September 2019 | VOL. 18, NO. 09| www.McGowan.pitt.edu
Dr. Eric Lagasse’s Work Highlighted in Forbes

As reported by Forbes contributor Robin Seaton Jefferson, LyGenesis, Inc., hopes to enter human trials in 2020 on a therapy that could potentially give patients with end stage liver disease hope for new livers without having to wait on donated organs. The technology, developed through research from McGowan Institute for Regenerative Medicine faculty member Eric Lagasse, PharmD, PhD, associate professor in the Department of Pathology at the University of Pittsburgh and LyGenesis’ Chief Scientific Officer, uses lymph nodes as bioreactors to regrow functioning organs within a patient’s own body. The research found that a variety of different cells types and tissues, including the liver, could engraft and actually grow within lymph nodes. The company is working on injecting cadaver cells into lymph nodes to grow secondary livers.
“Remarkably, we were able to get these transplanted cells to organize, proliferate and vascularize into functioning ectopic organs, which rescued animals from otherwise fatal cases of organ failure,” Dr. Lagasse said in a statement. The research is funded in part by the National Institutes of Health (NIH) and conducted at the McGowan Institute.
Michael Hufford, PhD, Chief Executive Officer of LyGenesis, said the technology could address the problem of the imbalance between organ supply and demand. Instead of one donated organ treating just one patient, one donated organ could serve as the seed to treat dozens of patients simultaneously, he said. And procedures using Lygenesis’ technology would not require major transplantation surgery, Dr. Hufford said in a statement, “but would instead use a minimally invasive outpatient endoscopy, decreasing costs while enabling patients considered too sick or having too many comorbidities to qualify for traditional organ transplantation to receive treatment.”
RESOURCES AT THE MCGOWAN INSTITUTE
October Histology Special
Mucins are a family of high molecular weight, heavily glycosylated proteins (glycoconjugates) produced by epithelial tissues in most metazoans. Mucins’ key characteristics include their ability to form gels; therefore, they are a key component in most gel-like secretions, serving functions that range from lubrication to cell signaling to forming chemical barriers. For example, mucins often serve a modulatory role by controlling mineralization and bone formation in vertebrates. Mucins bind to pathogens as part of the immune system, and overexpression of mucin proteins, especially MUC1, is associated with many types of cancer.
Alcian Blue / PAS
The combination of the alcian blue and various PAS techniques can be used to distinguish neutral mucins from acid mucins. In most protocols, sections are stained with the standard alcian blue (pH 2.5) method followed by the PAS technique. The alcian blue at a pH of 2.5 will stain all acid mucins deep blue but will not color the neutral mucins. The subsequent application of the PAS technique will stain the neutral mucins bright magenta. Tissues or cells that contain both neutral and acidic mucins may show a dark blue or purple coloration. The combined application of alcian blue and PAS is useful for several reasons. Changes in the distribution or pattern of expression of neutral and acid mucins are indicative of certain pathological conditions. In addition, the combined alcian blue/PAS technique is perhaps the most sensitive and comprehensive method for detection since all mucins should react regardless of the charge nature of the mucin.
You will receive 30% off your Alcian Blue or PAS staining or both in october when you mention this ad. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology work with the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
Potential Peripheral Nerve Repair Technology Receives NSF and NIH Funding

Renerva, LLC, a medical device company developing innovative technologies for peripheral nerve repair, announced recently that it has received two additional grants totaling $500,000 from the National Science Foundation (NSF) and the National Institutes of Health (NIH), rapidly accelerating development of their medical device products.
Renerva’s initial therapeutic focus is on the 500,000 Americans suffering from acute nerve injuries every year, with other indications including the treatment of chronic compressive nerve injuries and other conditions affecting the peripheral nerves. Renerva’s pioneering technology was developed in the laboratory of McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, Associate Professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh. Dr. Brown is the Chief Technology Officer at Renerva.
The NIH support will enable the company to expand the applications of its first product, Renerva Peripheral Nerve Matrix (PNM) – an injectable gel derived from porcine tissue that promotes and supports repair and regeneration in injured peripheral nerves. The NSF funding will support the development of Renerva’s second product, a novel graft for peripheral nerve gap applications (a $2 billion market today).
Renerva’s second product will offer surgeons the potential of an off-the-shelf graft with performance equivalent to or better than that of the gold standard nerve autograft. Renerva’s graft will spare patients the discomfort, loss of function, and potential complications associated with nerve autografts, while facilitating the return of motor and/or sensory function in patients affected by complex and debilitating nerve injuries.
Alexander Spiess, MD, Renerva’s Medical Advisory Board Member and Division Chief of Hand Surgery at the University of Pittsburgh Medical Center’s (UPMC), Department of Plastic Surgery, said: “This novel graft has the potential to become the new standard of care for patients requiring nerve bridges for long gap nerve injuries, nerve transfers, and muscle reinnervations. It has also the potential to open the door to new solutions in nerve surgical care.”
Jonathan Cheetham, PhD, VetMB, Associate Professor of Clinical Sciences at Cornell University, Chair of Renerva’s Scientific Advisory Board, and an affiliated faculty member of the McGowan Institute, said: “The NIH award will allow to test Renerva PNM in yet another application. Recurrent laryngeal nerve injury is a condition that results in vocal fold paralysis in up to 10% of patients receiving thyroid surgery. By implementing PNM in the repair of this type of injury, we will help demonstrate the broader scope of capabilities and applications of Renerva’s first product.” Through this testing, and with the support of the NIH award, Renerva’s technologies will expand their potential treatment applicability to ear, nose, and throat disorders.
“In addition to our dilutive capital, Renerva has now received $3 million in active, non-dilutive funding from three U.S. federal agencies (i.e., DoD, NSF, and NIH). This support reflects the comprehensive peer-reviewed vetting for our technology and team by the main government agencies supporting medical technology development, the rigor of the underlying science, and the significant unmet need for our growing product portfolio,” said Lorenzo Soletti, PhD, MBA, Chief Executive Officer of Renerva. “As Renerva transitions into a clinical stage company with our PNM product moving into first-in-human clinical trials next year, this support will help expand our core technology into other nerve injury applications and position us for Series A financing.”
First Ever State Sepsis Regulation Tied to Lower Death Rates

Death rates from sepsis fell faster in New York than expected—and faster than in peer states—following the introduction of the nation’s first state-mandated sepsis regulation, according to an analysis led by University of Pittsburgh researchers and published in JAMA. The policy requires all New York hospitals to quickly implement certain protocols when the deadly condition is suspected.
McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, professor and chair of Pitt’s Department of Critical Care Medicine and director of Pitt’s Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center is senior author on the study.
The finding is good news for the nearly dozen other states in varying stages of adopting similar policies to reduce deaths from sepsis, the leading cause of death in hospitalized patients. Sepsis is a life-threatening condition that arises when the body’s response to an infection injures its own tissues and organs.
“Rarely in the U.S. do we force hospitals to implement specific clinical protocols. Typically, quality improvement is achieved through financial incentives and public reporting,” said lead author Jeremy Kahn, MD, MS, professor in the Department of Critical Care Medicine at Pitt’s School of Medicine and the Department of Health Policy and Management at Pitt’s Graduate School of Public Health. “For the first time, state officials are enshrining in regulations that hospitals must follow certain evidence-based protocols when it comes to sepsis. And our study finds that, at least in New York, it seemed to work.”
Rory’s Regulations were issued by the New York State Department of Health in 2013 after 12-year-old Rory Staunton died of undiagnosed sepsis. The regulations require that hospitals in New York follow protocols for sepsis that include giving antibiotics within three hours and intravenous fluids within six hours of hospitalization. The hospitals also are required to regularly train staff in the protocols and to report adherence and clinical outcomes to the state.
Dr. Kahn and his team analyzed records of more than a million sepsis admissions in 509 hospitals in New York and four control states without a sepsis regulation: Florida, Maryland, Massachusetts and New Jersey. The team looked at dates from two years before Rory’s Regulations were adopted, and two years after.
In the years before the regulations went into place, 26.3% of the people diagnosed with sepsis in New York died while hospitalized, compared to a rate of 22% in the control states. Following the regulations, New York’s sepsis mortality rate dropped 4.3% to 22%, but the death rate only fell 2.9% to 19.1% in the control states.
After accounting for patient and hospital characteristics, as well as pre-existing sepsis trends in the states, New York’s sepsis death rate was 3.2% lower following the regulation than would have been expected, relative to the control states. This comparison was crucial to estimating the improvement and sets this study apart from prior work. Sepsis outcomes are known to improve over time—a study just looking in New York would not be able to differentiate the effects of the regulations from underlying trends. Because these improvements occurred more quickly in New York compared to other states, the researchers are more confident that the regulations are the source of the improvement.
“Sepsis is a tremendous global health burden, so developing proven ways to quickly recognize and treat people who have it is a top public health priority,” said Dr. Angus. “While every state should consider their specific population and needs when developing regulations, our analysis reveals that policies enforcing evidence-based clinical protocols for the timely recognition and treatment of sepsis saves lives.”
Strawberries May Be Key to Developing an Insulin Pill

More than 30 million Americans suffer from diabetes and must inject themselves with insulin two to four times daily. Researchers have been looking for ways to administer the drug orally, and researchers at Carnegie Mellon University (CMU) have now shown such a feat is possible.
McGowan Institute for Regenerative Medicine affiliated faculty member Kathryn Whitehead, PhD, a chemical engineering associate professor at CMU, and her team said the secret lies in an unlikely place: strawberries.
“The problem with insulin,” said CMU research assistant Nicholas Lamson, “is that it’s a protein. The human stomach is very adept at breaking down proteins — such as with food.”
For insulin to be therapeutic, the protein needs to be absorbed intact by the small intestine.
Researchers have developed many ways to encapsulate insulin molecules so that they can make it through the stomach to the small intestine. But what to do with them once the pill is there has been the biggest sticking point. Allowing the proteins to pass into the small intestine fully undigested means the insulin is too large to be absorbed through the intestine and into the blood stream. And while compounds already exist that can open the pores of the small intestine, few can do it without lasting damage.
“We took around 110 fruits and vegetables and screened them for an ability to open up the gaps between the cells of the intestine wide enough to allow the insulin to pass through,” Dr. Whitehead said.
That is where strawberries come in. The same chemical that makes strawberries red — pelargonidin — can dilate intestinal pores in a nontoxic way that later allows them to shrink back to normal.
Combine this molecule with an encapsulated insulin package and voilà — an insulin pill that can help diabetics manage their blood sugar with no negative side effects.
The research team has proven the pill’s efficacy in mice, but there is still a long way to go before an insulin pill is made available to human diabetic patients.
“A number of challenges must still be addressed,” Mr. Lamson said, “one of the biggest being the necessity of variable dosage. Diabetics must test their blood sugar throughout the day and administer an insulin dose appropriate for their blood sugar levels. This is easy to do with an injection, but much more difficult to do with a pill.”
Dr. Whitehead’s lab plans to extend their strawberry technology to proteins other than insulin. That means this technology can potentially be used with other protein therapies, many of which are used to treat conditions like leukemia, osteoporosis and autoimmune disease. Such an advance would revolutionize healthcare as we know it, removing the pain of injections and improving the daily lives of millions of patients.
Scientists Listed Ways of Applying Genetic Engineering to Treat Parkinson’s Disease
 Researchers of Sechenov University and University of Pittsburgh—including McGowan Institute for Regenerative Medicine affiliated faculty members
Researchers of Sechenov University and University of Pittsburgh—including McGowan Institute for Regenerative Medicine affiliated faculty members
- Charleen Chu, MD, PhD, Professor and Vice-Chair of Faculty Development and Mentorship in the Department of Pathology, University of Pittsburgh School of Medicine, where she holds the A. Julio Martinez Chair in Neuropathology, and
- Valerian Kagan, PhD, DSc, Professor and Vice-Chairman in the Department of Environmental and Occupational Health as well as a Professor in the Department of Pharmacology and Chemical Biology, the Department of Radiation Oncology, and the Department of Chemistry—
described the most promising strategies in applying genetic engineering for studying and treating Parkinson’s disease. This method can help evaluate the role of various cellular processes in pathology progression, develop new drugs and therapies, and estimate their efficacy using animal disease models. The study was published in Free Radical Biology and Medicine.
Parkinson’s disease is a neurodegenerative disorder accompanied by a wide array of motor and cognitive impairments. It develops mostly among elderly people (after the age of 55-60). Parkinson’s symptoms usually begin gradually and get worse over time. As the disease progresses, people may have difficulty controlling their movements, walking and talking and, more importantly, taking care of themselves. Although there is no cure for Parkinson’s disease, medicines, surgical treatment, and other therapies can often relieve some symptoms.
The disease is characterized by significant (up to 50-70%) loss of dopaminergic neurons, i.e. nerve cells that synthesize neurotransmitter dopamine which enables communication between the neurons. Another hallmark is the presence of Lewy bodies – oligomeric deposits of a protein called alpha-synuclein inside the neurons.
Scientists discovered several mechanisms that seem to trigger and exacerbate the development of Parkinson’s disease. In nearly 10% of all cases the disease is genetically predetermined, however, in most of the cases the disease is believed to involve both genetic and environmental risk factors, e.g. taking some drugs or pesticide/herbicide poisoning.
The authors of the study focused on the possible causes of the disease that are related to the oxidation-reduction (redox) reactions in cells as well as to mechanisms of cell death – apoptosis and ferroptosis. These processes were thoughtfully discussed in the context of direct manipulation (e.g. insertion or deletion of DNA sequences) with genetic material of living organisms or cells by applying a novel genome editing approach – CRISPR/Cas9 technology.
“Parkinson’s disease is the second most common neurodegenerative disorder in the world, and its incidence is growing with age. The disease affects patients’ quality of life and imposes significant social and economic burden on societies. CRISPR is a promising technology, a strategy to find new effective treatments to neurodegenerative diseases. The technology gives researchers ample opportunity to knock in and knock out any gene of interest, to define the processes it is involved in, and unearth entire metabolic pathways in order to understand the underlying mechanisms and causes of the disease. Special attention should be drawn to CRISPR editing applications for creation of cellular and animal models that fully reproduce pathological states observed in human patients, provide insights into the mechanisms underlying the disorder, and are applicable for drug discovery,” says Margarita Artyukhova, co-author of the work, 4th year student, Institute for Regenerative Medicine, Sechenov University, and member of Sechenov Biomedical Club.
The first group of mechanisms link the role of mitochondria with Parkinson’s disease pathology. Along with being powerhouses of the cell, mitochondria form reactive oxygen species (ROS) that play important roles in cell signaling and homeostasis. Damage to mitochondria or disruption of their work may lead to the extensive accumulation of ROS that may cause significant damage to cell structures. Because of the danger of having damaged mitochondria in the cell, the timely elimination of damaged and aged mitochondria is essential for maintaining the integrity of the cell. This turnover is known as mitophagy. Inefficient or excessive mitophagy may contribute to neurodegeneration.
Previous studies demonstrated that animals with mutations in the genes that encode for PINK1 and Parkin – proteins essential for proper mitochondrial functioning – developed mitochondrial dysfunction, muscle degeneration, and remarkable loss of dopaminergic neurons typical to Parkinson’s disease. Results from several research groups suggested that the role of these proteins could be much more complicated and may involve other partners that contribute to the execution and regulation of mitophagy, thus, the time for implementing genome editing to manipulate them is yet to come. However, there are several other proteins and lipids that impact mitophagy and mitochondrial function (DJ-1, alpha-synuclein, Fbxo7, and cardiolipin), and CRISPR/Cas9 can be used to search for yet unknown genes and their protein products involved in the disease progression and development. Moreover, generation of animal and cellular models with various mutations in mentioned genes will help to define the role of corresponding proteins in the disease pathology and make them targets for drug discovery.
The second group of cellular processes is related to iron homeostasis. A certain amount of iron and ROS is important for a proper functioning of the cell thus, the disruption of this balance may cause a chain of harmful and pathological alterations that affect crucial cellular processes. Iron can accumulate in brain tissues of elderly people and animals, in particular in the areas of the brain responsible for motor and cognitive functions. It was shown that a high level of iron in one of these areas (substantia nigra) is accompanied by death of dopaminergic neurons.
Furthermore, interaction between dopamine and iron can promote the production of toxic metabolites that cause damage to mitochondria and trigger aggregation of alpha-synuclein in neurons. Scientists also examine proteins involved in iron import and export within the neurons: tau protein, transferrin, transferrin receptor, ferroportin, and amyloid precursor protein. CRISPR/Cas9 can help in developing of drugs that will normalize iron homeostasis in affected tissues.
The third group of processes reviewed in the article are cell death programs, apoptosis and ferroptosis. During apoptosis, proteins and DNA of the cell are broken up with special enzymes and the cell itself disintegrates. Ferroptosis follows the iron-dependent oxidation of specific lipid classes; the products of oxidation accumulate and poison the cell. Both apoptosis and ferroptosis play a role in the development of neurological diseases, speeding up the death of dopaminergic neurons. In this case CRISPR/Cas9 will be useful in detailed studies of cell death pathways that promote Parkinson’s disease-associated loss of dopaminergic neurons as well as in the regulation of specific enzymes and proteins that are involved in the execution of these cell death programs.
Functional analysis of genome, a powerful approach to gain comprehensive information on the entire genome, helped obtain many of these results and has already proved its efficiency in research on schizophrenia, bipolar disorder and autism spectrum disorder. Paired with CRISPR/Cas9 it can contribute to the understanding of genetic basis of Parkinson’s disease, promote generation of individual cellular and animal models, as well as design of new drugs and treatment approaches.
“This is one important step of many toward bringing the promise of this new technology to patients with serious diseases like neurodegenerative disorders. It has already been used in human trials (in China, Germany, the USA) to treat patients with cancer at a late stage and beta-thalassemia. Such studies allow us to see vast potential of genome editing as a therapeutic strategy. It’s hard not to be thrilled and excited when you understand that progress of genome editing technologies can completely change our understanding of treatment of Parkinson’s disease and other neurodegenerative disorders,” added Margarita Artyukhova.
Runaway Mitochondria Cause Telomere Damage in Cells
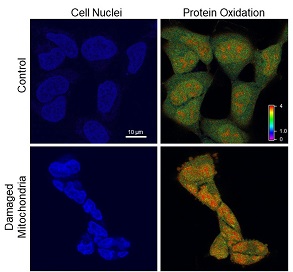
Researchers at the University of Pittsburgh Medical Center (UPMC) Hillman Cancer Center—including McGowan Institute for Regenerative Medicine affiliated faculty member Simon Watkins, PhD, founder and director of the Center for Biologic Imaging at the University of Pittsburgh, a member of the Pittsburgh Cancer Institute, and a distinguished professor and vice chairman within the Department of Cell Biology—provide the first concrete evidence for the long-held belief that sick mitochondria pollute the cells they’re supposed to be supplying with power.
The paper, published in the Proceedings of the National Academy of Sciences, involves a causal experiment to kick off a mitochondrial chain reaction that wreaks havoc on the cell, all the way down to the genetic level.
“I like to call it ‘the Chernobyl effect’ — you’ve turned the reactor on and now you can’t turn it off,” said senior author Bennett Van Houten, PhD, professor of pharmacology and chemical biology at the University of Pittsburgh School of Medicine and UPMC Hillman Cancer Center. “You have this clean-burning machine that’s now polluting like mad, and that pollution feeds back and hurts electron transport function. It’s a vicious cycle.”
Van Houten’s team used a new technology invented by Marcel Bruchez, Ph.D., of Carnegie Mellon University, that produces damaging reactive oxygen species — in this case, singlet oxygen — inside the mitochondria when exposed to light.
“That’s the Chernobyl incident,” Van Houten said. “Once you turn the light off, there’s no more singlet oxygen anymore, but you’ve disrupted the electron transport chain, so after 48 hours, the mitochondria are still leaking out reactive oxygen — but the cells aren’t dying, they’re just sitting there erupting.”
At this point, the nucleus of the cell is being pummeled by free radicals. It shrinks and contorts. The cell stops dividing. Yet, the DNA seems oddly intact.
That is, until the researchers start looking specifically at the telomeres — the protective caps on the end of each chromosome that allow them to continue replicating and replenishing. Telomeres are extremely small, so DNA damage restricted to telomeres alone may not show up in a whole-genome test, like the one the researchers had been using up to this point.
“If you imagine the chromosome as a car, the telomere would be the width of its license plate,” said study coauthor Patricia Opresko, PhD, professor of environmental and occupational health at the Pitt Graduate School of Public Health and UPMC Hillman.
So, to see the genetic effects of the mitochondrial meltdown, the researchers had to light up those tiny endcaps with fluorescent tags, and lo and behold, they found clear signs of telomeres’ fragility and breakage.
Then, in a critical step, the researchers repeated the whole experiment on cells with inactivated mitochondria. Without the mitochondria to perpetuate the reaction, there was no buildup of free radicals inside the cell and no telomere damage.
“Basically, we shut the machine off before it had a chance to do any damage,” Van Houten said.
These findings could be used for improving photodynamic cancer therapy, which involves bombarding solid tumors with reactive oxygen species using light delivered with fiber optic cables, Van Houten suggested.
One thing his team discovered in the course of these experiments is that inhibiting ATM — a protein that signals DNA damage — magnified the damaging effects of the reactive oxygen species spewed out by the mitochondria. The cells not only shriveled, but also died.
By combining photodynamic therapy with ATM inhibition, it may be possible to design a system that effectively kills cancer cells with light, Van Houten said.
Illustration: UPMC/PittHealthSciences/Hillman Cancer Center.
HERL Receives $50K Grant from Disabled Veterans National Foundation

The Disabled Veterans National Foundation (DVNF), a nonprofit veterans service organization that provides critically needed support to disabled and at-risk veterans who leave the military wounded—physically or psychologically—after defending our safety and our freedom, presented a grant of $50,000 to the Human Engineering Research Laboratories (HERL).
HERL is an organization at the forefront of groundbreaking technologies and adaptive devices that assist people with disabilities. HERL is directed by McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, FISA & Paralyzed Veterans of America (PVA) Professor and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Med & Rehab, and Orthopedic Surgery at the University of Pittsburgh. DVNF’s grant is intended to assist our veterans in obtaining services and programs that aid them in achieving their goals. HERL was presented with a check on the occasion of HERL’s 25th anniversary on July 25, 2019.
“I worked with Dr. Cooper during my time as the Wounded Warrior Regiment Sergeant Major, and I’m pleased to now continue to be a part of his work as DVNF’s CEO,” said Joseph VanFonda (USMC SgtMaj Ret.). “HERL has made such an impact on the lives of wounded and injured veterans, and DVNF is honored to be able to contribute to their life changing efforts.”
USMC SgtMaj VanFonda also expressed his appreciation to DVNF’s generous donors who helped make this grant possible. HERL is a joint collaboration between the University of Pittsburgh and the Department of Veterans Affairs, and often depends on outside organizations for support.
Researchers Create Soft, Flexible Materials with Enhanced Properties

A team of polymer chemists and engineers from Carnegie Mellon University (CMU) including McGowan Institute for Regenerative Medicine affiliated faculty member Krzysztof Matyjaszewski, PhD, the J.C. Warner Professor of Natural Sciences at CMU, has developed a new methodology that can be used to create a class of stretchable polymer composites with enhanced electrical and thermal properties. These materials are promising candidates for use in soft robotics, self-healing electronics and medical devices. The results are published in Nature Nanotechnology.
In the study, the researchers combined their expertise in foundational science and engineering to devise a method that uniformly incorporates eutectic gallium indium (EGaIn), a metal alloy that is liquid at ambient temperatures, into an elastomer. This created a new material — a highly stretchable, soft, multi-functional composite that has a high level of thermal stability and electrical conductivity.
Carmel Majidi, PhD, a professor of Mechanical Engineering at Carnegie Mellon and director of the Soft Machines Lab, has conducted extensive research into developing new, soft materials that can be used for biomedical and other applications. As part of this research, he developed rubber composites seeded with nanoscopic droplets of liquid metal. The materials seemed to be promising, but the mechanical mixing technique he used to combine the components yielded materials with inconsistent compositions, and as a result, inconsistent properties.
To surmount this problem, Dr. Majidi turned to Dr. Matyjaszewski, who developed atom transfer radical polymerization (ATRP) in 1994. ATRP, the first and most robust method of controlled polymerization, allows scientists to string together monomers in a piece-by-piece fashion, resulting in highly tailored polymers with specific properties.
“New materials are only effective if they are reliable. You need to know that your material will work the same way every time before you can make it into a commercial product,” Dr. Matyjaszewski said. “ATRP has proven to be a powerful tool for creating new materials that have consistent, reliable structures and unique properties.”
Drs. Majidi and Matyjaszewski and CMU Materials Science and Engineering Professor Michael Bockstaller, PhD, used ATRP to attach monomer brushes to the surface of EGaIn nanodroplets. The brushes were able to link together, forming strong bonds to the droplets. As a result, the liquid metal uniformly dispersed throughout the elastomer, resulting in a material with high elasticity and high thermal conductivity.
Dr. Matyjaszewski also noted that after polymer grafting, the crystallization temperature of eGaIn was suppressed from 15 C to -80 C, extending the droplet’s liquid phase — and thus its liquid properties — down to very low temperatures.
“We can now suspend liquid metal in virtually any polymer or copolymer in order to tailor their material properties and enhance their performance,” Dr. Majidi said. “This has not been done before. It opens the door to future materials discovery.”
The researchers envision that this process could be used to combine different polymers with liquid metal, and by controlling the concentration of liquid metal, they can control the properties of the materials they are creating. The number of possible combinations is vast, but the researchers believe that with the help of artificial intelligence, their approach could be used to design “made-to-order” elastomer composites that have tailored properties. The result will be a new class of materials that can be used in a variety of applications, including soft robotics, artificial skin and bio-compatible medical devices.
3D Printing of Soft Materials: New and Complicated Variables

While 3D printing soft materials, such as silicone or proteins, offers many advantages, it also introduces many new and complicated variables to consider when creating a new part, per Vanesa Listek for 3D Print in her report featuring the work of McGowan Institute for Regenerative Medicine affiliated faculty member Newell Washburn, PhD, professor of biomedical engineering and chemistry at Carnegie Mellon University (CMU). The existing soft materials that can be 3D printed commercially are somewhat limited since they don’t have all the properties that researchers need to fully advance their developments and they end up working within the constraints of the current technology.
One of the main problems with 3D printing a soft material is that it tends to deform under the forces that normally occur, sometimes even during the build, so they require support materials. According to researchers from the College of Engineering at CMU, that means that additive manufacturing of soft materials requires optimization of printable inks, formulations of these feedstocks, and complex printing processes that must balance a large number of disparate but highly correlated variables (such as metal powder particle size, melt pool shape and size or filament feeding rate, extrusion width, linear plotting speed and layer thickness or suspension viscosity). Due to the critical need for integrated methodologies, the researchers have come up with a hierarchical machine learning (HML) algorithm that optimizes parameters of these type of materials for 3D printing, using Freeform Reversible Embedding (FRE)–a recently developed method for 3D printing of liquid polymer precursors that involves controlled deposition of a fluid precursor into a supporting aqueous bath.
The team, led by Dr.Washburn, who along with colleagues from CMU including Adam Feinberg, PhD, associate professor of biomedical engineering and materials science and engineering (and an affiliated faculty member of the McGowan Institute); Barnabas Poczos, professor of machine learning at the School of Computer Science; and materials science and engineering doctoral student Aditya Menon, recently published a paper on their work, titled “Optimization of Silicone 3D Printing with Hierarchical Machine Learning,” in 3D Printing and Additive Manufacturing. The paper demonstrates how their new algorithm was designed to optimize high quality, soft material 3D prints.
“Increasingly, we’re starting to make more soft material-based components and devices for low-tech areas such as sensors and medical applications, and for high tech areas like soft robotics,” said Dr. Washburn. “But every time you add another component you exponentially raise the complexity of the manufacturing process. That’s where a machine learning algorithm could really help with optimization.”
Machine learning algorithms, which are a type of artificial intelligence algorithm, are designed to develop a relationship between the input variables and the outputs of a complex system based on training data. According to researchers at CMU, traditional algorithms in machine learning tools generally treat systems as black boxes and then attempt to define a response surface based on statistical analysis. This then requires extensive data sets to train these models, which can be difficult to generate, especially given the number of variables and time required to probe experimental processing parameters. But for the complex problem that the research team is working on, there isn’t a lot of data. Therefore, to account for this, Dr. Washburn and his team custom-built an HML algorithm that incorporates expert knowledge about how the physical systems and parameters operate with limited data available. Dr. Washburn assures that HML uses knowledge of the underlying physical system—in this case, the physics of the FRE 3D printing variables—to draw connections between the right variables, significantly cutting time and the amount of data needed.
“This new machine-learning algorithm is a powerful tool that we have designed to use in our research work for complex physical systems, it uses very small data sets allowing us to optimize their performance. For the 3D printing projects, the material and the technologies we are developing will allow us to optimize the 3D printing of entire organs where we are applying a complex mixture of components, materials, and cells. The overall goal of our research is to design materials for function and not just composition, leveraging the understanding of how these materials work,” Dr. Washburn claims.
The researchers were able to print 2.5 times faster, print with ink that previously didn’t work well, and the algorithm also identified a unique silicone formulation and printing parameters that had not been found previously through trial-and-error approaches. With these results, the researchers—some of whom are affiliated with both the Manufacturing Futures Initiative and the Bioengineered Organs Initiative—are optimistic that HML will have a broad application for planning and optimizing in the AM of many types of soft materials, such as for biomedical applications via the FRE method.
AWARDS AND RECOGNITION
Congratulations Dr. Wagner!

University of Pittsburgh Chancellor Patrick Gallagher announced that McGowan Institute Director William R. Wagner, PhD has been appointed to the special faculty rank of Distinguished Professor of Surgery.
The Distinguished category of faculty is reserved only for those who have made major contributions to science and the University and constitutes the highest honor that the University can accord a member of the professoriate.
Congratulations Dr. Wagner!
Dr. Youngjae Chun Receives American Heart Association’s 2020 Innovative Project Award

Coronary artery disease is a leading cause of death in the U.S., with about 370,000 Americans dying from the disease each year. Stents are a life-saving procedure used to prop open narrowing blood vessels; however, over time, tissue can regrow into the mesh stent and cause the artery to narrow again, putting the patient at risk.
Knowing as soon as possible that regrowth is happening is crucial in saving the patient’s life, but monitoring is a challenge. McGowan Institute for Regenerative Medicine affiliated faculty member Youngjae Chun, PhD, associate professor of industrial engineering and bioengineering at the University of Pittsburgh’s Swanson School of Engineering, has received a funding award from the American Heart Association for his project creating a stent that will use sensors to monitor for signs of restenosis and alert the patient’s doctor without the need for endless follow-up visits.
Dr. Chun’s project has been selected by the American Heart Association for its 2019 Innovative Project Award, which supports highly innovative, high-impact research that could lead to major advancements and discoveries that accelerate cardiovascular and cerebrovascular research. The award includes a total of $200,000 over two years and began on July 1, 2019.
“Stenting to treat coronary artery disease is a well-established and widely used interventional procedure. This new stent will minimize the follow-up imaging procedures that can be inconvenient, expensive, and sometimes invasive for the patient,” says Dr. Chun. “Our device would continuously monitor restenosis providing valuable information to the patients.”
This project will be conducted through a multidisciplinary collaboration with W. Hong Yeo, PhD, assistant professor of Department of Mechanical Engineering at Georgia Tech, and McGowan Institute affiliated faculty member John Pacella, MD, cardiologist at UPMC.
“Real-time surveillance would be critical for the patient whose stented blood vessels are re-narrowing, putting them at risk for heart attack or stroke,” says Dr. Chun. “The device would provide critical information directly to patients and their doctors and could potentially save many lives.”
Dr. Mangesh Kulkarni Receives $18K Award from Pitt’s Central Research Development Fund

McGowan Institute for Regenerative Medicine affiliated faculty member Mangesh Kulkarni, PhD, assistant professor of bioengineering at the University of Pittsburgh Swanson School of Engineering, was awarded $17,793 in funding from the Central Research Development Fund (CRDF) to support research for the treatment of inflammatory bowel disease (IBD).
Dr. Kulkarni’s project aims to develop an integrative treatment approach utilizing the interspecies interactions between innate immunity cells and gut microbiota, which is a collection of microorganisms in the intestine.
While these microorganisms can positively affect one’s health, a microbial imbalance – or dysbiosis – in the body has been associated with a variety of illnesses and diseases. Researchers speculate that dysbiosis may have an effect on the body’s immune system – specifically on macrophages, which are multifunctional white blood cells in the body.
“This project will seek to understand how dysbiosis during colitis affects the intestinal macrophages at the molecular level and get insights into pathways affected by colitis, focusing on those regulating the macrophage polarization,” said Dr. Kulkarni.
Macrophage polarization refers to the process in which macrophages adopt different functions in response to signals from their microenvironment. A functional imbalance among the macrophages may have a connection to a number of immunity-related diseases, including IBD.
“As a step towards developing a novel therapeutic avenue for colitis, we seek to examine the feasibility of specific and relevant miRNA therapy to modulate the macrophage polarization,” Dr. Kulkarni continued. “This strategy holds the promise of curbing the progression of intestinal inflammation and thus provide therapeutic benefit in IBD.”
Dr. Kulkarni joined the Department of Bioengineering in fall 2018 and works with McGowan Institute faculty member Bryan Brown, PhD, assistant professor of bioengineering.
“I’m very excited about the work that Dr. Kulkarni is doing,” said Dr. Brown. “This grant from the CRDF will enable him to perform studies that will inform the development of new therapeutic strategies for a disease which currently has few effective treatment options.”
The CRDF Small Grants Program aims to provide opportunities for faculty, especially early career faculty, at the University of Pittsburgh to engage in high-quality research, scholarship, and creative endeavors. The program provides seed funding to develop ideas to the point where external funding can be obtained and awards support for scholarship in areas where external funding is extremely limited.
Debski Lab Student Named the Bioengineering 2019 Wesley C. Pickard Fellow

Sene Polamalu, a bioengineering graduate student at the University of Pittsburgh in the Orthopedic Robotics Laboratory under the supervision of McGowan Institute for Regenerative Medicine affiliated faculty member Richard Debski, PhD, William Kepler Whiteford Faculty Fellow and Professor of Bioengineering and Orthopaedic Surgery, was named the 2019 Wesley C. Pickard Fellow by the Department of Bioengineering. Recipients of this award are selected by the department chair and chosen based on academic merit.
Mr. Polamalu began his studies in applied mathematics at Millersville University of Pennsylvania where he received his bachelor’s degree in two years. He then joined the mechanical engineering graduate program at the Swanson School of Engineering for one semester before receiving a STRIVE Fellowship and switching to the bioengineering program.
Mr. Polamalu is working on multiple biomechanical research projects in the Orthopedic Robotics Laboratory lead by Dr. Debski and Volker Musahl, MD, Blue Cross of Western Pennsylvania Professor in Orthopaedic Surgery and Chief of Sports Medicine.
“My primary research is focused on the association of tibiofemoral bony morphology and anterior cruciate ligament (ACL) injuries,” said Mr. Polamalu. “This research aims to improve ACL injury prevention and treatment by determining patient-specific bone shape parameters that increase the risk for ACL injury.”
The ACL is a major ligament that stabilizes the knee, and injury to this ligament is most commonly experienced during sports that involve sudden stops or changes in one direction.
Recovery from an ACL trauma can leave athletes out of practice for several months.
“I have always been passionate about sports-related injuries,” said Mr. Polamalu. “My research in biomechanics at Pitt has further fueled my interest in orthopaedic injury prevention and treatment. I am grateful for the support I have received from this award.”
Mr. Polamalu is starting his third year as a graduate student researcher in the Swanson School, and the Wesley C. Pickard Fellowship will provide funding for his research during the upcoming academic year.
“I’m incredibly proud of what Sene has accomplished during his time in the Orthopaedic Robotics Laboratory, and this fellowship is a well-deserved recognition of his work,” said Dr. Debski. “His project has the potential to determine which individuals are at more risk for ACL injury based on the bony morphology of their knee. Individualized treatments can then be developed to improve surgical success and reduce secondary injuries.”
Cui Lab Postdoc Receives Best Poster Presentation Award

A poster presented by Asiyeh Golabchi, PhD, a bioengineering postdoctoral research associate at the University of Pittsburgh, was selected as one of the best poster presentations at the 2019 Postdoctoral Data & Dine Symposium. Dr. Golabchi works in the Neural Tissue Engineering (NTE) Lab directed by McGowan Institute for Regenerative Medicine faculty member Xinyan Tracy Cui, PhD, professor of bioengineering in Pitt’s Swanson School of Engineering. Dr. Golabchi’s research focuses on developing a molecular-level understanding of neurobiological interactions to neural implants.
Hosted by the University of Pittsburgh Postdoctoral Association, the Data & Dine Symposium is an opportunity for postdoctoral associates and scholars at Pitt to present their research to colleagues, faculty, and administrators. The event recognizes 10 participants with a $750 travel award for the best poster presentations. Dr. Golabchi received an award for her work entitled, “Neuronal cell adhesion molecule L1 improves quality of the chronic neural recording in mouse visual cortex.”
Dr. Golbachi explained that these devices, which are used to record and stimulate the brain, have been an invaluable tool for neuroscience research and clinical applications, but their functional longevity has proven to be a hurdle for researchers.
“Neural implants have been used to help people who have lost abilities due to trauma or disease regain those abilities and improve their quality of life,” said Dr. Golabchi. “However, many medical, biological, and technical considerations have to be taken into account when using these devices.”
“Current neural implants are limited by the body’s natural conditions to safely operate and avoid toxicity and degradation,” she continued. “The requirements for a functional and stable long-term neural interface are still relatively unknown.”
Dr. Golabchi’s research develops biomaterial strategies and novel technologies to control neuroinflammatory responses, both acute and chronic, to implantable devices.
“Asiyeh is most deserving of this award from Pitt’s Postdoctoral Association. Her work with neural implant technology demonstrated the potential to improve neural recording stability and longevity through biomimetic coating,” said Dr. Cui. “Such coating may be optimized for commercial translation and benefit many implantable devices in both research and clinical settings.”
Dr. Golabchi received her PhD in neuroscience and brain technologies from Istituto Italiano di Tecnologia, in collaboration with the University of Genoa, under the supervision of Dr. Axel Blau. During Dr. Golabchi’s doctoral research, she used microfabrication methods to develop a flexible polymer-based microelectrode array for interfacing with neurons at a high spatiotemporal resolution for both in vivo and in vitro applications.
Illustration: University of Pittsburgh Swanson School of Engineering (Dr. Golabchi).
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#201 –– Dr. Garrett Coyan discusses the importance of clinical research and training in translational medicine.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Kahn JM, Davis BS, Yabes JG, Chang CH, Chong DH, Hershey TB, Martsolf GR, Angus DC
Title: Association Between State-Mandated Protocolized Sepsis Care and In-hospital Mortality Among Adults with Sepsis
Summary: IMPORTANCE: Beginning in 2013, New York State implemented regulations mandating that hospitals implement evidence-based protocols for sepsis management, as well as report data on protocol adherence and clinical outcomes to the state government. The association between these mandates and sepsis outcomes is unknown.
OBJECTIVE: To evaluate the association between New York State sepsis regulations and the outcomes of patients hospitalized with sepsis.
DESIGN, SETTING, AND PARTICIPANTS: Retrospective cohort study of adult patients hospitalized with sepsis in New York State and in 4 control states (Florida, Maryland, Massachusetts, and New Jersey) using all-payer hospital discharge data (January 1, 2011-September 30, 2015) and a comparative interrupted time series analytic approach.
EXPOSURES: Hospitalization for sepsis before (January 1, 2011-March 31, 2013) vs after (April 1, 2013-September 30, 2015) implementation of the 2013 New York State sepsis regulations.
MAIN OUTCOMES AND MEASURES: The primary outcome was 30-day in-hospital mortality. Secondary outcomes were intensive care unit admission rates, central venous catheter use, Clostridium difficile infection rates, and hospital length of 4stay.
RESULTS: The final analysis included 1 012 410 sepsis admissions to 509 hospitals. The mean age was 69.5 years (SD, 16.4 years) and 47.9% were female. In New York State and in the control states, 139 019 and 289 225 patients, respectively, were admitted before implementation of the sepsis regulations and 186 767 and 397 399 patients, respectively, were admitted after implementation of the sepsis regulations. Unadjusted 30-day in-hospital mortality was 26.3% in New York State and 22.0% in the control states before the regulations and was 22.0% in New York State and 19.1% in the control states after the regulations. Adjusting for patient and hospital characteristics as well as preregulation temporal trends and season, mortality after implementation of the regulations decreased significantly in New York State relative to the control states (P = .02 for the joint test of the comparative interrupted time series estimates). For example, by the 10th quarter after implementation of the regulations, adjusted absolute mortality was 3.2% (95% CI, 1.0% to 5.4%) lower than expected in New York State relative to the control states (P = .004). The regulations were associated with no significant differences in intensive care unit admission rates (P = .09) (10th quarter adjusted difference, 2.8% [95% CI, -1.7% to 7.2%], P = .22), a significant relative decrease in hospital length of stay (P = .04) (10th quarter adjusted difference, 0.50 days [95% CI, -0.47 to 1.47 days], P = .31), a significant relative decrease in the C difficile infection rate (P < .001) (10th quarter adjusted difference, -1.8% [95% CI, -2.6% to -1.0%], P < .001), and a significant relative increase in central venous catheter use (P = .02) (10th quarter adjusted difference, 4.8% [95% CI, 2.3% to 7.4%], P < .001).
CONCLUSIONS AND RELEVANCE: In New York State, mandated protocolized sepsis care was associated with a greater decrease in sepsis mortality compared with sepsis mortality in control states that did not implement sepsis regulations. Because baseline mortality rates differed between New York and comparison states, it is uncertain whether these findings are generalizable to other states.
Source: JAMA. 2019 Jul 16;322(3):240-250.
GRANT OF THE MONTH
Multi-PIs: Hang Lin and Michael Gold
Co-I: Douglas Weber
Title: Joint Pain on a Chip: Mechanistic Analysis Therapeutic Targets and an Empirical Strategy for Personalized Pain Management
Description: Life’s wear and tear can leave joints damaged. All too often, the result is the pain and disability of osteoarthritis (OA). Because pain is the most debilitating symptom of OA, it remains the primary target for therapeutic interventions that typically progress from non-steroidal anti-inflammatory drugs (NSAIDs) to weak opioids, and then to stronger opioids. If pain can’t be controlled, total joint replacement surgery remains the only viable long-term treatment option. However, the associated risks and costs will necessarily keep surgery an option of last resort. Thus, there is a critical need for the development of safe and effective methods for the treatment of OA-associated pain. Recently, our team successfully developed an in vitro multi-component joint on a chip (microJoint), in which engineered osteochondral complexes, synovium and adipose tissues were integrated. OA-like pathology has also been successfully modeled in the microJoint. In this new grant application, we propose to introduce sensory innervation into the microJoint. The result will be a more “complete” joint that enables the dynamic interplay between the peripheral nervous system and joint tissues. We hypothesize that a distinct combination of factors released from different cellular/tissue compartments within the joint mediate pain, hypersensitivity, and hyper-innervation of OA. Furthermore, we hypothesize that opioids not only increase the rate of joint degeneration but potentiate the release of pain producing mediators. In aim 1, a neuron-containing microfluidic ally will be developed to innervate the current microJoint (Neu-microJoint). This new bioreactor will be 3D printed and allow for the non-invasive assessment of neural activity via multi electrode arrays embedded in the tissue chamber, and high-speed optical recording. Human sensory neurons or induced pluripotent stem cell (iPSC)-derived sensory neuron progenitors, will be cultured in the new bioreactor chamber. In aim 2, our previsouly establised OA-model will be created in the Neu-microJoint system. We will assess the activation and/or sensitization of nociceptive afferents with electrophysiology, as well as neurite outgrowth. In aim 3, we will mechanically insult the Neu-microJoint and assess the emergence of “pain” in response to prolonged mechanical stress of the joint with the goal of creating a more natural OA-model. In aim 4, we will assess the impact of drugs used clinically for the management of OA on our OA models in the Neu-microJoint as a means of validating this platform for the assessment of therapeutic efficacy and toxicity of novel compounds. We will then use “omic” approaches to identify new biomarkers and therapeutic targets. Results from this unbiased screen may not only reveal an injury specific pain signature, but suggest medications approved for use in people that could be re-purposed for the treatment of OA pain. In aim 5, we will assess the impact of opioids on neural activity in the presence and absence of joint injury triggered with different stimuli, as well as the integrity of all joint elements. Our Neu-microJoint will enable identification of mechanisms responsible for pain associated with joint injury and therefore novel therapeutic targets, screening of therapeutic interventions, and the implementation of personalized therapeutic strategies.
Source: National Institute on Aging
Term: 2019-2021
Amount: $1.5 million for 2 years
