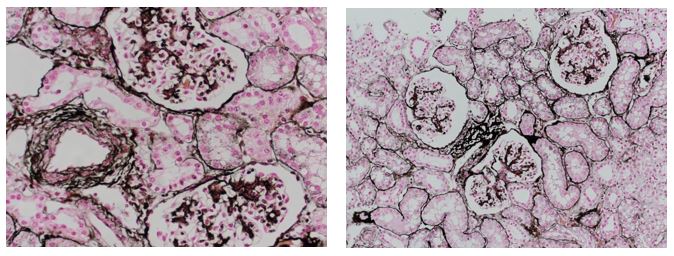
Jones Silver Stain demonstrates basement membrane and reticulin.
You’ll receive 25% off Jones Silver Stain in July when you mention this ad. Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: We ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you at the front door to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
Save the Date!
9th International Symposium on Regenerative Rehabilitation
 With a theme of “Where Applied Biophysics Meets Tissue Engineering & Cellular Therapies”, the Symposium is scheduled for December 3-5, 2020 at the University of Texas at Austin. The program features renowned researchers and clinicians from around the world, focusing on the emerging field of Regenerative Rehabilitation. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
With a theme of “Where Applied Biophysics Meets Tissue Engineering & Cellular Therapies”, the Symposium is scheduled for December 3-5, 2020 at the University of Texas at Austin. The program features renowned researchers and clinicians from around the world, focusing on the emerging field of Regenerative Rehabilitation. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
ICCAI’2020 Will Be Virtual

The 2020 International Conference on Complex Acute Illness (ICCAI’2020), titled “Meaningful AI in the Age of COVID-19”, will be held on September 10-11, 2020 entirely online, using Zoom teleconferencing technology. ICCAI is the annual meeting of the Society for Complex Acute Illness (SCAI), which, since its inception over 15 years ago, has successfully bridged the domains clinical practice of critical care medicine and quantitative approaches to understanding critical illness, with a strong translational focus. On the methodological side, it has emphasized eclecticism, covering, through its interdisciplinary membership, modeling approaches ranging from pure mechanistic differential equations models and agent-based models to purely data-driven machine learning techniques.
Please view the program and access the free registration site at http://iccai.org/
SCIENTIFIC ADVANCES
McGowan Institute – Regenerative Medicine Summer School 2020

The 7th Annual Regenerative Medicine Summer School was held virtually June 8-12, 2020, due to COVID-19. The program is led by Bryan Brown, PhD, Director of Educational Outreach, McGowan Institute for Regenerative Medicine. Dr. Brown is also Associate Professor in the Department of Bioengineering, Department of Obstetrics, Gynecology, and Reproductive Sciences, and the Clinical and Translational Science Institute at the University of Pittsburgh.
The McGowan Institute provides national and regional students with a week-long didactic learning experience addressing the science and engineering related to the multidisciplinary field of regenerative medicine. The program’s target audience is undergraduates, enrolled in a science or engineering program that completed their 3rd year of study. Also, exceptional candidates who completed their 2nd year of undergraduate study were considered.
This year’s lectures to 54 students (two students attended the program from Jordan and Taiwan) from 31 different academic institutions were live streamed via Zoom and addressed the areas of:
- Tissue Engineering
- Cell-Based Therapies
- Medical Devices
- Clinical Translation and Commercialization
The week’s program included a great cross-section of scientific topics given by McGowan Institute affiliated faculty and collaborators. They include:
Mechanical circulatory support for the failing heart
Harvey Borovetz, PhD
Distinguished Professor and former Chair (2002-2013), Bioengineering
Deputy Director of Artificial Organs and Medical Devices, MIRM
Tour of the Artificial Heart Program
Rick Schaub, PhD
Adjunct Assistant Professor, Bioengineering
Senior Director of the Artificial Heart Program, UPMC
Artificial lungs
Ryan Orizondo, PhD
Fellow, Clinical and Translational Science Institute
McGowan Institute for Regenerative Medicine
Center for Military Medicine Research – overview & project discussions
Ron Poropatich, MD
Professor, Pulmonary, Allergy, and Critical Care Medicine
Director, Center for Military Medicine Research, Health Sciences
Panel Discussion—Careers in Regenerative Medicine: Moderated by Bryan Brown, PhD
- Clint Skillen, Lab Technician, McGowan Institute
- Alexis Nolfi, PhD candidate, McGowan Institute
- Mangesh Kulkarni, PhD, Assistant Professor, Bioengineering
- Cecelia Yates, PhD, Associate Professor, Health Promotion & Development, School of Nursing, and Pathology, School of Medicine
Bioengineering of stem cells for diabetes therapy
Ipsita Banerjee, PhD
Associate Professor, Chemical and Petroleum Engineering, Bioengineering
Burns wound healing and autologous skin progenitor cell regenerative medicine R&D
Jörg Gerlach, MD, PhD
Professor, Surgery, and Bioengineering
Macrophage engineering: New approaches for drug delivery and diagnostics
Elizabeth Wayne, PhD
Assistant Professor, Biomedical Engineering and Chemical Engineering
Carnegie Mellon University
Growing a surrogate liver in a lymph node—from experimental approaches to potential clinical applications
Eric Lagasse, PharmD, PhD
Associate Professor, Pathology, Clinical and Translational Institute
Director, Cancer Stem Cell Center, McGowan Institute for Regenerative Medicine
Biomedical microscopy
Donna Stolz, PhD
Associate Director, Center for Biologic Imaging,
Associate Professor, Cell Biology and Pathology
Director, Cell Biology and Molecular Physiology Graduate Program
Biomaterials applied to support the failing heart
William Wagner, PhD
Professor, Surgery, Bioengineering, and Chemical Engineering
Director, McGowan Institute for Regenerative Medicine
Chairman, TERMIS – Americas
The extracellular matrix as an inductive scaffold for functional tissue reconstruction
Stephen Badylak, DVM, PhD, MD
Professor, Surgery
Director, Center for Pre-Clinical Tissue Engineering
Deputy Director, McGowan Institute for Regenerative Medicine
Tissue engineering strategies for severe peripheral nerve injuries
Kacey Marra, PhD
Professor, Plastic Surgery, and Bioengineering
Vice Chair of Research, Department of Plastic Surgery
Ocular drug delivery
Morgan Fedorchak, PhD
Assistant Professor, Ophthalmology, Chemical Engineering, and Clinical & Translational Sciences
Director, Ophthalmic Biomaterials Laboratory
Scaffold-free tissue engineering
Fatima Syed-Picard, PhD
Assistant Professor, Oral Biology, School of Dental Medicine
Airway tissue engineering
Riccardo Gottardi, PhD
Assistant Professor, Bioengineering and Biomaterials Laboratory
University of Pennsylvania, Children’s Hospital of Philadelphia
and
Soheila Ali Akbari Ghavimi, PhD
Postdoctoral Fellow, University of Pennsylvania, Children’s Hospital of Philadelphia
Regenerative medicine approaches in the study and treatment of human aortic disease
Julie Phillippi, PhD
Associate Professor, Cardiothoracic Surgery and Bioengineering
Director, Postdoctoral Research, Cardiothoracic Surgery
The new ex-vivo world and the business of science
Paulo Fontes, MD
Professor, Surgery
Director, Surgical Innovation & Research
West Virginia University
Brain tissue regeneration after stroke
Michel Modo, PhD
Professor, Radiology
Measurement, modeling, and modulation of the inflammatory response
Yoram Vodovotz, PhD
Professor, Surgery, Immunology, Computational & Systems Biology, Bioengineering, and Communication Disorders
Director, Center for Inflammation and Regenerative Modeling
Career lessons and pivots from an academic entrepreneur
Don Taylor, MBA, PhD
Assistant Vice Chancellor, Health Sciences Translation
Executive Director, sciVelo
Associate Director, Center for Medical Innovation
Associate Professor, Biomedical Informatics, Plastic Surgery, and Bioengineering
Adipose-derived therapies for regenerative medicine
Lauren Kokai, PhD
Assistant Professor, Plastic Surgery
Through the generosity of donors and sponsors, this year’s program was free to the participating students. The summer school program has been endorsed by the Society for Biomaterials.
Thanks to the faculty and staff for your contributions to this very successful event.
A Micro Look at Metastatic Environments in Ovarian Cancer

According to the American Cancer Society, a woman’s risk of being diagnosed with ovarian cancer during her lifetime is about one in 78. The majority of ovarian cancer patients are diagnosed with metastatic disease that spreads to other parts of the body and have a low five-year survival rate.
McGowan Institute for Regenerative Medicine affiliated faculty member Ioannis Zervantonakis, PhD, assistant professor of bioengineering at the University of Pittsburgh, received an award from the Elsa U. Pardee Foundation to develop microfluidic models of metastatic microenvironments in ovarian cancer and study mechanisms of cancer cell survival in these microenvironments.
The tumor microenvironment is the collection of cells, molecules, and blood vessels that surround tumor cells. Tumor growth, metastasis, and response to therapy is governed by a complex interaction network between tumor cells and those components.
“We hypothesize that during the early steps of metastasis formation, ovarian cancer cells recruit macrophages that in turn disrupt mesothelial barrier function to support adhesion and invasion,” said Dr. Zervantonakis, who runs the Tumor Microenvironment Engineering Laboratory in the Swanson School of Engineering.
In this project, they will use microfluidic technology, which allows researchers to create precise and controlled environments that can mimic human systems. Dr. Zervantonakis will develop a novel microfluidic device that will recreate a dynamic tumor-macrophage-mesothelial 3D metastatic microenvironment.
The research team will profile these metastatic microenvironments in vivo and evaluate the predictive capacity of the microfluidic device. They will also analyze ovarian cancer signaling in hopes of identifying targets that can be combined with current therapies to eliminate disease more effectively.
“Understanding cell behavior in native tumor microenvironments and developing new strategies to deliver therapeutics directly to tumor cells are critical in improving and extending patients’ lives,” said Dr. Zervantonakis.
Crafting a Better Graft

As the Baby Boomer generation gets older, the number of Americans over 65 continues to grow. With this growth, there is a need for improved medical technology that will help clinicians more effectively treat common age-associated conditions such as heart disease — a leading cause of death in the U.S.
McGowan Institute for Regenerative Medicine affiliated faculty member David Vorp, PhD, the Associate Dean for Research and the John A. Swanson Professor of Bioengineering at the University of Pittsburgh Swanson School of Engineering, received a $394,300 award from the National Institutes of Health to address this issue and will lead a project to improve technology for bypass grafting and hemodialysis.
Dr. Vorp’s Vascular Bioengineering Lab produces small-diameter, tissue engineered vascular grafts (TEVGs), which can be used to replace blood vessels damaged by coronary heart disease or to remove and return blood during dialysis.
A TEVG consists of a scaffold that provides a framework for seeded cells, which when given environmental cues, will promote tissue regeneration. These devices are an improvement on synthetic grafts which often become obstructed and fail, especially at small diameters. The degree of openness, known as patency, is a measure that defines the success of these devices.
“There is a lot of promise for this technology; however, we have only observed its effects in young recipients, despite the fact that older patients are the demographic most commonly in need of arterial bypass or hemodialysis,” said Dr. Vorp.
In this project, he seeks to understand how age affects the successful implantation of their small-diameter TEVG.
“A major complication is that older populations typically have high quantities of the plasma protein plasminogen activator inhibitor-1 (PAI-1), which could jeopardize the success of the device,” he said.
“We believe that elevated PAI-1 may compromise TEVG remodeling and patency,” he continued. “If increased levels of PAI-1 are associated with age, we want to determine if middle-aged and older recipients are capable of generating a successful TEVG.”
PAI-1 is a protein that comes with complications: it is important because it helps prevent premature clot removal after injury, but in elevated levels, it also increases risks for cardiovascular disease. There are FDA-approved drugs that inhibit PAI-1 production to help mitigate these issues, and as part of this project, Dr. Vorp will examine whether pharmacological intervention using these drugs will improve patency of their TEVGs.
“We hope that the results of this project will not only be foundational for tailoring a translatable TEVG for those who are most in need – elderly patients – but may also be paradigm-shifting in how TEVGs and other tissue engineering-based therapies are tested preclinically,” said Dr. Vorp.
“You Make Me Sick” Board Game Teaches Students About COVID-19
COVID-19 is highlighted in a newly updated board game, “You Make Me Sick,” to teach students about the immune system, infectious diseases, and good health practices.
Designed by Duquesne University’s Partnership in Education, the game is available as a free download and optimized to print from the organization’s website.
With funding from a Science Education Partnership Award from the National Institutes of Health, “You Make Me Sick” challenges players to successfully fight off and recover from common infectious diseases by learning about immunology and healthy habits. The game is designed for two to four players and recommended for children age 11-15 years old. The game provides a learning opportunity for the whole family and can be used by parents who are home schooling their children during the coronavirus pandemic.
“The game offers instructors and parents a fun, educational way to teach children steps on how to prevent the spread of diseases, including COVID-19,” said McGowan Institute for Regenerative Medicine affiliated faculty member John Pollock, PhD, professor of biological sciences at Duquesne University and the game’s creator. “The game informs players about bacteria and viruses, and highlights the importance of healthy habits, such as good nutrition and exercise, to successfully fight and prevent disease.”
An award-winning educator, Dr. Pollock originally developed the game in 2007 and updated it now with information about the coronavirus.
“Students, like many of us, have questions about how diseases spread and affect the body,” he said. “This game helps to explain the importance of white blood cells and how they work to eliminate germs from your body.”
As director of the university’s Partnership in Education, Dr. Pollock has developed a wide range of educational and multimedia resources for school children, including Emmy® Award winning educational television programs, apps, animated movies and teaching curriculum, most of which is available for free to educators.
Illustration: The “You Make Me Sick” board game teaches students about diseases, including COVID-19. Duquesne University.
NeuBase Therapeutics Announces Positive, Preclinical Data Validating its Novel Genetic Therapy PATrOL™ Platform

NeuBase Therapeutics, Inc., a biotechnology company developing next-generation antisense oligonucleotide (“ASO”) therapies to address genetic diseases, recently announced positive preclinical data from its pharmacokinetics studies in non-human primates (“NHPs”) and in vitro pharmacodynamics data in patient-derived cell lines. NeuBase believes these data validate the key advantages of the proprietary NeuBase peptide-nucleic acid (“PNA”) antisense oligonucleotide (PATrOL™) platform and support the Company’s decision to advance the development of its Huntington’s disease (“HD”) and myotonic dystrophy type 1 (“DM1”) programs, as well as the potential expansion of its therapeutic pipeline into other indications.
“We believe the PATrOL™ platform has the potential to create drugs that are easy for patients to take at infrequent intervals after they have tested positive for a genetic disease but before symptoms emerge,” said McGowan Institute for Regenerative Medicine affiliated faculty member Dietrich Stephan, PhD, chief executive officer of NeuBase. “We believe the best way to effectively manage degenerative genetic diseases is to get ahead of the disease process, and we believe that can only be achieved with early diagnosis coupled with well-tolerated, effective, and easily administered therapies.”
Dr. George Church, professor of genetics at Harvard Medical School and member of the National Academy of Sciences, stated, “Given the activity and broad biodistribution observed in these studies and the potential for easier target definition, I believe the PATrOL™ technology may have a potent impact on the future of drug development and treatment of genetic diseases.”
Non-Human Primate Pharmacokinetic Study
Quantitative whole-body autoradiography was performed on NHPs. A PATrOL™-enabled compound was radio-labeled, and the resulting material was injected into NHPs at 5 mg/kg via a bolus tail vein injection. The major conclusions from this study include:
- Rapid uptake of compound out of the body’s circulation after systemic intravenous administration, with a half-life in circulation of approximately 1.5 hours;
- Compound penetrates every organ system studied, including the central nervous system and skeletal muscle;
- Compound crosses the blood-brain barrier and into the key deep brain structures, including the caudate, supporting a key capability for the development of the Company’s lead program in HD;
- Delivery of the compound to skeletal muscle, the primary organ system that is affected in DM1;
- Because both HD and DM1 have manifestations outside of the primary affected organ, the broad biodistribution of the compounds may enable a potential whole-body therapeutic solution in both indications.
- Therapeutically relevant doses persist for greater than one week in NHPs after single-dose injection;
- 96% of administered compound remained in vivo after a one-week period (latest timepoint tested);
- Redistribution over one week after administration between organ systems enriches concentrations in key brain regions up to two-fold, including in those deep brain structures most relevant for HD;
- Retention of ~90% of compound concentrations achieved in skeletal muscle over the course of one-week post-single-dose administration; and
- Sustained concentrations in many other key organ systems throughout the body may indicate the potential for durable therapeutic responses and an infrequent dosing cadence.
Patient-Derived Huntington’s Cell Line Pharmacodynamic Studies
Multiple Huntington’s disease candidate compounds were incubated with HD-derived cells and assayed for their toxicity and their ability to selectively knock down mutant huntingtin protein (“mHTT”) expression by engaging with the CAG repeat expansion in the huntingtin (“HTT”) gene transcript. Multi-well plates were seeded with cells and candidates were added to the culture at various concentrations. The major conclusions from this study include:
- Activity in engaging target disease-causing transcripts and knocking-down resultant malfunctioning mHTT protein levels preferentially over normal HTT protein knock-down; and
- Dose limiting toxicities were not observed relative to a control either at or above the doses demonstrating activity in human cells in vitro.
In addition, PATrOL™ enabled compounds were generally well-tolerated in vivo after systemic administration, both after single dose administration in NHPs and multi dose administration in mice for over a month.
Dr. Robert Friedlander, chief medical officer of NeuBase and member of the National Academy of Medicine, stated, “An allele specific approach that can be systemically administered and cross the blood brain barrier would be an ideal drug profile for many untreatable genetic diseases. I believe that NeuBase is moving towards realizing this goal.”
The intersection of the NHP pharmacokinetic data and the in vitro patient-derived pharmacodynamic data provides a roadmap to create a pipeline of therapeutic candidates which can reach target tissues of interest after systemic administration and achieve the desired activity at that dose. NeuBase believes the data from these studies support the advancement of the Company’s HD and DM1 programs into lead optimization and subsequent IND-enabling studies, as well as provide a roadmap for the future expansion of the Company’s therapeutic pipeline into other indications, including oncology.
Dr. Sam Broder, former Director of the National Cancer Institute of the National Institutes of Health and member of the National Academy of Sciences, stated, “I believe that the NeuBase strategy of targeting transcripts before they become dangerous mutant proteins has the potential to deliver a dramatic improvement in our collective capabilities to effectively treat a wide range of genetic diseases, including some of the most deadly cancers, by targeting driver mutations and accelerating immunotherapy capabilities.”
European Society of Cataract & Refractive Surgeons Podcast: Dr. Jenny Yu

McGowan Institute for Regenerative Medicine affiliated faculty member Jenny Yu, MD, is the Vice Chair of Clinical Operations for the Department of Ophthalmology, UPMC Eye Center. Dr. Yu also serves on the Orbital, Oculoplastic, and Aesthetic Surgery Service, and is a Clinical Assistant Professor of Ophthalmology and Otolaryngology at the University of Pittsburgh.
Dr. Yu recently spoke with Sean Henahan of the European Society of Cataract & Refractive Surgeons podcast. Dr. Yu discussed minimally invasive surgical approaches to complications when dealing with the orbit, facial structures, and the skull base. Listen here.
Dr. Yu talks about the multidisciplinary team approach used to solving surgical problems, whether they be fractures, tumors, abscesses, or imbedded foreign bodies. She also highlights the challenges pediatric patients present and potential surgical complications, for instance from hematomas. CT scans are important imaging sources as well as pathology input during surgery when necessary.
Fountain Therapeutics Closes $6 Million Series A-1 Financing

McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Rando, MD, PhD, is the co-founder and chair of the board of directors of Fountain Therapeutics. At Fountain Therapeutics, the research team has combined the expertise of leaders in aging research and computation to build a pipeline of therapeutics aimed at reversing cellular aging. The company’s mission is to decouple aging from disease and significantly extend human health span.
Fountain Therapeutics recently announced the closing of a $6 million Series A-1 financing, bringing its total Series A funding to $11 million. The round was led by Khosla Ventures, with participation from Nan Fung Life Sciences. Fountain previously closed on a $5 million Series A financing with Nan Fung Life Sciences in May 2018.
“We appreciate Nan Fung Life Sciences and Khosla Ventures’ strong support and confidence in our technology platform and experienced team,” said John Dimos, PhD, chief executive officer of Fountain Therapeutics. “Over the last two years we have built and validated our platform technology, which includes an AI-based drug screening platform and animal models for disease modeling and preclinical testing. This has allowed us to generate unprecedented insights into the cellular aging process. Today, thanks to the help of our investors, we are in a strong financial position to rapidly discover and develop a pipeline of novel therapeutics to target and reverse the biological process of aging with the potential to improve the quality of life for our aging population.”
Dr. Rando added, “I have worked in the field of aging research for over 20 years and there is now enough scientific evidence to demonstrate that aging itself can be approached therapeutically to improve quality of life. I founded Fountain with the belief that targeting the underlying mechanisms of aging will be the most powerful way to treat diseases in the aging population. Fountain’s technology allows us to solve the challenges of aging with an unbiased and phenotypic-driven approach. This, together with the outstanding team of experts in aging research, places the company in a unique position to reveal new possibilities and radically transform the landscape of drug discovery and development for the treatment of age-related health conditions.”
Proceeds from the financing will be used to expand the senior leadership team and enhance the capabilities of Fountain’s technology platform. Proceeds will also be used to advance novel therapeutics identified by the platform through preclinical testing in animal models.
Dr. Rando is a Professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine. In addition, he is the Director, Glenn Laboratories for the Biology of Aging, Stanford University, and the Deputy Director, Stanford Center on Longevity, also at Stanford University. He serves as the Chief of Service, Neurology Service, and the Director, REAP Program, both at the VA Palo Alto Health Care System.
Recent Podcasts with McGowan Affiliated Faculty

The McGowan Institute podcasts feature interviews with scientists, clinicians, and patients. The series addresses emerging science and new therapies, where the primary strategy is to repair/replace tissue or organs that have been damaged by disease, trauma, or congenital issues vs. the classical approach that is used today to treat the symptoms of the insult to the body. The following are some of the recent podcasts:
SURGICAL SUPPORT PRIOR TO BIRTH WITH DR. STEPHEN EMERY
Fetal obstructive hydrocephalus causes permanent brain damage due to increased intracerebral pressure. Because of obstruction of the flow of cerebrospinal fluid (CSF), CSF accumulates within the cerebral ventricles, causing increased intracerebral pressure, which leads to decreased blood flow, as well as damage from stretch of neurons. In-utero relief of increased intracerebral pressure may result in normal brain development, thereby preventing lifelong disability.
McGowan Institute for Regenerative Medicine affiliated faculty member Stephen Emery, MD, Associate Professor, Department of Obstetrics, Gynecology, & Reproductive Sciences, Maternal Fetal Medicine, at Magee-Womens Hospital of UPMC, and the Director of the Center for Innovative Fetal Intervention at Magee-Womens Hospital of UPMC, recently spoke with Regenerative Medicine Today about the multiple research protocols through the North American Fetal Therapy Network (NAFTNet).
Listen to the conversation here.
WHAT’S BEING DONE FOR ACUTE KIDNEY STRESS/INJURY WITH DR. JOHN KELLUM
Acute kidney injury is common in hospitalized patients, particularly those in intensive care units and older adults, and refers to a sudden episode of kidney failure or damage that happens within a few hours or days. It causes a build-up of waste products in the blood that can affect other organs, including the brain, heart, and lungs.
McGowan Institute for Regenerative Medicine affiliated faculty member John Kellum, MD, Professor in the Departments of Critical Care Medicine, Medicine, Bioengineering, and Clinical and Translational Science at the University of Pittsburgh, recently spoke with Regenerative Medicine Today about:
- The importance of clinical tracking of the measures of kidney function,
- Acute kidney stress/injury and the NephroCheck® Test System and new treatment development opportunities, and
- The kidneys as a major target of sepsis.
Listen to the conversation here.
HEMOLUNG® RAS HISTORY, COVID-19 PATIENTS, AND WHAT’S ON THE HORIZON
A respiratory dialysis system that was developed at the McGowan Institute for Regenerative Medicine Medical Devices Lab has been used to treat 16 COVID-19 patients, in conjunction with non-invasive or mechanical ventilation. The core technology developed at the Medical Devices Lab, was licensed to the spinout ALung Technologies, and ALung has designed and produced a clinically viable device, which is called the Hemolung® Respiratory Assist System (RAS).
McGowan Institute faculty member William Federspiel, PhD, the William Kepler Whiteford Professor in the Department of Bioengineering, Chemical Engineering, and Critical Care Medicine, and ALung’s Chairman and CEO Peter DeComo spoke with Regenerative Medicine Today about:
- The development history of the Hemolung® RAS,
- How the Hemolung® RAS has been successfully used in helping COVID-19 patients, and
- The new technology—Modular Extracorporeal Lung Assist System (ModELAS)—being advanced in the Medical Devices Lab today.
Listen to the conversation here.
COMPUTATIONAL AND EXPERIMENTAL DATA COMPLEMENTATION
In the Mechanics of Morphogenesis Lab, team members carry out interdisciplinary research at the interface of physics, engineering, mathematics, and developmental biology. What you cannot follow in real time in the body, these researchers follow with sophisticated micromechanical test devices and imaging systems. Once this data is gathered, they then create elaborate computer modeling simulations which can be easily and frequently changed to produce knowledge out of the reach of mathematical analysis or natural experimentation alone.
McGowan Institute for Regenerative Medicine affiliated faculty member Lance Davidson, PhD, Professor and Wellington C. Carl Faculty Fellow of Bioengineering in the University of Pittsburgh’s Swanson School of Engineering and an Adjunct Faculty Member in Developmental Biology and Computational and Systems Biology at Pitt and in Biomedical Engineering at Carnegie Mellon University, is the head of the Mechanics of Morphogenesis Lab and recently spoke with Regenerative Medicine Today about:
- Computational and experimental data complementation and how mechanics affect cell biology shape and tissue movement,
- Today’s tools used in the laboratory can highlight very nuanced differences in basic research studies, and
- Broadening scientific training opportunities for modern research.
Listen to the conversation here.
AWARDS AND RECOGNITION
Dr. Charleen Chu Wins Distinguished Educator Award

McGowan Institute for Regenerative Medicine affiliated faculty member Charleen Chu, MD, PhD, professor of pathology and the A. Julio Martinez Endowed Chair in Neuropathology, received the 2020 Robbins Distinguished Educator Award from the American Society for Investigative Pathology (ASIP). The award recognizes individuals whose exemplary contributions to education in pathology have demonstrated a manifest impact at a national and international level.
Dr. Chu’s research focuses on mechanisms of neurodegeneration and neuroprotection in Parkinson’s Disease (PD) and related neurodegenerative diseases. A major focus is delineating why adaptive cellular mechanisms fail to protect neurons, with emphasis on mitochondrial dysfunction, alterations in kinase signaling and autophagy. Molecular and biochemical studies in cell culture and mouse models are integrated with studies of diseased human brain tissues. She and her team are particularly interested in potentially reversible mechanisms by which mutations in PD-linked genes affect dendritic extension/retraction and synaptic dysfunction.
Her work has been recognized with other honors, including the Carnegie Science Emerging Female Scientist Award, election to the American Society for Clinical Investigation Honor Society, and the ASIP Outstanding Investigator Award.
Congratulations, Dr. Chu!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#210 –– Dr. Ron Poropatich discusses his work with the Center for Military Medicine Research at the University of Pittsburgh, such as remote medical robotics, and the strategic partnerships that make their research possible.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Kazuki Takeishi, Alexandra Collin de l’Hortet, Yang Wang, Kan Handa, Jorge Guzman-Lepe, Kentaro Matsubara, Kazutoyo Morita, Sae Jang, Nils Haep, Rodrigo M Florentino, Fangchao Yuan, Ken Fukumitsu, Kimimasa Tobita, Wendell Sun, Jonathan Franks, Evan R Delgado, Erik M Shapiro, Nicolas A Fraunhoffer, Andrew W Duncan, Hiroshi Yagi, Tomoji Mashimo, Ira J Fox, Alejandro Soto-Gutierrez
Title: Assembly and Function of a Bioengineered Human Liver for Transplantation Generated Solely from Induced Pluripotent Stem Cells
Summary: The availability of an autologous transplantable auxiliary liver would dramatically affect the treatment of liver disease. Assembly and function in vivo of a bioengineered human liver derived from induced pluripotent stem cells (iPSCs) has not been previously described. By improving methods for liver decellularization, recellularization, and differentiation of different liver cellular lineages of human iPSCs in an organ-like environment, we generated functional engineered human mini livers and performed transplantation in a rat model. Whereas previous studies recellularized liver scaffolds largely with rodent hepatocytes, we repopulated not only the parenchyma with human iPSC-hepatocytes but also the vascular system with human iPS-endothelial cells, and the bile duct network with human iPSC-biliary epithelial cells. The regenerated human iPSC-derived mini liver containing multiple cell types was tested in vivo and remained functional for 4 days after auxiliary liver transplantation in immunocompromised, engineered (IL2rg-/-) rats.
Source: Cell Reports. 2020 Jun 2;31(9):107711.
GRANT OF THE MONTH
PI: David Vorp
Title: The Role of Fibrinolysis in Tissue Engineered Vascular Grafts for Aged Individuals
Description: As our aging population grows, so does the national need for a readily available and dependable small-diameter conduit for bypass grafting (for coronary and other small arteries) and for hemodialysis access, as current options are limited. While small-diameter tissue engineered vascular grafts (TEVGs) have shown great clinical promise, our current understanding of them has been almost exclusively derived from implantations into young recipients, even though older patients are the demographic most commonly in need of arterial bypass or hemodialysis. Further, these older patients also typically have high levels of the plasma protein plasminogen activator inhibitor- 1 (PAI-1), which could jeopardize the success of a TEVG. Therefore, the goals of this proposal are to understand how our small diameter TEVG performs differently in aged vs young recipients and to identify an intervention that will improve performance in aged individuals. The proposed work has two Specific Aims, each with their own testable hypothesis: Specific Aim 1 – Evaluate how recipient age affects the success of TEVG implantation and levels of circulating PAI-1. We hypothesize that TEVG remodeling and patency are compromised in elderly recipients in comparison to grafts in young or middle-aged recipients, and that elevated PAI-1 levels will be associated with increasing age. The important outcome of this aim will be determining whether middle-aged or elderly recipients are competent for generating a successful TEVG, and if increased plasma PAI-1 is associated with TEVG failure. Specific Aim 2 – Test the effect of pharmacological PAI-1 antagonism on the success of TEVG implantation in aged animals. PAI-1 plays a critical physiological role by preventing premature clot removal after injury, yet chronic elevation of PAI-1 is associated with increased incidence of cardiovascular disease. Metformin, an FDA-approved drug for type 2 diabetes, has been shown to inhibit PAI-1 production. In preliminary work, we show that Klotho, a protein linked to human lifespan extension, can lower age-associated PAI-1 elevation in injured muscle. We hypothesize that antagonism of PAI-1 in aged animals by supplementation with Metformin or Klotho will restore the success (patency rate) of our TEVGs to that of younger animals. Innovation: Despite the disproportionate occurrence of vascular disease in elderly individuals, pre- clinical testing of TEVGs rarely uses aged animal models. The innovation of the proposed work includes the unique combination of an off-the-shelf cell-free tubular scaffold, assessment of PAI-1 levels and TEVG performance in different aged recipients, and evaluation of a pharmacological intervention using the FDA- approved diabetes drug Metformin and longevity-associated protein Klotho to improve age-associated deficient TEVG performance. The carefully-chosen combination of studies proposed here will not only be foundational for tailoring a translatable TEVG for those who are most in need – elderly patients – but may also be paradigm- shifting in how TEVGs and other tissue engineering-based therapies are tested preclinically.
Source: National Institute on Aging
Term: May 1, 2020 – Feb 22, 2022
Amount: $394,300
