April 2020 | VOL. 19, NO. 4| www.McGowan.pitt.edu
Medical Devices Lab Technology May Help COVID-19 Patients

Under the direction of McGowan Institute for Regenerative Medicine faculty member William Federspiel, PhD, the William Kepler Whiteford Professor in the Department of Bioengineering at the University of Pittsburgh, the Institute’s Medical Devices Laboratory is developing clinically significant devices for the treatment of pulmonary and other cardiovascular ailments by utilizing the engineering principles of fluid flow and mass transfer.
The Lab’s primary success story is the respiratory dialysis device—now known as the Hemolung Respiratory Assist System (RAS)—developed from technologies in the Lab and licensed to the commercial spin-out, ALung Technologies, Inc. Respiratory dialysis refers to extracorporeal carbon dioxide removal (ECCO2R). This technology is a great example of an academic/industrial partnership. The collaboration has expedited the translation of engineering principles developed in the Medical Devices Lab into a highly successful clinical tool.
ECCO2R provides an alternative to invasive mechanical ventilation, which involves patient sedation and use of a breathing machine, by removing carbon dioxide directly from the blood using techniques similar to kidney dialysis. The procedure can be performed as part of comprehensive treatment for acute lung failure in the intensive care unit.
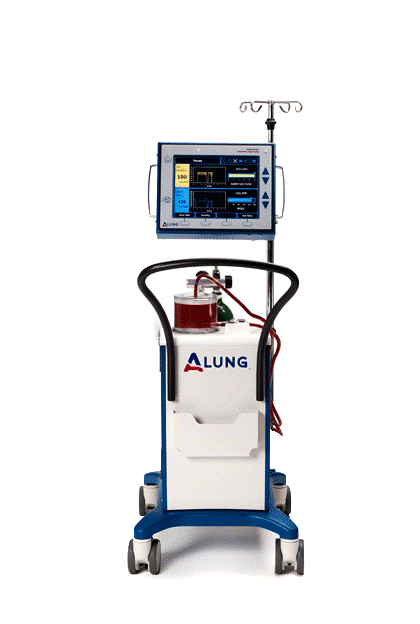 The global incidence of acute respiratory failure exceeds 1 million cases per year. Many of these patients end up in intensive care due to viral infections, like the current coronavirus, COVID-19. The Hemolung device is beginning to be used to treat some COVID 19 patients and there is continued interest by clinicians in using it for some of their patients. ALung ubmitted a petition to the FDA requesting an “emergency use authorization” that would make it available to COVID-19 patients. The FDA recently approved the request. How the Hemolung RAS can help COVID-19 patients is explained by Dr. Federspiel and ALung’s CEO Peter DeComo in this recent interview by ABC’s WTAE news. Watch here.
The global incidence of acute respiratory failure exceeds 1 million cases per year. Many of these patients end up in intensive care due to viral infections, like the current coronavirus, COVID-19. The Hemolung device is beginning to be used to treat some COVID 19 patients and there is continued interest by clinicians in using it for some of their patients. ALung ubmitted a petition to the FDA requesting an “emergency use authorization” that would make it available to COVID-19 patients. The FDA recently approved the request. How the Hemolung RAS can help COVID-19 patients is explained by Dr. Federspiel and ALung’s CEO Peter DeComo in this recent interview by ABC’s WTAE news. Watch here.
Chronic obstructive pulmonary disease (COPD) affects 30 million Americans and is the third leading cause of death in the United States behind cancer and heart disease. Acute exacerbations, defined as a sudden worsening of COPD symptoms, are a major cause of morbidity and mortality in COPD patients. For these patients, mechanical ventilation is used as a life saving measure, but mechanical ventilation has many side effects, and in-hospital mortality remains around 30%. ECCO2R therapy with ALung’s Hemolung (RAS) allows carbon dioxide to be removed from the blood independently of the lungs in order to avoid invasive mechanical ventilation.
 The Hemolung RAS is approved for clinical use in Europe and is currently undergoing a one-of-a-kind clinical trial in the US (Vent-Avoid) to gain FDA approval. The U.S. FDA’s approval of its Investigational Device Exemption (IDE) makes ALung’s trial the first pivotal trial of ECCO2R for treating patients with COPD exacerbations. Forty hospitals will enroll up to 800 patients in the trial, which was launched in 2017.
The Hemolung RAS is approved for clinical use in Europe and is currently undergoing a one-of-a-kind clinical trial in the US (Vent-Avoid) to gain FDA approval. The U.S. FDA’s approval of its Investigational Device Exemption (IDE) makes ALung’s trial the first pivotal trial of ECCO2R for treating patients with COPD exacerbations. Forty hospitals will enroll up to 800 patients in the trial, which was launched in 2017.
Illustrations: McGowan Institute for Regenerative Medicine (Federspiel)/ALung Technologies, Inc. (Hemolung RAS).
View a Hemolung application in the fight against Covid-19.
RESOURCES AT THE MCGOWAN INSTITUTE
May Histology Special
Mast cells play a key role in the inflammatory process.
A mast cell is a myeloid derived cell and is part of the immune system. Mast cells contain distinctive granules rich in histamine and heparin. Although best known for their role in allergy and anaphylaxis, mast cells play an important protective role as well, being intimately involved in angiogenesis, defense against pathogens, and wound healing.
Toluidine blue is one of the most common stains for acid mucopolysaccharides and glycoaminoglycans, both of which are components of mast cells granules.
You will receive 25% off Toluidine blue Staining for the entire month of May when you mention this ad.
Contact Julia at the McGowan Core Histology Lab and ask about our staining specials. Email Hartj5@upmc.edu or call 412-624-5265. As always, you will receive the highest quality histology work with the quickest turn-around time.
Save the Date!
9th International Symposium on Regenerative Rehabilitation
 With a theme of “Where Applied Biophysics Meets Tissue Engineering & Cellular Therapies”, the Symposium is scheduled for December 3-5, 2020 at the University of Texas at Austin. The program features renowned researchers and clinicians from around the world, focusing on the emerging field of Regenerative Rehabilitation. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
With a theme of “Where Applied Biophysics Meets Tissue Engineering & Cellular Therapies”, the Symposium is scheduled for December 3-5, 2020 at the University of Texas at Austin. The program features renowned researchers and clinicians from around the world, focusing on the emerging field of Regenerative Rehabilitation. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
SCIENTIFIC ADVANCES
Mimicking Cancer to Avoid Transplant Rejection

Inspired by a tactic cancer cells use to evade the immune system, University of Pittsburgh researchers have engineered tiny particles that can trick the body into accepting transplanted tissue as its own. McGowan Institute for Regenerative Medicine affiliated faculty members involved in the research team include:
- Steven Little, PhD, Chairman of the Department of Chemical and Petroleum Engineering and is the William Kepler Whiteford Endowed Professor in the Departments of Chemical and Petroleum Engineering, Bioengineering, Immunology, and Ophthalmology
- Hēth Turnquist, PhD, Associate Professor in the University of Pittsburgh School of Medicine, Department of Surgery, T.E. Starzl Transplantation Institute, with a secondary appointment in the Department of Immunology
- Mario Solari, MD, Assistant Professor in the Department of Plastic Surgery with a secondary appointment in the Department of Otolaryngology, both within University of Pittsburgh’s School of Medicine
- Vijay Gorantla, MD, PhD, Associate Professor of Surgery, Ophthalmology and Bioengineering at the Wake Forest School of Medicine
Rats that were treated with the cell-sized microparticles developed permanent immune tolerance to grafts — including a whole limb — from a donor rat, while keeping the rest of their immune system intact, according to a paper published in Science Advances.
“It’s like hacking into the immune system borrowing a strategy used by one of humanity’s worst enemies to trick the body into accepting a transplant,” said senior author Steven Little, PhD, William Kepler Whiteford Endowed Professor and Chair of chemical and petroleum engineering in the Swanson School of Engineering at Pitt. “And we do it synthetically.”
The advantage of a synthetic approach rather than cell-based therapy, which is currently in clinical trials, is that the treatment logistics are much simpler.
“Instead of isolating cells from a patient, growing them up in the lab, injecting them back in and hoping they find the right location, we’re packaging it all up in an engineered system that recruits these naturally occurring cells right to the transplanted graft,” said lead author James Fisher, MD, PhD, a postdoctoral researcher in the Pitt School of Medicine.
The microparticles work by releasing a native protein secreted by tumors, CCL22, which draws regulatory T cells (Treg cells) to the site of the graft, where they tag the foreign tissue as “self” so that it evades immune attack.
Microparticle-treated animals maintained healthy grafts for as long as they were monitored — a little under a year, equivalent to about 30 human years. All it took was two shots to effect seemingly permanent change.
In a companion paper published recently in PNAS, the researchers showed that these engineered microparticles can train the immune system of one strain of rat to accept a donor limb from a different strain. This new paper shows that the effects are specific to the intended donor. Skin grafts from a third strain were rapidly rejected.
Today, transplant patients take daily doses of immunosuppressant drugs to avoid rejection, leaving them vulnerable to cancer, diabetes, infectious diseases and a host of other ailments that come along with a weakened immune system.
“These drugs hammer the immune system into submission so it can’t attack the transplanted organ, but then it can’t protect the body either,” said coauthor Stephen Balmert, PhD, a postdoctoral researcher in the Pitt School of Medicine. “We’re trying to teach the immune system to tolerate the limb, so that a transplant recipient can remain immunocompetent.”
The risks of lifelong immunosuppression are particularly problematic when the transplant isn’t a life-saving procedure. Doctors and patients have to consider whether the benefits outweigh the risks.
“The ability to induce transplant tolerance while avoiding systemic immunosuppression, as demonstrated in these innovative studies, is especially important in the context of vascularized composite transplantation where patients receive quality-of-life transplants, such as those of hands or face,” said coauthor Angus Thomson, PhD, professor of surgery and immunology in the Thomas E. Starzl Transplantation Institute at Pitt.
Illustration: White Rat, Black Leg: This white rat received a donor leg from a black rat – a complete mismatch – and yet his body has been trained to accept the new limb as if it came from his twin. Best of all, his immune system remains intact. UPMC.
Dr. Jelena Janjic Helps Lead Nanomedicine Effort in Transplantation

McGowan Institute for Regenerative Medicine affiliated faculty member and Duquesne University Associate Professor of Pharmaceutics Jelena Janjic, PhD, is working with a multidisciplinary team of researchers to explore the use of nanomedicine in organ transplantation and regenerative surgery.
Dr. Janjic, who created the first inflammatory pain nanomedicine that directly targets the pain source, is working with researchers from Wake Forest University and inStem (the Institute for Stem Cell Science and Regenerative Medicine) to investigate how nanomedicine can improve patient outcomes for individuals whose devastating injuries are treated with vascularized composite allotransplantation (VCA).
“Nanomedicine has begun to revolutionize cancer treatment and holds tremendous potential in regenerative medicine and transplantation,” said Dr. Janjic, founder and co-director of Duquesne University’s Chronic Pain Research Consortium. “Unlike other treatments, nanomedicine offers an approach that personalizes treatment while reducing adverse effects that often arise with current transplantation supportive treatments.”
Thanks to a $1.5 million grant from the U.S. Department of Defense (DoD), Dr. Janjic is working with McGowan Institute affiliated faculty member Vijay Gorantla, MD, PhD, a leading VCA expert from the Wake Forest School of Medicine, and Praveen Kumar Vemula, PhD, from inStem, which is part of India’s Department of Biotechnology, to develop novel diagnostic and treatment procedures for VCA patients.
A major challenge with transplantation is tissue rejection, which is complicated by the inability to deliver current anti-rejection drugs directly to the affected organ. The researchers use nanomedicine to target anti-rejection therapies predominantly to the transplanted tissue, which, if effective, could also reduce the medications’ side effects.
A second part of the team’s research identifies potential problems to transplantation by using nanoimaging to see the response of a patient’s immune system to transplantation.
“Nanomedicine can deliver low-dose medication to cells or graft tissues, while nanoimaging allows us to monitor donor and recipient cells in the patient’s immune system,” Dr. Janjic said. “This would allow us to personalize therapies while reducing the adverse effects seen with current treatments.”
Drs. Janjic and Gorantla have been partnering for the past few years to develop new therapies in pain and regenerative medicine. At the time they received the DoD grant, they were conducting collaborative research with the U.S. Air Force’s (USAF) largest medical research wing, the 59th Medical Wing (MDW). The unit works to identify and develop transformative therapies and products that can help the military both in the field and hospital settings.
Dr. Janjic is an Oak Ridge Institute for Science and Education faculty fellow/principal scientist, and Dr. Gorantla serves as the chief scientific consultant in reconstructive transplantation for the 59th MDW. Dr. Janjic is the first Duquesne faculty fellow with the 59th MDW and has initiated multiple collaborative projects with USAF.
The DoD grant is titled “A Novel Graft Implanted Macrophage-Enzyme Responsive Immunosuppressive Therapy to Prevent Chronic Rejection in Vascularized Composite Allotransplantation.” The award number is W81XWH-19-1-0828. Drs. Gorantla, Janjic and Vemula share the partner grant, and Dr. Gorantla serves as the initiating principal investigator.
Exercise Restores Youthful Properties to Muscle Stem Cells of Old Mice

According to a new study by researchers at the Stanford School of Medicine, a nightly jaunt on the exercise wheel enhances muscle-repair capabilities in old mice. Only older mice saw this benefit, which the researchers found is due to the rejuvenation of the animals’ muscle stem cells.
“The effect in old animals is very significant,” said McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Rando, MD, PhD, professor of neurology and neurological sciences and director of Stanford’s Glenn Center for the Biology of Aging. “We found that regular exercise restores youthfulness to tissue repair. Their muscle stem cells start to look and behave like those of much younger animals.”
The researchers also identified a molecular pathway involved in turning back the clock on the cells. Drugs that manipulate the pathway might be an effective substitute for exercise, they suggest.
Dr. Rando is the senior author of the study, which was published in Nature Metabolism. Medical student Jamie Brett, PhD, postdoctoral scholar Marina Arjona, PhD, and visiting scholar Mika Ikeda, PhD, are the lead authors.
Unlike embryonic or induced pluripotent stem cells, which can give rise to any tissue in the body, tissue-specific stem cells are restricted in their potential. Muscle stem cells wait in the wings along the muscle fibers in a resting state known as quiescence until called upon to repair damage.
“Studies conducted by us and others have shown that tissue regeneration decreases with age, and that this is due to declining function in adult stem cells,” Dr. Rando said. “Many researchers are looking for a way to restore youthfulness.”
Benefits of lifestyle adjustments
While no researchers have discovered a reliable fountain of youth, it’s well known that certain lifestyle adjustments can be beneficial.
“Exercise is known to reduce the risk of a wide variety of age-related problems, including cardiovascular disease, cancer and perhaps even Alzheimer’s disease,” Dr. Rando said. “There’s a lot of interest in understanding how exercise confers these health benefits.”
In particular, the researchers wanted to know whether and how voluntary exercise affects the function of muscle stem cells in mice. They gave mice that were about 20 months old, the equivalent of being 60-70 years old in humans, and mice that were 3 to 4 months old, the equivalent of 20- to 30-year-old humans, access to an exercise wheel and allowed them to run at will. Young mice averaged about 10 kilometers each night, and the older mice covered about 5 kilometers. Two other groups of young and old mice were given wheels that didn’t rotate to serve as controls.
“The animals were exercising at the intensity levels at which they were comfortable,” Dr. Rando said, “much like what people do for their own health. This is a less stressful situation than resistance training or intense endurance exercise, which may themselves affect muscle stem cell function.” Subsequent analysis showed that the muscle stem cells of the exercising animals remained quiescent, and that the animals did not develop significant numbers of new muscle fibers in response to the exercise.
After three weeks of nightly aerobics for the active groups, the researchers compared the ability of the animals to repair muscle damage. They found that, as expected, the aged, sedentary mice were significantly less able to repair muscle damage than younger sedentary mice. However, the older animals that had exercised regularly were significantly better at repairing muscle damage than were their counterparts that did not exercise. This exercise benefit was not observed in the younger animals.
Similar results were obtained when muscle stem cells from older mice that had exercised were transplanted into younger mice. The stem cells from the exercising animals contributed more to the repair process than did those from their sedentary peers.
Benefit of young blood
The researchers also showed that injecting blood from an old mouse that had exercised into an old mouse that hadn’t conferred a similar benefit in stem cell function, suggesting that exercise simulates the production of some factors that then circulate in the blood and enhance the function of older stem cells.
“That’s really fascinating,” Dr. Rando said, noting that the result mirrors those from earlier studies jointly conducted by him and Tony Wyss-Coray, PhD, a professor of neurology and neurological sciences at the School of Medicine, indicating that blood from a young mouse appears to somehow enhance the tissue-specific stem cells in an older animal.
Further studies indicated that the exercise-induced rejuvenation observed by the researchers could be mimicked by increasing the expression of a signaling molecule called cyclin D1, which is involved in rousing resting muscle stem cells in response to damage. The discovery suggests that it may one day be possible to artificially activate this pathway to keep aging muscle stem cells functioning at their youthful best.
“If we could develop a drug that mimics this effect, we may be able to experience the benefit without having to do months of exercise,” Dr. Rando said.
Illustration: Stanford University.
Dr. Bryan Brown Featured in the Products of Pittsburgh Podcast

Soundcloud’s Products of Pittsburgh podcast is about the people in Pittsburgh – innovators, scientists, community leaders – and the remarkable stories behind how they came to be and the work they have produced.
Recently, McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, Associate Professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh, spoke to Mike Flock, PhD, of Products of Pittsburgh. Dr. Flock is the Innovation Core Manager at Pitt.
Their conversation provides insight into Dr. Brown’s early life, his education path, his new spinoff company, Renerva, and his latest effort with CyteSolutions Lens. In the end, in one word, Dr. Brown feels he’s been very lucky with all the opportunities afforded him in Pittsburgh.
Listen to the podcast here.
Dr. Brown graduated from the University of Pittsburgh with a BS in Mechanical Engineering in 2005 and a PhD in Bioengineering in 2010. He then completed postdoctoral training in the Departments of Biomedical Engineering and Clinical Sciences at Cornell University prior to joining the University of Pittsburgh as a faculty member in 2011. Dr. Brown has also served as a visiting researcher at Tsinghua University in Beijing China (2005-2006), a NSF East Asia and Pacific Summer Institutes Fellow at Tokyo Women’s Medical University Institute for Advanced Biomedical Sciences (2008), and most recently as a Building Interdisciplinary Research Careers in Women’s Health Scholar (NIH K12) at Magee Women’s Research Institute.
The Brown Laboratory seeks to couple a mechanistic understanding of the host inflammatory response in injury and disease with the development of context-dependent biomaterials for regenerative medicine strategies. The focus of the Brown Laboratory is upon clinical applications where few effective solutions currently exist, with increasing emphasis upon unmet clinical needs in women’s health. Since 2011, the Brown Laboratory has received research funding for these efforts from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Aging, National Institute of General Medical Science, Office of Research on Women’s Health, the Wallace H. Coulter Foundation, and multiple industry partners.
Smoking Relapse Common in Weight-Loss Surgery Patients

Although 1 in 7 adults smoke cigarettes the year prior to undergoing weight-loss surgery, nearly all successfully quit at least a month before their operation. However, smoking prevalence steadily climbs to pre-surgery levels within seven years, according to new research led by the University of Pittsburgh Graduate School of Public Health. McGowan Institute for Regenerative Medicine affiliated faculty member Steven Belle, PhD, MScHyg, Professor in the Department of Epidemiology and a Co-Director in the Epidemiology Data Center, Graduate School of Public Health at the University of Pittsburgh, is a co-author on this work.
The findings — reported in the Annals of Surgery — suggest that there may be missed opportunities to engage patients in interventions to improve long-term smoking cessation rates, particularly at regular post-surgery checkups.
“Smoking cessation prior to surgery is strongly recommended to reduce surgical complications,” said lead author Wendy King, PhD, associate professor of epidemiology at Pitt Public Health. “But there isn’t the same emphasis on maintaining cessation after surgery. Our findings show that there is a need for ongoing support in order to reduce and quickly respond to relapses.”
Dr. King and her colleagues followed 1,770 adults who underwent Roux-en-Y gastric bypass surgery — a procedure that reduces the size of the stomach and bypasses part of the small intestine — for seven years post-surgery, annually surveying them about their smoking habits. The participants were enrolled in the National Institutes of Health-funded Longitudinal Assessment of Bariatric Surgery-2 (LABS-2), a prospective, observational study of patients undergoing weight-loss surgery at one of 10 hospitals across the United States.
More than 45% of the participants reported a history of smoking prior to surgery, with 14% still smoking in the year before surgery, which fell to 2% in the month before surgery. The rate rebounded to nearly 10% in the year following surgery and steadily climbed back to 14% by seven years post-surgery.
“Interestingly, the people who picked up smoking post-surgery weren’t just the people who quit smoking in the year prior to surgery, presumably to prepare for the operation. Many had never smoked to begin with,” said co-author Gretchen White, PhD, assistant professor in Pitt’s School of Medicine, explaining that 2 out of 5 people who smoked after surgery had quit more than a year before their operation or hadn’t ever smoked.
Additionally, people who identified as smokers post-surgery smoked more, going from an average of a dozen cigarettes per day in the year before surgery to more than 15 cigarettes per day seven years post-surgery. These findings contrast with concurrent reductions in smoking prevalence and intensity in the general U.S. population.
The researchers hypothesized that weight control would be a key reason weight-loss patients took up smoking after surgery, but found that the prevalence of smoking for weight control was actually fairly stable over time, at about 2% pre- and post-surgery, and did not appear to be related to smoking more cigarettes. Dr. King noted that “this surprised everyone, as there is a general assumption that weight control is a main motivator for smoking.”
While the study was not designed to find a biological reason for the results, the researchers did observe that gastric bypass patients were more likely to smoke post-surgery than patients who underwent gastric banding, where a silicone belt is surgically inserted around the stomach to reduce the amount of food it can hold. A recent study showed that gastric bypass increases exposure to the psychoactive nicotine metabolite cotinine. Just as gastric bypass increases the risk of alcohol use disorder due to changes in alcohol metabolism that lead to higher and quicker elevation of blood alcohol levels, it may also increase risk of smoking via nicotine metabolism, Dr. King suggested.
The scientists identified several factors that predict which patients would be most likely to take up smoking after surgery. Not surprisingly, a prior history of smoking was the greatest risk factor. In addition, younger age, poverty, being married or living as married, and drug use were each associated with increased risk.
COVID-19 Vaccine Candidate Shows Promise

University of Pittsburgh School of Medicine scientists announced a potential vaccine against SARS-CoV-2, the new coronavirus causing the COVID-19 pandemic. When tested in mice, the vaccine, delivered through a fingertip-sized patch, produces antibodies specific to SARS-CoV-2 at quantities thought to be sufficient for neutralizing the virus.
The paper appeared in EBioMedicine, which is published by The Lancet, and is the first study to be published after critique from fellow scientists at outside institutions that describes a candidate vaccine for COVID-19. The researchers were able to act quickly because they had already laid the groundwork during earlier coronavirus epidemics.
“We had previous experience on SARS-CoV in 2003 and MERS-CoV in 2014. These two viruses, which are closely related to SARS-CoV-2, teach us that a particular protein, called a spike protein, is important for inducing immunity against the virus. We knew exactly where to fight this new virus,” said co-senior author Andrea Gambotto, MD, associate professor of surgery at the Pitt School of Medicine. “That’s why it’s important to fund vaccine research. You never know where the next pandemic will come from.”
“Our ability to rapidly develop this vaccine was a result of scientists with expertise in diverse areas of research working together with a common goal,” said co-senior author Louis Falo, MD, PhD, professor and chair of dermatology at Pitt’s School of Medicine and UPMC and a McGowan Institute for Regenerative Medicine affiliated faculty member.
Compared to the experimental mRNA vaccine candidate that just entered clinical trials, the vaccine described in this paper — which the authors are calling PittCoVacc, short for Pittsburgh Coronavirus Vaccine — follows a more established approach, using lab-made pieces of viral protein to build immunity. It’s the same way the current flu shots work.
The researchers also used a novel approach to deliver the drug, called a microneedle array, developed in the Falo Lab, to increase potency. This array is a fingertip-sized patch of 400 tiny needles that delivers the spike protein pieces into the skin, where the immune reaction is strongest. The patch goes on like a Band-Aid and then the needles — which are made entirely of sugar and the protein pieces — simply dissolve into the skin.
“We developed this to build on the original scratch method used to deliver the smallpox vaccine to the skin, but as a high-tech version that is more efficient and reproducible patient to patient,” Dr. Falo said. “And it’s actually pretty painless — it feels kind of like Velcro.”
The system also is highly scalable. The protein pieces are manufactured by a “cell factory” — layers upon layers of cultured cells engineered to express the SARS-CoV-2 spike protein — that can be stacked further to multiply yield. Purifying the protein also can be done at industrial scale. Mass-producing the microneedle array involves spinning down the protein-sugar mixture into a mold using a centrifuge. Once manufactured, the vaccine can sit at room temperature until it’s needed, eliminating the need for refrigeration during transport or storage.
“For most vaccines, you don’t need to address scalability to begin with,” Dr. Gambotto said. “But when you try to develop a vaccine quickly against a pandemic that’s the first requirement.”
When tested in mice, PittCoVacc generated a surge of antibodies against SARS-CoV-2 within two weeks of the microneedle prick.
Those animals haven’t been tracked long term yet, but the researchers point out that mice who got their MERS-CoV vaccine produced a sufficient level of antibodies to neutralize the virus for at least a year, and so far the antibody levels of the SARS-CoV-2 vaccinated animals seem to be following the same trend.
Importantly, the SARS-CoV-2 microneedle vaccine maintains its potency even after being thoroughly sterilized with gamma radiation — a key step toward making a product that’s suitable for use in humans.
The authors are now in the process of applying for an investigational new drug approval from the U.S. Food and Drug Administration in anticipation of starting a phase I human clinical trial in the next few months.
“Testing in patients would typically require at least a year and probably longer,” Dr. Falo said. “This particular situation is different from anything we’ve ever seen, so we don’t know how long the clinical development process will take. Recently announced revisions to the normal processes suggest we may be able to advance this faster.”
Illustration: Microneedle Array Vaccine: The vaccine is delivered into the skin through a fingertip-sized patch of microscopic needles. UPMC.
UPMC-Led Global Effort Fast Tracks Testing of COVID-19 Therapies

Launched recently at UPMC is a novel clinical trial developed by researchers at the University of Pittsburgh School of Medicine to address one of the most important debates during the COVID-19 pandemic: How should doctors decide between quickly adopting new therapies, such as the anti-malarial drug hydroxychloroquine, and waiting until they are tested in longer clinical trials?
“The solution is to find an optimal tradeoff between doing something now, such as prescribing a drug off-label, or waiting until traditional clinical trials are complete,” said Derek Angus, MD, MPH, professor and chair, Department of Critical Care Medicine at Pitt and UPMC, and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “We’ve developed a way to do that with an adaptive clinical trial model that relies on a type of artificial intelligence known as reinforcement learning to identify the best, evidence-backed therapy for COVID-19 much faster than using the traditional scientific approach.”
Before COVID-19 emerged, Dr. Angus and a wide range of international collaborators had developed a platform, called REMAP-Community Acquired Pneumonia (REMAP-CAP), designed to find optimal treatments for severe pneumonia both in non-pandemic and pandemic settings. When COVID-19 began circulating, REMAP-CAP was rapidly adapted, as per its intent, to incorporate additional treatment regimens specifically targeting the SARS-CoV-2 virus. The international team describes the REMAP-CAP platform in a manuscript published in the Annals of the American Thoracic Society (AnnalsATS).
REMAP (randomized, embedded, multi-factorial, adaptive platform) allows researchers to rapidly test multiple treatment approaches simultaneously at a lower cost and with fewer patients than traditional clinical trials. The REMAP design, first described in a paper by Dr. Angus in 2015 in the Journal of the American Medical Association (JAMA), is a flexible version of what are called “adaptive platform trials.” “Adaptive platform trials are rapidly being endorsed by the U.S. Food and Drug Administration, the Bill & Melinda Gates Foundation and others as a long-needed revolution in clinical trials,” said Dr. Angus, who holds the Mitchell P. Fink Endowed Chair at Pitt.
He compares the REMAP approach to a chef offering a fixed price menu with appetizer, main course and dessert. The chef may try various combinations, serving sizes and options, sometimes leaving out the appetizer or dessert, and adjusting on the fly as plates come back scraped clean or barely touched, until hitting on the combination that sells best.
The UPMC-REMAP-COVID19 trial, built on the backbone of the REMAP-CAP platform, will be particularly powerful because it is being integrated with the electronic health record system at UPMC, noted Dr. Angus. “In a pandemic, doctors will not have the time to debate the pros and cons of every possible clinical trial. By building this one-stop solution at the point-of-care, we are rolling out an approach that can assure that every patient admitted with COVID-19, if they choose to, can be enrolled in the program.”
“We must throw out old ways of thinking and fuse clinical care and clinical research into one extremely efficient system,” said Dr. Angus, who authored a recent viewpoint in JAMA advocating for the “learning while doing” approach. “This is an unprecedented pandemic and we need an unprecedented response.”
UPMC-REMAP-COVID19 will open across UPMC’s 40-hospital system and begin with multiple treatments tested simultaneously in different combinations — including hydroxychloroquine, steroids and medications called immunomodulators that alter the responsiveness of the immune system. If new drugs need to be tested, they are simply rolled into the platform as study amendments, rather than tested in separate free-standing trials. All participants will receive the current standard of care, and most also will receive one, two or three of the experimental treatment options. This means that, at launch, only 12.5% of participants will be strictly assigned to the placebo arm of the trial and, within weeks, researchers expect that about 99% of patients will be receiving one or more active therapies specifically targeting COVID-19.
Furthermore, because the UPMC-REMAP-COVID19 platform is connected to the worldwide REMAP-CAP, the trial learns from the entire international experience. REMAP-CAP is enrolling patients with COVID-19 in North America, Europe, Australia and New Zealand, and expanding rapidly.
“The trial design uses a machine-learning model that incorporates data from patients enrolled across the world to continuously learn which therapies and combinations of therapies are performing best,” explained AnnalsATS co-author Scott Berry, PhD, president and senior statistical scientist of Berry Consultants, who worked with Dr. Angus and his colleagues to build the statistical model. “Last week, the Chief Medical Officer of the United Kingdom’s National Health Service urged every hospital in the country to participate in this trial. As more institutions join, the model learns faster.”
If one of the treatments shows early signs of performing better than the others, patients are automatically enrolled more often into that treatment option. Physicians can be assured that they are always betting on the winning horse in the moment, and poorly performing options are quickly discontinued.
“This allows us to always rapidly identify which treatment works best, while keeping the number of patients needed to achieve statistical significance low,” said Dr. Angus. “It also means we get the best treatment to the most patients right out of the gate.”
The design and implementation of the UPMC-REMAP-COVID19 trial is led by Dr. Angus and colleagues at Berry Consultants (Austin, Texas), and supported by UPMC Enterprises.
The design and implementation of REMAP-CAP worldwide is supported by multiple governments and institutions. A complete list of the collaborating authors, institutions and funding agencies can be found in the study.
The Future of Human Healing May Lay in the Brain of a Starfish

The potential benefits of stem cell therapy have been widely discussed for decades. Potential benefits include reduction of the pain of arthritis and help patients heal faster after surgery. But stem cell therapies can be prohibitively expensive. At the projected costs, these potential life-changing treatments could be far out of range of the vast majority of those who need them. But thanks to Carnegie Mellon University’s Kris Dahl, PhD, professor of chemical engineering, and Veronica Hinman, PhD, head of CMU’s Department of Biological Sciences, stem cell therapy could get a lot less expensive. And the key to this approach lies in the incredible regenerative powers of starfish.
Stem cells are able to become any type of cell the body needs: muscle, skin, blood and more. In humans, when stem cells differentiate into these other cells, they are unable to change back, or de-differentiate. But this is not the case with starfish. Notably, if a starfish loses one of its arms, it can grow it back just the same. This is due to the starfish’s unique cells, which can de-differentiate themselves from skin or muscle cells back into stem cells. While this regenerative capability in itself is incredible, study co-author Dr. Hinman’s preliminary research has shown an even more incredible ability.
“In the larval state, starfish have a distinctive head that contains their brain,” says Dr. Dahl, an affiliated member of the McGowan Institute for Regenerative Medicine. “If the head is removed or damaged, the differentiated cells that are definitely not neural cells will de-differentiate, crawl up to the head region, and regrow into neurons. To not only do this in the larval state, but to regrow something as complex as a brain — this is an amazing regenerative capability.”
Supported by funding from the DSF Charitable Foundation, Drs. Hinman and Dahl are working to understand just what in the starfish causes their cells to do this. While Dr. Hinman is focused on the fundamental science, Dr. Dahl’s lab is delving into the structure of the cells, cell crawling, and the biomechanics of cellular regeneration.
“While regenerative medicine is great, there’s still a lack of understanding of the fundamentals that govern how cells respecify themselves,” says Dr. Dahl. “The hope is that by studying a model organism like the starfish, and combining what we learn with our knowledge of human stem cells, we can use comparative genomics to understand the gene expression that allows starfish cells to respecify their programming.”
To do this, Dr. Dahl is creating an artificial model of the starfish’s larval system to map the cells as they crawl to their new destination. With this artificial model, Dahl and her team can manipulate the chemical and mechanical factors that exist in the starfish embryo, blocking them one at a time until they find exactly what it is that tells the cells to de-differentiate back into stem cells, crawl up to the brain region, and become neuronal tissue. Once this factor has been isolated, the goal is to then be able to apply it to human cells, to tell those cells to de-differentiate so they can become whatever the patient needs.
Current therapies require stem cells to be harvested from a patient, then cultured over the course of days, in order to have enough to be reinjected back into the patient to help speed healing. But with this method, cells could be taken from any part of the body, de-differentiated back into stem cells, then re-differentiated into therapeutic cells. This could make the process of preparing stem cell therapy faster, easier, and most importantly, cheaper.
“If you could reduce the costs of stem cell therapies—it would touch nearly every person’s life,” says Dr. Dahl. “Surgeons could include a stem cell injection with every major or minor surgery, helping patients heal 100 times faster. It’s quick healing; it’s reduced scarring. This could be like penicillin. I see it becoming the standard of care in the next 10 years.”
Uncovering Stimulation’s Impact on Neurons
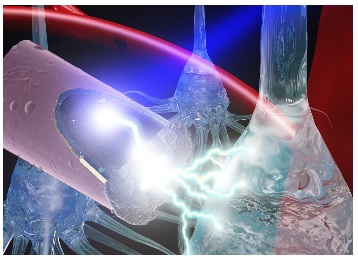 Using electrodes smaller than a human hair, researchers are able to connect mind to machine and interact with the human brain in revolutionary ways. Brain-computer interfaces have helped rehabilitate neurodegenerative diseases and restore function to individuals with brain damage. This cutting-edge technology, however, comes with complications.
Using electrodes smaller than a human hair, researchers are able to connect mind to machine and interact with the human brain in revolutionary ways. Brain-computer interfaces have helped rehabilitate neurodegenerative diseases and restore function to individuals with brain damage. This cutting-edge technology, however, comes with complications.
McGowan Institute for Regenerative Medicine affiliated faculty member Takashi Kozai, PhD, assistant professor of bioengineering at the University of Pittsburgh, received a $437,144 CAREER award from the National Science Foundation to improve the integration of the brain and technology in order to study long-standing questions in neurobiology and improve clinical applications of these devices.
One of the challenges remaining with this technology is achieving long-term and precise stimulation of a specific group of neurons. Dr. Kozai has designed a wireless, light-activated electrode that enables precise neural circuit probing while minimizing tissue damage. In this project, he will further improve this technology.
“Our first objective is to design a coating technology that will be applied to the wireless axon and release biomolecules during simulation,” said Dr. Kozai, who leads the Bio-Integrating Optoelectric Neural Interface Cybernetics Lab in the Swanson School of Engineering. “These specific biomolecules can control the activity of a small population of neurons, and the device will recharge by drawing upon intrinsically produced biomolecules.”
Developing this coating will help Dr. Kozai achieve the main research goal of this CAREER project, which is to establish the relationship between different types of stimulation and their impact on excitability of neuronal populations.
“In order for the brain to properly function, there needs to be a balance between excitatory and inhibitory neuronal activity,” explained Dr. Kozai, “but we don’t know how stimulation impacts this balance.”
According to Dr. Kozai, an imbalance between excitatory and inhibitory neuronal activity can lead to cognitive dysfunctions and is a hallmark of autism spectrum disorder. Moreover, brain injuries such as traumatic brain injuries, stroke, and microelectrode implantation have also been shown to disrupt this balance.
“We believe that different types of stimulation will differentially alter excitatory and inhibitory neuronal activity, which will in turn alter the long-term excitability of nearby neurons in different capacities,” said Dr. Kozai. “To better understand the relationship between stimulation and neuronal activity, we will use optical and optogenetic methods to determine the excitability of neurons, which will give us a better physiological understanding of the activated brain region.”
The research team will use in vivo two-photon microscopy and genetically encoded fluorescent indicators to investigate this relationship. They will collect images across 12 weeks and examine the number, distance, timing and neuronal subtype densities before, during and after electrical stimulation. This method will allow them to track stimulation-induced changes over time with high spatial resolution near the electrodes.
Dr. Kozai expects that this work will impact the future design of neural interfaces and give researchers an improved tool to answer neurobiological questions. A better understanding of how stimulation affects long-term neural excitability will hopefully advance BCI technology and impact the rehabilitation of neurodegenerative disease and brain damage.
As part of this CAREER award’s educational goal, Dr. Kozai will target underrepresented minority students with an outreach program designed to demonstrate how science and engineering converge at the neural interface. In an effort to better disseminate neurobiology and neural engineering resources, he will provide an early platform for lecture videos, protocols and training materials. Dr. Kozai will also develop a virtual “Education in Biological and Neuroelectronic Interface Community” (eBioNIC.org).
Illustration: A laser shining onto an untethered, ultrasmall carbon fiber electrode to stimulate neurons via the photoelectric effect. Photo credit: J. Mater. Chem. B, 2015,3, 4965-4978 – Reproduced by permission of The Royal Society of Chemistry.
Developing a Valve for Developing Hearts

Approximately one in every 125 babies in the U.S. is born with a congenital heart defect (CHD), making it the country’s most common birth defect. Heart valves developed for adults have been used on infants to treat CHDs, but the large devices sometimes require open heart surgery, presenting a severe risk to infants and young children. Additionally, infants and children grow quickly, but the artificial valve does not, resulting in repeated surgeries that increase risks.
To address this issue, McGowan Institute for Regenerative Medicine affiliated faculty member Youngjae Chun, PhD, an associate professor of industrial engineering and bioengineering at the University of Pittsburgh, is developing a new type of metallic frame for pediatric heart valves that could not only be placed by a minimally invasive catheter-based procedure but would also grow with the child, eliminating the need for follow-up surgeries. The project recently received an award of $120,000 from the Children’s Heart Foundation’s Liam Ward Fund.
“Using a heart valve developed for an adult on an infant or young child is considered an emerging technology, but they’re bulky and typically require open heart surgery. Often, these patients are already too weak or ill to undergo such major surgery,” explains Dr. Chun. “Our goal is to develop a novel metallic valve frame that would eliminate the need for multiple heart surgeries and their associated hospital stays, and one that would actually grow with the patient.”
The proposed new valve will use two types of novel metallic biomaterials: superelastic nitinol and biodegradeable metals like magnesium and iron. Nitinol, an alloy of nickel and titanium, is known for its ability to flex and return to its original shape. This flexibility allows the valve to be compressed and placed by a small catheter inserted into a vein, rather than through open heart surgery, presenting much less risk to the patient. Magnesium and iron, on the other hand, would degrade over time, giving the valve the ability to change and expand with the surrounding heart tissue as the patient grows.
“No one wants to see their child go through multiple surgeries before they’re even able to walk, but that’s the reality for thousands of families every year,” says Dr. Chun. “With improved devices for these young patients, we can give them a better quality of life and give their parents greater peace of mind.”
If the project proves to be successful, Dr. Chun will collaborate with William Wagner, PhD, director of Pitt’s McGowan Institute for Regenerative Medicine, and Antonio D’Amore, PhD, research assistant professor in the departments of Surgery and Bioengineering, to develop it further.
Illustration: University of Pittsburgh Swanson School of Engineering.
Push for Artificial Intelligence Innovation in Medical Imaging Project Wins University of Pittsburgh Scaling Grant

Led by Shandong Wu, PhD, associate professor in the Department of Radiology at Pitt, and supported by McGowan Institute for Regenerative Medicine David Vorp, PhD, associate dean for research and John A. Swanson Professor of Bioengineering, the “Pittsburgh Center for Artificial Intelligence Innovation in Medical Imaging” project won a University of Pittsburgh Scaling Grant. The Scaling Grant provide $400,000 over two years to support detailed project planning, gathering proof-of-concept results, and reduction of technical risk for teams pursuing an identified large extramural funding opportunity. The Scaling Grants are part of the University’s Pitt Momentum Funds, which offer funding across multiple stages of large, ambitious projects.
The project is a collaboration between Pitt’s Departments of Radiology, Bioengineering, Biomedical Informatics, and Computer Science. This work, led by Dr. Wu, aims to use artificial intelligence (AI) to reshape medical imaging in radiology and pathology.
Through the Pittsburgh Health and Data Alliance, the region is already at work using machine learning to translate “big data” generated in health care to treatments and services that could benefit human health.
“The advancement in AI, especially in deep learning, provides a powerful approach for machine learning on big healthcare data,” said Dr. Wu. “Deep learning enables large-scale data mining with substantially increased accuracy and efficiency in data analysis.”
The multidisciplinary research team will work to develop AI imaging methodology and translational applications with the ultimate goal of creating tools that are clinically useful, accurate, explainable and safe.
“AI can substantially improve quantitative analysis to medical imaging data and computational modeling of clinical tasks using medical images for disease diagnosis and outcome prediction,” explained Dr. Wu.
Dr. Vorp will help facilitate this collaboration in engineering.
“Artificial intelligence nicely complements bioengineering and medical research,” said Dr. Vorp. “My lab uses AI with CT scans to help predict the prognosis and improve treatment of aortic aneurysm, and that is just one example of how this cutting-edge technology can be applied to medical images. Rather than relying on the naked eye, we can use AI to analyze these images and have a more sensitive detector to identify disease, improve health and save lives.”
The group’s long-term vision is to combine the computational expertise and clinical resources across Pitt, UPMC and Carnegie Mellon University to build a center for innovative AI in clinical translational medical imaging.
AWARDS AND RECOGNITION
Badylak Lab Student Madeline Cramer Receives NIH F31 Award

University of Pittsburgh graduate student Madeline Cramer received an F31 award from the National Institutes of Health for her regenerative medicine research that may help improve outcomes in cardiac disease. Ms. Cramer studies bioengineering in the Swanson School of Engineering and works in the lab of McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor of surgery at Pitt.
Dr. Badylak’s lab focuses on the use of biologic scaffolds composed of extracellular matrix (ECM) to facilitate functional tissue and organ reconstruction. Present within all tissues and organs, ECM provides essential structural support and also initiates biochemical and biomechanical cues. Ms. Cramer’s project will look at myocardial infarction (MI) and examine how a specific protein embedded within the ECM may affect the underlying mechanisms behind the scaffold’s therapeutic response.
“Following myocardial infarction, cardiomyocyte death initiates an intense inflammatory response which is necessary to clear the debris of the dead cells,” explained Ms. Cramer. “However, a prolonged pro-inflammatory state is associated with immune-driven fibrosis that can progress to heart failure.”
Heart failure is a costly condition that affects millions of adults in the United States. Tissue engineered biologic scaffolds derived from ECM have been shown to promote an anti-inflammatory phenotype in macrophages and reduce fibrosis after MI in pre-clinical and clinical studies, but the underlying mechanisms driving this response are only partially understood.
“Previous work in the Badylak lab showed that ECM is an abundant source of extra-nuclear interleukin-33 (IL-33), a protein that is stored and protected from degradation within matrix-bound nanovesicles (MBV),” said Ms. Cramer. “My research aims to delineate the roles of MBV-associated IL-33 in mediating the pro-remodeling effects of ECM through in vitro and in vivo models of myocardial infarction.”
Demonstrating that IL-33 containing MBV can dampen the fibrotic response following MI may prove to be a significant advancement in the treatment of MI and the prevention of subsequent heart failure.
“Maddie has worked extremely hard and is very deserving of this award,” said Dr. Badylak. “I’m confident that the results of her work will have a significant impact upon the field.”
Illustration: University of Pittsburgh.
AIMBE Announces 2020 College of Fellows Inductees

The American Institute for Medical and Biological Engineering (AIMBE) has announced the induction of these McGowan Institute for Regenerative Medicine affiliated faculty members to its College of Fellows:
- Christopher Bettinger, PhD: For outstanding contributions to integrating biomimetic materials and flexible electronics into biomedical devices and implants.
- Kris Dahl, PhD: For providing foundational structure-function information about the cell nucleus that changes how that organelle is viewed in mechanotransduction.
- Douglas Weber, PhD: For outstanding contributions to neurorehabilitation engineering, translational neuroscience, and leadership in the field of neural engineering.
Election to the AIMBE College of Fellows is among the highest professional distinctions accorded to a medical and biological engineer. The College of Fellows is comprised of the top two percent of medical and biological engineers. College membership honors those who have made outstanding contributions to “engineering and medicine research, practice, or education” and to “the pioneering of new and developing fields of technology, making major advancements in traditional fields of medical and biological engineering, or developing/implementing innovative approaches to bioengineering education.”
While most AIMBE Fellows hail from the United States, the College of Fellows has inducted Fellows representing 34 countries. AIMBE Fellows are employed in academia, industry, clinical practice and government.
AIMBE Fellows are among the most distinguished medical and biological engineers including 3 Nobel Prize laureates, 18 Fellows having received the Presidential Medal of Science and/or Technology and Innovation, and 173 also inducted to the National Academy of Engineering, 84 inducted to the National Academy of Medicine and 37 inducted to the National Academy of Sciences.
AIMBE is the authoritative voice and advocate for the value of medical and biological engineering to society. AIMBE’s mission is to recognize excellence, advance the public understanding, and accelerate medical and biological innovation. No other organization can bring together academic, industry, government, and scientific societies to form a highly influential community advancing medical and biological engineering. AIMBE’s mission drives advocacy initiatives into action on Capitol Hill and beyond.
Congratulations, all!
Weber Lab Student Awarded with 2020 NSF Graduate Research Fellowship

Jordyn Ting, a bioengineering graduate student, works in the Rehab Neural Engineering Labs with McGowan Institute for Regenerative Medicine affiliated faculty member Douglas Weber, PhD, where her work focuses on investigating the spared connection between the motor cortex and muscles. Ms. Ting is one of twelve University of Pittsburgh students who were awarded a 2020 National Science Foundation Graduate Research Fellowship. This is the highest number of students to receive this competitive award since 2015 when the University had a total of 13 recipients. An additional sixteen Pitt students also earned an honorable mention.
The NSF Graduate Research Fellowship Program (GRFP) is designed to ensure the vitality and diversity of the scientific and engineering workforce in the United States. GRFP supports the graduate study of U.S. citizens, nationals and permanent residents attaining research-based master’s and doctoral degrees in science, technology, engineering and mathematics (STEM) or in STEM education at institutions located in the United States. Fellows receive a three-year annual stipend of $34,000 as well as a $12,000 cost-of-education allowance for tuition and fees.
Dr. Weber is an Associate Professor in the Department of Bioengineering at the University of Pittsburgh with secondary appointments in the Department of Physical Medicine and Rehabilitation, the Department of Rehabilitation Science and Technology, and the Center for the Neural Basis of Cognition.
Congratulations, Ms. Ting!
Illustration: Rehab Neural Engineering Labs (Ting)/McGowan Institute for Regenerative Medicine (Weber).
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#208 –– Mr. Ryan Pruchnic discusses the history of Cook MyoSite as well as the research currently being conducted there.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Fisher JD, Zhang W, Balmert SC, Aral AM, Acharya AP, Kulahci Y, Li J, Turnquist HR, Thomson AW, Solari MG, Gorantla VS, Little SR
Title: In situ recruitment of regulatory T cells promotes donor-specific tolerance in vascularized composite allotransplantation.
Summary: Vascularized composite allotransplantation (VCA) encompasses face and limb transplantation, but as with organ transplantation, it requires lifelong regimens of immunosuppressive drugs to prevent rejection. To achieve donor-specific immune tolerance and reduce the need for systemic immunosuppression, we developed a synthetic drug delivery system that mimics a strategy our bodies naturally use to recruit regulatory T cells (Treg) to suppress inflammation. Specifically, a microparticle-based system engineered to release the Treg-recruiting chemokine CCL22 was used in a rodent hindlimb VCA model. These “Recruitment-MP” prolonged hindlimb allograft survival indefinitely (>200 days) and promoted donor-specific tolerance. Recruitment-MP treatment enriched Treg populations in allograft skin and draining lymph nodes and enhanced Treg function without affecting the proliferative capacity of conventional T cells. With implications for clinical translation, synthetic human CCL22 induced preferential migration of human Treg in vitro. Collectively, these results suggest that Recruitment-MP promote donor-specific immune tolerance via local enrichment of suppressive Treg.
Source: Sci Adv. 2020 Mar 13;6(11):eaax8429. doi: 10.1126/sciadv.aax8429. eCollection 2020 Mar.
GRANT OF THE MONTH
PI: Takashi Kozai
Title: CAREER: Uncovering the Impact of Traditional and Novel Chronic Stimulation Modalities on Neural Excitability and Native Neuronal Network Function
Description: The ability to selectively stimulate a small group of neurons has been long desired for basic neuroscience studies as well as for clinical applications. To address this need, the investigator has developed a wireless technology that can precisely stimulate a distinct population of neurons by electrical or light stimulation. Because a balance between excitatory and inhibitory neural activity is important for perception in the brain, a key question is how stimulation impacts this balance. An imbalance between excitatory and inhibitory neuronal activity can lead to cognitive dysfunctions and is a hallmark of autism spectrum disorder. Moreover, brain injuries such as traumatic brain injuries, stroke, and microelectrode implantation have also been shown to disrupt this balance. Therefore, the research goal of this CAREER project is to establish the relationship between different types of stimulation and their impact on excitability of neuronal populations. The project’s educational goal is to train the next wave of investigators with the multidisciplinary skills needed to solve the chronic neural interface challenge. This will be achieved through: 1) integrating examples from this research into an outreach program to Underrepresented Minority Students that focuses on introducing the fundamental principles of the scientific method and engineering design controls and criteria and on demonstrating how science and engineering converge at the neural interface; 2) making neural interface knowledge more widely accessible by the formation of the virtual “Education in Biological and Neuroelectronic Interface Community” (eBioNIC.org) that will be a focal point for providing videos and other training materials; and 3) providing an early platform for hands-on education on integrating Neurobiology and Neural Engineering.
The Investigator’s long-term career vision is to seamlessly integrate the brain and technology in order to enable new approaches to studying long-standing neurobiology questions such as how to repair brain injuries and neurodegenerative diseases. Towards this vision, this CAREER project?s specific goals are to break through traditional limitations of neurostimulation by engineering wireless axons that use specific biomolecules to modulate the activity of a small population of neurons in the brain, and then apply this technology to modulate excitatory-inhibitory neuronal imbalances. The project will employ new optical technologies to solve long-standing questions on the relationship between stimulation technologies and changes to the brain?s excitatory-inhibitory balance. This will be achieved using optical and transgenic methods to determine the cell-type specificity of excitatory and inhibitory neuronal activity, an important parameter that can enhance our physiological understanding of the activated brain region. The project’s guiding hypothesis is that different stimulation modalities will differentially alter spatio-temporal excitatory and inhibitory neuronal activity, which will in turn alter the long-term excitability of nearby neurons in different capacities. The Research Plan is organized under two objectives. THE FIRST Objective is to further engineer this wireless stimulation technology to reliably and repeatably release specific biomolecules including neurotransmitters. Coating technologies will be applied to the wireless axons to release biomolecules during stimulation and recharge by drawing upon endogenously produced biomolecules. THE SECOND Objective is to investigate how stimulation with electrical, optical, wireless-axon, and wireless neurochemical modalities impacts long-term excitatory and inhibitory neuronal excitability using in vivo 2-photon microscopy and genetically encoded fluorescent indicators. In vivo images will be collected from awake head-fixed mice at increasing intervals daily for two weeks and then once a week until 12 weeks. The number, distance, timing and neuronal subtype densities before, during and after electrical stimulation will be examined over time. The method enables tracking of stimulation-induced dynamic changes with high spatial resolution near the electrodes. Research outcomes are expected to have a significant impact on the future design of neural interfaces through the engineering of chronic selective neural stimulation tools with ultra-small free-floating implants that will provide scientists with a new tool for interrogating neuronal networks and creating different sensations in BCIs and through visualization of how stimulation with electrical, optical, wireless, and wireless neurochemical modalities impacts long-term excitatory and inhibitory neural excitability.
Source: National Science Foundation
Term: July 1, 2020 – June 30, 2025 (Estimated)
Amount: $437,144
