December 2021 | VOL. 20, NO. 12| www.McGowan.pitt.edu
Study Identifies Factor in ‘Young Blood’ That Helps Rejuvenate Aged Mouse Muscle

As we age, our muscles gradually become smaller, weaker, and less able to heal after injury. In a new study, University of Pittsburgh and UPMC researchers pinpoint an important mediator of youthfulness in mouse muscle, a discovery that could advance muscle regeneration therapies for older people.
Published in Nature Aging, the study demonstrates that circulating shuttles called extracellular vesicles, or EVs, deliver genetic instructions for the longevity protein known as Klotho to muscle cells. Loss of muscle function and impaired muscle repair in old mice may be driven by aged EVs, which carry fewer copies of these instructions than those in young animals.
The findings are an important advance in understanding why the capacity for muscles to regenerate dwindles with age.
“We’re really excited about this research for a couple of reasons,” said senior author and McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, MPT, director of rehabilitation for UPMC International and associate professor of physical medicine and rehabilitation at Pitt. “In one way, it helps us understand the basic biology of how muscle regeneration works and how it fails to work as we age. Then, taking that information to the next step, we can think about using extracellular vesicles as therapeutics to counteract these age-related defects.”
The new study builds on decades of research showing that when old mice are given blood from young mice, youthful features are restored to many cells and tissues. But until now, it was unclear which components of young blood confer these rejuvenating effects.
“We wondered if extracellular vesicles might contribute to muscle regeneration because these couriers travel between cells via the blood and other bodily fluids,” said lead author Amrita Sahu, PhD, postdoctoral fellow in Dr. Ambrosio’s Lab in the Department of Physical Medicine and Rehabilitation at Pitt. “Like a message in a bottle, EVs deliver information to target cells.”
Dr. Ambrosio and her team collected serum, the fraction of blood that remains after removing blood cells and clotting factors, from young mice and injected it into aged mice with injured muscle. Mice that received young serum showed enhanced muscle regeneration and functional recovery compared to those that received a placebo treatment, but the serum’s restorative properties were lost when EVs were removed, indicating that these vesicles mediate the beneficial effects of young blood.
Delving deeper, the researchers found that EVs deliver genetic instructions, or mRNA, encoding the anti-aging protein Klotho to muscle progenitor cells, a type of stem cell that is important for regeneration of skeletal muscle. EVs collected from old mice carried fewer copies of the instructions for Klotho than those from young mice, prompting muscle progenitor cells to produce less of this protein.
With increasing age, muscle doesn’t heal as well after damage because scar tissue is deposited instead of restoring original muscle structure. In earlier work, Dr. Ambrosio and her team showed that Klotho is an important regulator of regenerative capacity in muscle progenitor cells and that this protein declines with age.
The new study shows for the first time that age-related shifts in EV cargo contribute to depleted Klotho in aged stem cells, suggesting that EVs could be developed into novel therapies for healing damaged muscle tissue.
“EVs may be beneficial for boosting regenerative capacity of muscle in older individuals and improving functional recovery after an injury,” said Dr. Ambrosio. “One of the ideas we’re really excited about is engineering EVs with specific cargoes, so that we can dictate the responses of target cells.”
Beyond muscles, EVs also could help reverse other effects of aging. Previous work has demonstrated that young blood can boost cognitive performance of aged mice. Dr. Ambrosio and coauthor Radosveta Koldamova, MD, PhD, professor of environmental and occupational health at Pitt’s Graduate School of Public Health, have a grant to explore the potential of EVs for reversing age-related declines in cognition.
RESOURCES AT THE MCGOWAN INSTITUTE
January Histology Special – Frozen Sections
Safranin O staining is used for the detection of cartilage, mucin, and mast cell granules on formalin-fixed, paraffin-embedded tissue sections or frozen sections. The cartilage and mucin will be stained orange to red, and the nuclei will be stained black. The background is stained bluish green.
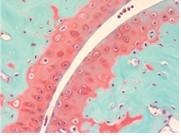
You’ll receive 30% off your Safranin O staining in January when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
Dr. William Wagner Points to Bright Future for Regenerative Medicine

The McGowan Institute for Regenerative Medicine has been one of the more prolific research centers at the University of Pittsburgh over the past 25 years, particularly in terms of its impact from translating discoveries from the lab to the clinic/market.
With more than 1,000 invention disclosures submitted, 220 patents issued, and 203 licenses executed, including 34 spinout companies, the approximately 250 McGowan Institute-affiliated faculty and their students have established a track record for commercial translation unrivaled at Pitt.
The director of the McGowan Institute, William Wagner, PhD, recently spoke to a gathering of regional investment professionals at a luncheon hosted by the Pittsburgh Venture Capital Association to outline the exciting pipeline of life science innovation from McGowan Institute researchers that could one day be produced in Pittsburgh, or even in outer space.
Dr. Wagner’s presentation comes on the heels of the announcement of the $100 million grant from the Richard King Mellon Foundation to establish the Pitt BioForge, a new facility for Pitt researchers, clinicians, and industry to collaborate on the development and production of personalized cellular therapeutics, viral vectors, gene therapies, antigens for vaccine development, and more.
“If it doesn’t get to the patient, we haven’t fulfilled our mission,” Dr. Wagner said, explaining that all new McGowan Institute researchers receive mentoring with a clinical and entrepreneurial focus so that from day one they are thinking not only about their science, but how to ensure that their research crosses the Rubicon into the clinic.
Dr. Wagner traced the progression of the McGowan Institute from the legacy of organ transplantation pioneered by Thomas Starzl at Pitt in the 1980s, to today, where Pitt researchers continue groundbreaking research to restore organ and tissue function.
As Pittsburgh became a global center for transplants, Dr. Wagner recounted, it resulted in an abundance of people awaiting organs. This created an organic opportunity for Pitt researchers and clinicians to begin to develop methods to try to keep the patients alive as they awaited donor organs.
Dr. Wagner pointed specifically to two innovations originating at the McGowan Institute that have made a huge impact on patients, and others recently spun out into companies and preparing for clinical trial, that are transforming patient care for those with impaired organ function.
The first was when Pitt researchers partnered with a California turbine company to create a rotary blood pump in the 1990s. That pump now has long-term survival rates in some people of more than 10 years, approaching the effectiveness of a transplant. A next-generation version being brought to market, initially in India, employs magnetics to avoid some of the issues of blood protein damage resulting from the heat generated by the current pumps.
The second is the Hemolung Respiratory Assist System, from the lab of Pitt professor of bioengineering William Federspiel, PhD, which received FDA approval in November. The device, marketed by ALung Inc., can be used as a bridge to lung transplant and received emergency use from the FDA last year to treat critically ill COVID patients. The device removes carbon dioxide from the blood and recirculates it to the body.
More recently the McGowan Institute approach has combined both medical devices and human tissue engineering, often in tandem. Dr. Wagner called out several recent Pitt startups originating from the McGowan Institute that are taking a next-generation approach to organ and tissue repair.
- LyGenesis Inc. is using liver hepatocyte cells and introducing them into lymph nodes, which act as bioreactors to grow functional liver tissue.
- Renerva Inc. is developing a peripheral nerve matrix (PNM), an injectable gel derived from porcine tissue that promotes and supports repair and regeneration in injured peripheral nerves.
- ECM Therapeutics is employing biologic materials composed of extracellular matrix as surgical meshes and topical wound products with an initial focus on esophageal and colorectal diseases.
- Neoolife is working to replace artificial or animal heart valve replacements with polymeric tissue-engineered biomimetic valves designed to enable a person’s own tissue to regenerate.
Another key to the McGowan Institute’s success has been its ability to cultivate multi-investigator and multi-institution consortiums to produce focused innovation around specific tissues and/or patient populations.
A few examples include:
Armed Forces Institute for Regenerative Medicine (AFIRM)
Pitt and Wake Forest University formed a consortium as part of the larger AFIRM initiative to explore restoring muscle animation and promoting scar healing for soldiers injured by IEDs.
Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center
This partnership focuses on oral, dental, and craniofacial research and development.
Pennsylvania Pediatric Device Consortium
Pitt and the Children’s Hospital of Philadelphia (CHOP) received a $5 million grant from the FDA to develop medical devices, particularly for pediatric patients.
Pitt is leading a $22 million grant funded by the Defense Advanced Projects Research Agency to create an artificial intelligence-based platform for healing muscle injuries. Other members include Carnegie Mellon University, Northwestern University, University of Vermont, University of Wisconsin, and Walter Reed National Military Medical Center.
NSF Engineering Research Center for Revolutionizing Metallic Biomaterials
This consortium is exploring the creation of implantable systems employing degradable biomaterials for tissue reconstruction and regeneration. Partners include North Carolina A&T and the University of Cincinnati.
Alliance for Biomanufacturing in Low Earth Orbit
The McGowan Institute is pioneering the exploration of low earth orbit for regenerative medicine.
If you would like to learn more about collaborating with the McGowan Institute researchers, contact the Institute. Or you can explore McGowan Institute-developed regenerative medicine innovations available for licensing.
Pitt Researchers Identify Molecular Underpinnings of Liver Disease in Patients with Cystic Fibrosis

Persistent coughing, frequent lung infections, fatigue – these are some of the most common symptoms of cystic fibrosis, a devastating genetic disorder without a cure that affects more than 70,000 people worldwide.
But along with classic symptoms of cystic fibrosis, a small subset of patients also develops liver disease, which causes liver inflammation and scarring due to perturbations in bile flow and can lead to cirrhosis and liver failure. Doctors don’t know why some patients develop liver disease and some don’t, but the mechanism of liver disease development in patients with cystic fibrosis became clearer thanks to a new paper published by University of Pittsburgh scientists in the journal eLife.
The researchers discovered in mice and humans with cystic fibrosis, that cells called cholangiocytes – which line bile ducts that crisscross the liver tissue and serve as pipelines that shunt toxic byproducts of body metabolism – lack a critical interaction between proteins that control cell regeneration and inflammation. Without that interaction, cholangiocytes become inflamed and fibrotic – offering an explanation as to why cystic fibrosis pathology in the liver manifests the way it does.
“This missing interaction is at the core of the pro-inflammatory environment in the fibrotic liver – it adds insult to injury and makes the disease worse,” said senior author and McGowan Institute for Regenerative Medicine faculty member Satdarshan (Paul) Monga, MD, professor at Pitt’s Department of Medicine. “This discovery sheds light on how bile duct cells that are not malignant by nature can suddenly turn against the body.”
Cystic fibrosis – a genetic disorder diagnosed in about a thousand new patients in the United States each year – starts with one faulty gene. Hundreds of mutations in the genetic code for a protein called CFTR, or cystic fibrosis transmembrane conductance regulator, can render this molecule inactive, leading to a buildup of thick mucus in the lungs and symptoms in other parts of the body.
Building on experiments in mice, Pitt researchers realized that the inability of the mutated CFTR to regulate cellular location of two proteins controlling inflammation and cell repair might have something to do with clogged bile ducts in cystic fibrosis patients.
The group discovered that under normal circumstances, CFTR acts as a molecular anchor, tightly tethering itself and the two other proteins together, preventing them from spontaneous activation. But when CFTR is gone, or when its ability to link up to its partners is disrupted because of mutations, the other two proteins become active and cause overgrowth of bile duct lining and progressive inflammation.
The discovery wasn’t an instant “aha moment,” though.
“For me, this is the joy of science. No one can tell you where to look for answers – you have to look hard to find them – but when things fall into place, it’s the most gratifying feeling,” said first author Shikai Hu, a visiting scholar at Pitt and a member of the first cohort of MD-PhD students in the joint program between Pitt and Tsinghua University in China. “Doing an experiment and confirming for the first time that what we saw in mice was also what we see in patients with cystic fibrosis was very exciting and validating.” The researchers emphasize that there are still many questions that are left unanswered – but they are optimistic that one day, the full picture of molecular changes caused by cystic fibrosis in the liver will reveal itself.
“Observing changes in tissue morphology can only take us so far,” said co-corresponding author Sungjin Ko, DVM, PhD, assistant professor in the Department of Pathology. “It takes a lot of effort to correlate visually observed changes to their molecular signatures, but understanding these intricacies is key to developing better therapies.”
When Demand Outstrips Capacity, Injections of COVID-19 Treatment Instead of IV Are Justified

Giving monoclonal antibodies to outpatients with mild to moderate COVID-19 via a series of four injections under the skin is essentially as effective at preventing severe hospitalization and death as giving the treatment in one intravenous (IV) infusion and is superior to not giving the treatment at all, according to new results announced by UPMC and the University of Pittsburgh School of Medicine. It is the first clinical study to compare the different methods of administering the treatment.
The findings mean that when surging COVID-19 cases and staffing shortages make it logistically impossible to administer monoclonal antibodies through long IV infusions, health care providers can revert to quicker subcutaneous injections to equitably continue providing the treatment to as many patients as possible. Injections take less time to administer, result in shorter appointments for patients and can be administered by more health care staff than IV infusions.
To guide health care providers on best practices during the recent nationwide upswing in COVID-19 cases, the physician-scientists published the findings in medRxiv, a preprint journal, and shared the results ahead of peer-reviewed publication. McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, executive vice president and chief innovation officer, UPMC, and distinguished professor holding the Mitchell P. Fink Endowed Chair in Critical Care Medicine at the University of Pittsburgh, is a co-author on this study.
“When we began to see a surge in COVID-19 cases due to the Delta variant three months ago, we realized it would be impossible to accommodate the demand for monoclonal antibodies with IV infusions,” said lead author Erin McCreary, PharmD, infectious diseases pharmacist at UPMC and clinical assistant professor in Pitt’s School of Medicine. “We can more than double our outpatient appointments for antibodies when using the subcutaneous route, so we began administering the treatment through injections when needed to treat as many patients as possible.”
The Food and Drug Administration’s emergency use authorization of Regeneron’s combination treatment of the monoclonal antibodies casirivimab and imdevimab allows for subcutaneous injection only when IV infusion “is not feasible and would lead to delay in treatment.” Although only the IV route of administration had been studied in randomized clinical trials, Dr. McCreary and colleagues knew that without using injections to expand capacity during the Delta surge, many patients would experience delays in treatments or not receive them at all. UPMC switched most of its outpatient infusion centers to administering the treatment by subcutaneous injection on Sept. 9, 2021.
The patients who received injections of Regeneron’s monoclonal antibodies were followed for 28 days and compared to a matched population of patients who received an IV infusion of the drug, as well as those who were eligible for treatment but did not receive it.
The study team determined that the COVID-19 patients who received subcutaneous injections had a 3.4% likelihood of being hospitalized or dying in the following four weeks, compared to 7.8% in matched patients who were not treated. Patients who received subcutaneous injections had a similar chance of avoiding hospitalization and death compared to those who received IV infusions; the adjusted risk was within the pre-determined, clinically acceptable threshold.
“There was little to no evidence that subcutaneous administration was associated with a higher risk of death or severe hospitalization compared to IV administration, and it was associated with an estimated 56% lower risk of being hospitalized or dying within 28 days compared to no monoclonal antibody treatment,” said senior author Kevin Kip, PhD, vice president of clinical analytics at UPMC. “Looking at these real-world results, I would confidently recommend that any health care provider struggling to provide casirivimab and imdevimab infusions in a timely manner switch to subcutaneous injections rather than turn patients away.”
Intracerebral Cell Therapy and the Impact of Physical Therapy

Recently published in the Journal of Cerebral Blood and Metabolism, the article entitled “Physical therapy exerts sub-additive and suppressive effects on intracerebral neural stem cell implantation in a rat model of stroke” investigates how physical therapy affects neural stem cells implanted into stroke damage. McGowan Institute for Regenerative Medicine affiliated faculty members and collaborative authors on this work are Michael Modo, PhD, Professor in the Department of Radiology at the University of Pittsburgh, and Fabrisia Ambrosio, PhD, MPT, Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh.
This study used the neural stem cells undergoing clinical trials for stroke by ReNeuron (PISCES trials). A key question in these trials was whether patients should get physical therapy or not as it was unclear if this was required for these cells to form Hebbian synapses or if both might act on the same target. Researchers found both sub-additive and suppressive effects here which potentially can have significant implications for the design of future clinical trials.
The abstract from this article follows:
Intracerebral cell therapy (CT) is emerging as a new therapeutic paradigm for stroke. However, the impact of physical therapy (PT) on implanted cells and their ability to promote recovery remains poorly understood. To address this translational issue, a clinical-grade neural stem cell (NSC) line was implanted into peri-infarct tissue using MRI-defined injection sites, two weeks after stroke. PT in the form of aerobic exercise (AE) was administered 5 × per week post-implantation using a paradigm commonly applied in patients with stroke. A combined AE and CT exerted sub-additive therapeutic effects on sensory neglect, whereas AE suppressed CT effects on motor integration and grip strength. Behavioral testing emerged as a potentially major component for task integration. It is expected that this study will guide and inform the incorporation of PT in the design of clinical trials evaluating intraparenchymal NSCs implantation for stroke.
This study was funded in part by the Alliance for Regenerative Rehabilitation Research and Training (AR3T).
Uncovering a Promising Use Case for Exosomes

Extracellular vesicles, or exosomes as they are more commonly known, continue to be a curious research focus for the scientific community. Once assumed to be waste materials secreted by cells, exosomes have recently been identified as mail carriers, serving an essential role in cell-to-cell communication by acting as delivery vehicles between cells. New research from Carnegie Mellon University (CMU) and UPMC explores a new use case for exosomes: delivering growth factors like bone morphogenetic proteins (BMPs) for bone healing.
Exosomes resemble nano-sized bubbles and are secreted by every cell in the body. Functionally, they carry messages from one cell to another, for example, proteins, DNA, RNA, and other biomolecules that can help determine how cells behave and function. In simple terms, if exosomes are produced by bad cells, such as cancer cells, they will carry bad messages, and vice versa, they will carry good messages if produced by healthy cells. Exosomes are now being explored as biomarkers and for various therapeutic-specific applications.
“In thinking through the evolutionary role of exosomes, we started wondering whether or not we could extract and load them with growth factors and use them as trojan horses for carrying therapeutic cargo,” said Saigopalakrishna “Sai” Yerneni, PhD, postdoctoral researcher at CMU. “We focused our initial efforts on one type of growth factor, BMP2. BMP2-engineered exosomes could be used for bone healing and aiding people who have traumatic bone injuries.”
Growth factors as proteins have a significant effect in altering cell behavior and typically behave in a lock-and-key manner. In other words, for a growth factor to effect cell behavior or have a therapeutic benefit, it must initially bind to it a receptor, which is found on the cell’s surface. Over the course of the group’s study, they found that when BMP2 was engineered into exosomes, this lock and key mechanism could be bypassed.
“While this may seem trivial at the outset, the discovery holds promise to change our current understanding of signaling processes of growth factors,” according to CMU’s Phil Campbell, PhD, research professor of biomedical engineering, member of the Engineering Research Accelerator, and an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
To date, very few growth factors have been approved for clinical use by the FDA. This is due in part to the generally high concentration of growth factors that are required for therapeutic benefit in humans. BMP2 delivered via engineering exosomes, however, was found to produce a therapeutic effect with an extremely low concentration. This finding offers new hope for future clinical applications of growth factor deliveries via exosomes.
“Beyond continuing to pursue the biological aspects of extracellular vesicle-cell communication, we also plan on expanding the exosome-based delivery approach to other growth factors that could potentially help in regenerative medicine and in controlling diseases such as cancer,” said Dr. Campbell.
$1 Million Magee Prize Awarded to Dr. Pamela Moalli and Team

During the Magee-Womens Summit, the Magee-Womens Research Institute (MWRI) awarded the $1 million Magee Prize to an international team led by McGowan Institute for Regenerative Medicine affiliated faculty member Pamela Moalli, MD, PhD, director of urogynecology and pelvic reconstruction surgery at UPMC Magee-Womens Hospital and MWRI. The team is working to develop new biomaterials to repair tissue loss in women with compromised vaginal structure and function.
“We believe we are the right team to achieve the aims of the proposal, and we look forward to a productive collaboration in the future,” said Dr. Moalli in accepting the award.
An increasing number of women are seeking vaginal reconstruction to repair tissue loss after surgery for pelvic cancers or following ovariectomy, chemotherapy and radiation, which can result in the vagina becoming contracted, thinned, and painful. After noticing a limited range of biomaterials for these procedures, Dr. Moalli was inspired to develop better solutions.
She assembled a team that includes McGowan Institute affiliated faculty member Kyle Orwig, PhD, MWRI and University of Pittsburgh School of Medicine, and Caroline Gargett, PhD, Monash University, Australia. Their project seeks to identify stem cell populations that restore vaginal structure and function and isolate the cellular ecosystems needed for stem cell survival, proliferation, and engraftment. The researchers will use a novel bioassay to develop vaginal organoids from these stem cell populations.
“I am beyond excited about this study because it provides a viable solution to young girls and women in whom vaginal structure and function has been compromised. Stem cell technologies for use in tissue biofabrication is where the future of reconstructive surgery lies,” Dr. Moalli said.
The Magee Prize, which is funded by the Richard King Mellon Foundation, is among the largest grants of its kind in women’s health that is awarded by a non-government institution. To qualify for the prize, teams must include at least one MWRI researcher and at least one international collaborator.
Illustration: The winning team of the 2021 Magee Prize. Magee-Womens Research Institute.
LyGenesis Adds Inborn Errors of Metabolism, Orphan Pediatric Indications with Large Unmet Needs, to its Drug Development Pipeline

LyGenesis is a clinical-stage biotechnology company whose cell therapies use patients’ lymph nodes as bioreactors to regrow functioning ectopic organs. LyGenesis’s lead allogeneic cell therapy program is currently in a Phase 2a clinical trial for patients with end stage liver disease. LyGenesis was built on nearly a decade of groundbreaking academic research by McGowan Institute for Regenerative Medicine faculty member Eric Lagasse, PharmD, PhD, Associate Professor in the Department of Pathology at the University of Pittsburgh and LyGenesis Founder and Chief Scientific Officer.
LyGenesis’s drug development pipeline includes cell therapies that can produce an ectopic thymus (for aging and multiple other potential indications), pancreas (for Type 1 diabetes), and kidney (for end stage renal disease). LyGenesis announced that it has achieved positive in vitro results of a novel combination drug-biologic product for patients with inborn errors of metabolism, adding these orphan pediatric indications to its drug development pipeline.
McGowan Institute affiliated faculty member Paulo Fontes, MD, LyGenesis Co-Founder and Chief Medical Officer, noted: “While our lead therapy is focused on patients with end stage liver disease, we have also been developing a novel hydrogel with growth factors and a proprietary patent-pending surgical catheter to engraft our cell therapy under the liver capsule of patients, enabling a large mass of cells to be engrafted and nurtured by the patient’s liver. Our early in vitro work supports this approach, and we are now rapidly moving into preclinical studies so that we can advance this to the clinic for our pediatric patients born with catastrophic and often fatal inborn errors of metabolism.”
Inborn errors of metabolism are genetic disorders in which the body cannot properly metabolize food into energy. Comprised of over two dozen separate genetic defects, inborn errors of metabolism can produce profound brain damage and death if not identified and treated soon after birth. The lead indication for this new combination product will focus on Maple syrup urine disease (MSUD), a rare disorder, affecting 1 in 185,000 newborns. Untreated, it leads to brain injury in days and death occurs in weeks or months. Full liver transplant cures MSUD, but it is associated with both surgical risk, lifetime immunosuppression, and a risk of complications post-transplant.
C. Andrew Bonham, MD, an Associate Professor of Surgery and Abdominal Transplantation at Stanford University, commented, “New therapeutic approaches for the treatment of inborn errors of metabolism are desperately needed. Currently children suffering from these diseases risk catastrophic neurologic complications. The only cure is with a liver transplant, a rather draconian procedure to correct an enzymatic defect. LyGenesis combines cell therapies with a deep understanding of anatomy and how structures within the body – whether lymph nodes or the liver capsule – can be used to create a niche for cells to expand and thrive to correct the metabolic defect and help patients. I look forward to helping accelerate this new therapy into the clinic so that we can help patients born with MSUD and other metabolic defects as quickly as possible.”
Greg Bailey, MD, CEO and Director of Juvenescence Limited, added, “Juvenescence is delighted that LyGenesis has reached another important milestone expanding its regenerative cellular therapies platform following a successful path to the clinic with its lead program for liver regeneration, which should start a phase 2a clinical trial shortly.”
Novel Computational Pipeline Could Help Repurpose Cancer Drugs for Rare Diseases

By combining computational and experimental approaches, University of Pittsburgh School of Medicine and Prairie View A&M University researchers identified cancer drugs that show promise for treating pulmonary hypertension, or PH, a rare and incurable lung disease.
Published in Science Advances, the study used a new algorithm to identify candidate cancer drugs for PH. Two of these compounds improved markers of the disease in human cells and rodents. The findings support broader use of this drug-repurposing platform for other non-cancerous conditions that don’t yet have effective treatments. McGowan Institute for Regenerative Medicine affiliated faculty member Mauricio Rojas, MD, professor of internal medicine and the associate vice chair of research, Department of Internal Medicine, in the Division of Pulmonary, Critical Care, & Sleep Medicine of the Davis Heart & Lung Research Institute, at The Ohio State University, is a co-author on the study.
“Repurposing drugs can cut down the time and cost of developing treatments for rare diseases, which historically don’t receive much investment into research and drug development,” said senior author Stephen Chan, MD, PhD, professor of medicine and director of the Vascular Medicine Institute at Pitt and UPMC. “Pulmonary hypertension is an example of a rare disease where there is an unmet need for new treatments, given its devastating consequences. We developed this pipeline to rapidly predict which drugs are effective for PH and get these treatments to patients faster.”
Pulmonary hypertension is a type of high blood pressure that occurs in the vessels that transport blood from the heart to the lungs. As the disease progresses and the heart must strain harder against these high pressures, it can lead to heart failure, multi-organ dysfunction and death. PH affects people of all ages but hits young women more often than men.
One of these young women is Allison Dsouza, a 24-year-old nurse who not only lives with the condition herself but also treats PH patients in the UPMC Lung Transplant Program. She was diagnosed with PH as a high school senior after she started having trouble walking to her car and doing hobbies like horseback riding. According to Ms. Dsouza, she was the sickest patient with the highest lung pressures that her doctors had seen.
PH is thought to be triggered by environmental and genetic factors that damage the endothelial cells that line blood vessels, leading to inflammation and abnormal repair that restrict blood flow or cause the loss of the thinnest branches of the lung vessel tree.
The drugs currently available for PH dilate or relax these blood vessels, which can give relief from symptoms and prolong the time it takes for the disease to progress, but they’re not curative. Ms. Dsouza receives a drug called Remodulin that is delivered continuously by a pump inserted under her skin. Within months of starting treatment, she walked a 5-kilometer race, started playing polo on horseback and enrolled in college to study nursing.
“The treatments are phenomenal, but they’re also a huge burden on life,” said Ms. Dsouza. “Subcutaneous treatments cause severe pain, and intravenous treatments have a huge risk of infection that people can actually die from. The medication is lifesaving, but the side effects can kill you. And for some people, the therapies don’t work.”
According to Dr. Chan, there is a need for drugs that target the origins of pulmonary hypertension.
Given evidence that PH and cancer share numerous features, Dr. Chan hypothesized that vast molecular data from cancer studies could be leveraged to predict which cancer drugs may also target PH. To make those predictions, Dr. Chan collaborated with co-senior author Seungchan Kim, PhD, chief scientist and executive professor of electrical and computer engineering at Prairie View A&M University. They built a computational platform that analyzed gene expression data from 800 cancer cell lines exposed to hundreds of cancer therapeutics and assessed rewiring of gene networks associated with drug responses in these cells.
“When we overlay these networks with PH-specific gene networks, we can predict which drugs may be effective in treating PH,” Dr. Kim explained.
The platform ranked each drug in terms of how its action depended on rewiring of PH-specific gene networks, and the researchers chose to further investigate two highly ranked compounds: I-BET762 and BRD2889.
In human pulmonary endothelial cells, I-BET762 and BRD2889 modulated PH gene networks predicted by the platform—genes that drive cell death under low-oxygen and inflammatory conditions, processes that promote PH.
A drug in the same class as I-BET762 currently is being tested for PH in clinical trials led by Laval University coauthors. The computational analysis and experiments identified new molecular pathways for this drug class, indicating that the platform can offer novel insights into drugs already under study. Another promising compound, BRD2889—which is an analog of piperlongumine, a compound derived from long pepper plants that has anti-cancer properties—had not previously been investigated for treating PH.
When Dr. Chan and co-senior author Imad Al Ghouleh, PhD, assistant professor of medicine at Pitt, gave BRD2889 to mice and rats with PH, disease symptoms were reversed, suggesting that this compound has potential as a new PH drug. The researchers have applied for a provisional patent for BRD2889, and they plan to move the compound into clinical trials in the future.
With evidence that drug candidates identified by the algorithm are effective at treating PH in animals, the researchers plan to go back and look more closely at other compounds predicted as potential treatments for the disease. And according to Dr. Chan, the applications of this study go far beyond PH.
“With this algorithm in hand, we may be able to repurpose existing cancer drugs for the treatment of other rare and emerging diseases,” said Dr. Chan.
Illustration: The Ohio State University.
Surviving COVID: Comorbidities Don’t Tell the Full Story

Advanced age, being a man, and several types of preexisting health conditions have been associated with poor outcomes among hospitalized patients with COVID, but a new study suggests that other biological factors may be more important in determining prognosis and survival than these factors.
Improved understanding of the biological underpinnings of different responses to SARS-CoV-2 infection could improve treatment for patients.
Most studies seeking to identify risk factors for COVID have grouped patients by age, sex, and preexisting conditions such as obesity, diabetes, and heart failure. “But that can potentially obscure contributions from underlying factors and obscure the potential that a single comorbidity can be associated with different outcomes,” says Soojin Park, MD, the study’s senior author, associate professor of neurology at Columbia University Vagelos College of Physicians and Surgeons, and a neurocritical care physician at Columbia University Irving Medical Center/NewYork-Presbyterian. Three McGowan Institute for Regenerative Medicine affiliated faculty members are co-authors of this study:
- Michael Pinsky, MD, professor of critical care medicine, bioengineering, cardiovascular diseases, clinical and translational science, and anesthesiology at the University of Pittsburgh
- Gilles Clermont, MD, a professor of critical care medicine, industrial engineering, and mathematics, and the medical director of the Center for Inflammation and Regenerative Modeling, University of Pittsburgh
- Yoram Vodovotz, PhD, professor of surgery, computational and systems biology, bioengineering, immunology, communication science and disorders (of the School of Health and Rehabilitation Science), clinical and translational science, and the director of the Center for Inflammation and Regeneration Modeling
To identify underlying factors that predict outcome, Dr. Park and her team used machine learning to analyze patient laboratory data collected throughout the hospitalizations of 528 patients treated at a single hospital during the first few months of the pandemic in 2020.
The analysis identified four unique groups of patients that had vastly different outcomes not expected based on classic risk factors such as age, sex, and preexisting conditions.
The group with the highest number of comorbidities, for example, did not have the worst outcomes and had a relatively low mortality rate, indicating that comorbidities alone are not responsible for variable COVID clinical courses.
The group of patients with the worst outcomes and most deaths had fewer comorbidities than expected but had higher levels of circulating inflammatory markers than the other three groups.
“Having certain high- or low-risk conditions does not guarantee a certain outcome,” says Dr. Park. “Rather, this highlights that healthy patients can still have poor COVID outcomes and patients with numerous chronic diseases can still have good outcomes.”
“Given the risk of poor outcome in a group of patients who were previously quite healthy, vaccination remains essential for everyone,” say Benjamin Ranard, MD, the study’s first author and a pulmonary and critical care fellow at Columbia.
Further examination of the different groups has potential to yield clinical and pathobiological insight into what is driving the vastly different clinical courses experienced by patients with COVID.
Bridging Campuses and Increasing Opportunities in STEM

A new, multi-institutional MS-to-PhD program will help strengthen collaboration between the University of Pittsburgh and Carnegie Mellon University (CMU) campuses and expand access to graduate education in the growing fields of artificial intelligence, robotics, and neural engineering. McGowan Institute for Regenerative Medicine affiliated faculty member Douglas Weber, PhD, professor of mechanical engineering and neuroscience at Carnegie Mellon University, is a co-principal investigator of this program along with Gelsy Torres-Oviedo, PhD, associate professor of bioengineering at the University of Pittsburgh.
The two-year BRIDGE Program, recently funded by the U.S. Department of Defense (DoD), will actively recruit individuals currently underrepresented in STEM fields, including student veterans and children of veterans. It will provide a hands-on research experience and target individuals who have not had similar research opportunities at their undergraduate institutions.
“The research is the focus of this program,” said Dr. Torres-Oviedo. “The BRIDGE Program will prepare students to compete for PhD positions at top-tier research institutions.”
In addition to the rigorous curriculum and research, students will participate in a summer internship at one of nine participating national laboratories — an experience that the program leaders hope will generate a pipeline of talent into these labs and the private sector.
“The global economy relies heavily on highly qualified personnel having advanced degrees in science and engineering,” said Dr. Weber. “For the US to remain competitive, especially in rapidly developing sectors like robotics, artificial intelligence, autonomous systems, and neural engineering, participation in research-intensive graduate education must be expanded.”
The program will leverage the complementary strengths of both universities and utilize established resources like the Center for the Neural Basis of Cognition (CNBC).
“CMU and Pitt share a long and enviable history of providing graduate students with research and training opportunities that span both campuses, leveraging the unique strengths of each. The BRIDGE program builds on this history to expand access to graduate training opportunities in these high-demand areas,” said Dr. Weber.
While similar MS-to-PhD programs exist across the country, none are focused on engineering or neuroscience and actively recruit students underrepresented in STEM. Black and Hispanic individuals continue to earn a smaller proportion of degrees in STEM relative to their share of the U.S. population.
“I have been fortunate to attract talented engineers from Hispanic background into my research group, and it has been a privilege to facilitate their transition from skilled technicians to independent thinkers,” Dr. Torres-Oviedo said. “This program is aimed at supporting the intellectual growth and broadening the professional opportunities for many other students like them.”
More information about the program can be found here. Interested students can apply now. The first cohort will begin this program in the Summer of 2022.
Illustration: University of Pittsburgh Swanson School of Engineering.
Pitt Researchers Receive Grant to Explore Wireless Technology for Those with Disabilities

We use voice commands for smart devices in our cars, homes, and offices to turn on lights, send text messages, or call someone. For people with disabilities who require assistance completing daily activities, wireless technologies can improve their ability to control their environment and quality of life – but implementing these systems remains a challenge.
To help overcome these obstacles, the University of Pittsburgh has received a six-year, $4.6 million grant from the Department of Health and Human Services, through the National Institute on Disability, Independent Living, and the Administration for Community Living, to create a Rehabilitation Engineering Research Center (RERC).
McGowan Institute for Regenerative Medicine affiliated faculty member Dan Ding, MD, an associate professor and researcher at the Human Engineering Research Laboratories (HERL), will be the grant’s principal investigator.
Many of the project’s key investigators are affiliated with the HERL — a partnership between the VA Pittsburgh Healthcare System, the University of Pittsburgh, and UPMC.
Pitt’s School of Health and Rehabilitation Sciences (SHRS) will host the RERC, to be named PROMISE: Promoting Mainstream Wireless Inclusion through Technology Services. Researchers hope to improve accessibility and promote mainstream wireless inclusion through technology services.
“This grant is a great platform for engaging diverse investigators and partners in implementing a holistic systems approach for addressing the challenges of wireless technology access and use by people with disabilities,” Dr. Ding said.
Dr. Ding acknowledges that smart technologies are advancing at a much faster rate than regulations, reimbursement systems, clinician education, and the capacity of people with disabilities and families can keep up.
Joining Dr. Ding as the co-principal investigator is Brad Dicianno, MD, a professor of physical rehabilitation and medicine at Pitt. They will tap highly qualified investigators with expertise in engineering, rehabilitation training, and funding/policy. The researchers will also leverage their close ties with providers, industry, and disability organizations.
“I am excited about this project because it brings together a fantastic team of people with disabilities, community organizations, investigators, and other stakeholders,” said Dr. Dicianno. “The projects provide us with an opportunity to develop better service delivery models for wireless technologies.”
New Game to Help Reduce Vaccine Hesitancy for Teens, Children

A new game being developed at Duquesne University hopes to reduce anxiety about COVID-19 and combat vaccine misinformation for kids, parents, and teachers. The tabletop board game challenges players to tackle a hypothetical global pandemic by researching, developing, testing, and distributing a vaccine to save humanity.
The game is part of a five-year Science Education Partnership Award (SEPA) from the National Institute of General Medicine (NIGMS) at the National Institute of Health (NIH) that focuses on educating children and teens on how to manage pain, anxiety, and stress in a healthy way.
During the COVID-19 pandemic, John Pollock, PhD, director of Duquesne’s Partnership in Education program and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, surveyed parents and children about their everyday stressors. He found that children are much more worried about the health effects of COVID-19 than parents realized.
“We found a disconnect between the things parents thought were stressing children, as opposed to the things that were actually causing children’s own stress,” he said. “When we surveyed children, their greatest stressors did not align with the perceptions of parents and teachers.”
The new board game will allow for cooperative play, where players must work together to save humanity from a global pandemic. Players model the real-life steps of vaccine development, manage both monetary and time constraints, evaluate various vaccine types, and consider how to produce and distribute the vaccine to meet FDA and WHO approval. They will also combat misinformation and pseudoscientific obstacles during gameplay.
The game’s development follows the success of 2020’s You Make Me Sick, another SEPA-funded and award-winning board game from the Partnership in Education that teaches students about infectious diseases, including COVID-19.
Director of Education and Multimedia Development Brinley Kantorski and Lead Artist and Designer Sarah Will have guided the Partnership in Education’s successful development of several other SEPA-funded games, including the app Dermis Defense and apps on sports-related concussion and sleep. Ms. Kantorski and Will said their passion for games has been an important element in their success.
“Educational games first and foremost need to be fun and engaging before they can be vehicles for learning,” Ms. Kantorski said. “Once you’re having fun, you may not even notice that you’re learning!”
Ultimately, the goal of the new game is to reduce vaccine hesitancy by providing children, parents and teachers with solid, evidence-based scientific information, Dr. Pollock said.
Dr. Pollock, who has developed a wide range of educational and multimedia resources for school children, received one of only a dozen new NIGM awards aimed at developing innovative educational resources that address SARS-CoV-2 vaccine hesitancy. With his most recent funding, he has received a total award of more than $1.4 million from the NIGMS SEPA program for this project. It is the fourth SEPA award to Dr. Pollock and the Partnership in Education – NIH funding of $6.3 million has anchored the program since 2001.
“Our mandate is to educate pre-kindergarten to 12th grade students and community audiences to fight disinformation about the safety and value of vaccination,” Dr. Pollock said.
In addition to his Partnership in Education team, Dr. Pollock is working with Paul Duprex, PhD, director of the Center for Vaccine Research at the University of Pittsburgh School of Medicine and members of the center, and Cathy Morton, EdD, of West Virginia University’s Health Sciences & Technology Academy (HSTA), a SEPA-supported mentoring program that helps high school students in underserved areas enter STEM-based degree programs.
Duquesne’s Partnership in Education program reflects the university’s commitment to providing education and training programs that help improve equity and opportunity in the region.
Pittsburgh Startups to Watch in 2022

Nate Doughty, writer for Pittsburgh Inno, reports that Pittsburgh’s youngest companies aren’t just raising money and hiring workers. They’re changing the very way the world works, developing technologies capable of improving human health and even business itself.
To better inform you of the diverse and unique work being done in the region’s startup scene, Pittsburgh Inno has launched its inaugural list of Startups to Watch — an annual list that profiles startup companies poised for significant developments over the course of the next year. This list isn’t meant to be interpreted as definitive, but rather a glance at the wide array of talent and ideas that permeate the region’s budding startup scene and the companies that are poised to have a breakout year to come.
One of Pittsburgh Inno’s 2022 startups to watch is Ariel Precision Medicine Inc., founded in 2015 by CEO Jessica Gibson, MBA, and Chief Scientific Officer David Whitcomb, MD, PhD. Dr. Whitcomb is the Giant Eagle Foundation Professor of Cancer Genetics, a Professor of Medicine, Cell Biology and Physiology, and Human Genetics, Director of the Precision Medicine Service at UPMC, and an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
Ms. Gibson and Dr. Whitcomb’s company is helping doctors treat patients with chronic health conditions through the use of its Advance Platform technology, an AI platform that can take genetic and clinical data and turn it into insights for the proper identification, management, and advanced therapeutic development of various chronic health conditions. Its main focus is on identifying causes of digestive and metabolic diseases, starting with the pancreas.
The startup has received several accolades, placing multiple times in the Pittsburgh Technology Council’s Tech 50 competition, most recently as a finalist in the innovator of the year: life sciences category for 2021, and being ranked among Data Magazine’s 101 best bioinformatics startups and companies. In 2022, the company is looking to improve the efficiency and effectiveness of physician diagnosis and care plans in an effort it hopes will result in improved patient outcomes. It has raised about $8 million in funding so far from institutional investors, venture capital firms, patient advocacy groups, and angel investors. The current employee count is at 25, 10 of whom work full-time at the company. McGowan Institute affiliated faculty member David Finegold, MD, Professor of Human Genetics at the University of Pittsburgh, is Ariel Precision Medicine’s Lab Director.
Ms. Gibson said the startup’s biggest goals for the next year include “(raising) our Series A to scale our Advance Platform and accelerate the development of our Phase 2 asset for pancreatitis. … We are moving ‘beyond genetics’ into a place where genetics are a framework for understanding integrated risk, and we can model all of the other factors that can contribute to when a disease starts, how rapidly or severely it progresses, and transform that data into insights to prevent or mitigate severe disease.”
Live in 2021, Pittsburgh Inno covers, connects, and catalyzes the city’s ecosystem, producing digital media, events and intelligence about the entrepreneurs, executives, startups, businesses, trends, and topics that are shaping the present and future of Pittsburgh’s economy. Through a twice-a-week newsletter, weekly editorial, quarterly events, directories, data and more, Pittsburgh Inno is building a portal to and for the city’s thriving startup and tech communities.
AWARDS AND RECOGNITION
Dr. Steven Little Selected to the 2021 Fellow Class of NAI

McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, has been selected as a member of the 2021 Fellow class of the National Academy of Inventors (NAI), the highest professional distinction accorded to academic inventors.
Dr. Little is a Distinguished Professor and the William Kepler Whiteford Professor of Chemical and Petroleum Engineering, in addition to being the department chair. He has appointments to the departments of Bioengineering, Immunology, and Ophthalmology. Dr. Little is a co-founder of Pitt spinout company Qrono Inc., which is developing therapies that change the way cancer tumor cells and immune cells interact in a manner that enables immune T cells to infiltrate metastatic tumors. In 2020 he co-authored a study in the journal Science Advances that utilized a similar microparticle therapy that “hacks” the immune system to accept transplanted tissue.
Dr. Little is a pioneering innovator in drug development and delivery with innovations for treatments of cancer, cystinosis, glaucoma, transplantation, dry eye disease, periodontal disease, pulmonary hypertension and more.
Since joining the Pitt faculty in 2006 after his doctoral training, Dr. Little has secured nearly $30 million in peer-reviewed funding from federal sources and has been issued 12 U.S. patents that have been licensed six times. Through his seven-year tenure as Chair, the Department of Chemical and Petroleum Engineering has seen over a two-fold increase in grant proposal submissions and a nearly two-fold increase in peer-reviewed publications.
Dr. Little’s initial two patents were licensed by Zycos Inc. for their DNA vaccine pipeline and were subsequently acquired by MGI Pharma. His patents on thermoresponsive hydrogels containing polymer particles led to the founding of OTERO Tx.
Dr. Little has been recognized with the University of Pittsburgh’s Chancellor’s Distinguished Teaching Award followed by the Chancellor’s Distinguished Research Award and the Chancellor’s Distinguished Public Service Award. He is the only individual in the University of Pittsburgh Swanson School of Engineering’s history to receive more than one of the Chancellor’s awards, and the only individual to receive all three of the Chancellor’s Distinguished Awards in the history of the university.
Dr. Little has been recognized by national and international awards including the Curtis W. McGraw Research Award from the ASEE, elected as a fellow of the BMES and AIMBE, a Carnegie Science Award for Research, the Society for Biomaterials’ Young Investigator Award, named a Camille Dreyfus Teacher Scholar, named an Arnold and Mabel Beckman Young Investigator, and elected to the Board of Directors of the Society for Biomaterials.
The Controlled Release Society, which is the premier society world-wide for delivery science and technology, has recognized Dr. Little with three of its top honors: Distinguished Service Award (2021), College of Fellows (2020), and Young Investigator Award (2018).
“Dr. Little has focused on innovation, entrepreneurship and technology translation for his entire career,” said Nicholas Peppas, Professor and Director of the Institute for Biomaterials, Drug Delivery and Regenerative Medicine at the University of Texas, and also an inaugural Fellow of the NAI. “As a leader of an academic department, he has made improvements to his department’s education programs in innovation and entrepreneurship, which has led to student-driven founding of companies and winning local and national prizes.”
The NAI Fellows Program highlights academic inventors who have demonstrated a spirit of innovation in creating or facilitating outstanding inventions that have made a tangible impact on the quality of life, economic development, and the welfare of society. The 164 members of the 2021 Fellow class hail from 116 research universities and governmental and non-profit research institutes worldwide. They collectively hold over 4,800 issued U.S. patents.
The 2021 new Fellows will be inducted at the Fellows Induction Ceremony at the 11th Annual Meeting of the National Academy of Inventors June 2022 in Phoenix, Arizona.
11th Annual Michael G. Wells Student Healthcare Competition Winners

Treatments for pain that do not involve the prescription of addictive opioids are greatly needed. Nerve stimulation devices have proven to be effective at treating pain, but they are expensive, require surgeries to implant and remove and have a high failure rate.
A team of Pitt innovators are developing an injectable nerve stimulator that biodegrades inside the body. The team, under the name Vanish Therapeutics, took the top prize in the 11th annual Michael G. Wells Student Healthcare Competition, which includes a check for $20,000 to help the team accelerate on its path to commercialization and impact.
“This was the best field we have ever had,” declared Michael Wells, the Pitt trustee and healthcare investor who sponsors the competition’s top award and each year serves as one of the judges.
“I serve as a judge on the Wharton Business School’s annual pitch competition, the oldest in the nation, and I can say the quality of the presentations this year was right on par,” he added.
Pitt innovation teams over the past 11 years have used the Wells competition as a launchpad. Of the 60 finalist teams, nearly 30 percent have gone on to form a startup company, raising a combined $30 million in additional capital along the way.
Kevin Woeppel, a bioengineering PhD student, who served as the entrepreneurial lead for the Vanish team, said the winnings from the competition will be used to do bio-compatibility testing experiments as they work towards clinical trials.
Also, co-founding Vanish is Trent Emerick, MD, MBA, assistant professor of anesthesiology and designated pain specialist for the Pittsburgh Steelers, and Pitt bioengineering professor and McGowan Institute for Regenerative Medicine faculty member Xinyan Tracy Cui, PhD.
Two other teams receiving $10,000 each included NextGen ET, which is developing an improved endotracheal tube designed to prevent ventilator-associated pneumonia, a common problem associated with current tubes that results in approximately 300,000 deaths each year.
Mr. Wells commented that entrepreneurial lead for NextGen ET, PhD student Lauren Grice, was one of the most polished presenters in a student pitch competition that he has encountered.
The innovation comes from the lab of McGowan Institute affiliated faculty member Carl Snyderman, MD, MBA, professor of otolaryngology and neurological surgery. The team includes Garrett Coyan, MD, MS (formerly from the lab of McGowan Institute director William Wagner, PhD), an entrepreneurial postdoctoral fellow in cardiothoracic surgery who has been part of several innovation teams in his time at Pitt, along with Ross Beresford, who is pursuing a master’s degree in medical product innovation.
The other $10,000 winner was Reach Neuro, which is developing a spine stimulation device that has demonstrated in two live patients the ability to provide partial motor skill recovery in stroke patients. A video of one of those people being able to grasp an object in her hands for the first time in several years following her stroke amazed Mr. Wells and the other judges.
The principal investigator for Reach Neuro is Marco Capogrosso, PhD, assistant professor of neurosurgery and director of the Spinal Cord Stimulation Laboratory, and the entrepreneurial lead is a PhD student in his lab, Marc Powell. Rounding out this multi-disciplinary and multi-institution team is McGowan Institute affiliated faculty member Douglas Weber, PhD, professor of mechanical engineering and neuroscience at Carnegie Mellon University, Jordyn Ting, a PhD student in bioengineering at Pitt, and Peter Gerszten, MD, professor of neurosurgery.
Another finalist team in the 2021 Wells Competition included:
Aneurysm Prognosis Classifier (the lab of McGowan Institute affiliated faculty member David Vorp, PhD, associate dean for research, Swanson School of Engineering, University of Pittsburgh): About 4.5 million people have abdominal aneurysms. The average cost is $40,000 over 4 years for imaging surveillance of these aneurysms. The team is developing a new blood test to measure risk of rupture that would dramatically reduce these costs.
Congratulations, all!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#228 –– Dr. Fabrisia Ambrosio discusses the International Consortium of Regenerative Rehabilitation as well as her research in extracellular vesicles and their effects on aging.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Amrita Sahu, Zachary J. Clemens, Sunita N. Shinde, Sruthi Sivakumar, Abish Pius, Ankit Bhatia, Silvia Picciolini, Cristiano Carlomagno, Alice Gualerzi, Marzia Bedoni, Bennett Van Houten, Mita Lovalekar, Nicholas F. Fitz, Iliya Lefterov, Aaron Barchowsky, Radosveta Koldamova & Fabrisia Ambrosio
Title: Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles
Summary: Heterochronic blood exchange (HBE) has demonstrated that circulating factors restore youthful features to aged tissues. However, the systemic mediators of those rejuvenating effects remain poorly defined. We show here that the beneficial effect of young blood on aged muscle regeneration was diminished when serum was depleted of extracellular vesicles (EVs). Whereas EVs from young animals rejuvenate aged cell bioenergetics and skeletal muscle regeneration, aging shifts EV subpopulation heterogeneity and compromises downstream benefits on recipient cells. Machine learning classifiers revealed that aging shifts the nucleic acid, but not protein, fingerprint of circulating EVs. Alterations in subpopulation heterogeneity were accompanied by declines in transcript levels of the prolongevity protein α-Klotho (Klotho), and injection of EVs improved muscle regeneration in a Klotho mRNA-dependent manner. These studies demonstrate that EVs play a key role in the rejuvenating effects of HBE and that Klotho transcripts within EVs phenocopy the effects of young serum on aged skeletal muscle.
Source: Nature Aging. 06 December 2021.
GRANT OF THE MONTH
PIs: Pamela Moalli, Kyle Orwig, and Caroline Gargett
Title: Vaginal Stem Cells: The Missing Link in Vaginal Reconstruction
Description: An increasing number of women are seeking vaginal reconstruction surgery to repair tissue loss after surgery for pelvic cancers or following ovariectomy, chemotherapy and radiation, which can result in the vagina becoming contracted, thinned and painful. After noticing the limited range of biomaterials for these procedures, Moalli was inspired to develop better solutions. This project seeks to identify stem cell populations that restore vaginal structure and function and isolate the cellular ecosystems needed for stem cell survival, proliferation and engraftment. The researchers will use a novel bioassay to develop vaginal organoids from vaginal stem cells.
Source: Magee-Womens Research Institute, funded by the Richard King Mellon Foundation
Term: 10/1/21 – 6/30/22
Amount: $1,000,000
