November 2021 | VOL. 20, NO. 11| www.McGowan.pitt.edu
Hemolung Respiratory Assist System Receives FDA De Novo Clearance
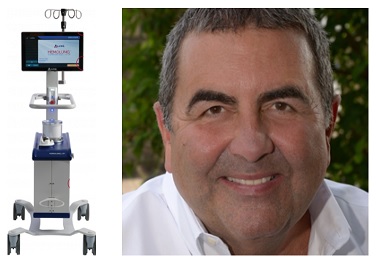
ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, announced that the Food and Drug Administration (FDA) has granted the company De Novo clearance for the Hemolung Respiratory Assist System. This system was developed at the McGowan Institute for Regenerative Medicine by a team of researchers led by faculty member William Federspiel, PhD, the company’s cofounder and professor of bioengineering, chemical engineering, critical care medicine, and the Clinical Translation Institute at the University of Pittsburgh. Dr. Federspiel is also the director of the Medical Devices Laboratory at the McGowan Institute.
The Hemolung System is the first and only ECCO2R device cleared by the FDA. The Hemolung is indicated for respiratory support that provides extracorporeal carbon dioxide (CO2) removal from the patient’s blood for up to 5 days in adults with acute, reversible respiratory failure for whom ventilation of CO2 cannot be adequately or safely achieved using other available treatment options and continued clinical deterioration is expected.
“The FDA De Novo review process is designed to determine that the clinical benefits outweigh the clinical risks associated with a medical device when there is not a substantially equivalent predicate device. As a result, the De Novo process is quite rigorous. In order to demonstrate benefit over risk, ALung submitted data to FDA from over 1,000 Hemolung patient treatments on clinical safety and over 230 Hemolung patient treatments on clinical performance outcomes. This clinical data along with all of their pre-clinical data demonstrated to the FDA that the clinical benefits of the Hemolung for ECCO2R therapy has been substantiated. We look forward to introducing this new and valuable technology to the clinical market,” stated Peter DeComo, chairman and CEO of ALung.
“The Hemolung will be an important new treatment modality for acute respiratory failure to avoid or mitigate the harms from invasive mechanical ventilation. FDA clearance of the Hemolung is a game-changer for the treatment of these critically ill patients and will usher in a new era of extracorporeal lung support,” said Steven Conrad, MD, PhD, MCCM, medical director at ALung and professor of medicine at Louisiana State University Health Sciences Center.
The Hemolung is a first-of-its-kind, comprehensive, all-in-one system intended to provide minimally invasive, low-flow ECCO2R. Low-flow ECCO2R with the Hemolung is a lung-independent ventilatory support therapy for removal of CO2 waste molecules from venous blood via extracorporeal circulation through a single, 15.5 French, central venous catheter at blood flows of 350 – 550 mL/min. Respiratory failure patients often experience the need for CO2 removal without the need for supplemental oxygen. Historically, when extracorporeal therapy was indicated for the removal of CO2, extracorporeal membrane oxygenation (ECMO) devices were utilized because other alternatives were not available. ECMO is typically necessary when respiratory failure patients have a significant oxygenation issue. ECMO systems are complex, invasive, require high blood flows and as a result, require specialty personnel to monitor both the technology and the patient. The Hemolung offers ICU physicians, nurses, respiratory therapists and perfusionists a new tool to treat respiratory failure patients in a less complex, costly, and invasive manner.
ALung has been conducting the VENT-AVOID pivotal clinical trial for the treatment of Chronic Obstructive Pulmonary Disease (COPD) exacerbations and providing the Hemolung for the treatment of COVID-19 patients under an Emergency Use Authorization (EUA) granted by the FDA. The objective of the VENT-AVOID trial is to demonstrate that the Hemolung can avoid or minimize the need for invasive mechanical ventilation. The trial sites have enrolled 111 subjects making VENT-AVOID the largest ECCO2R trial ever conducted for this indication. ALung has also been working with numerous hospitals to treat COVID-19 patients with acute respiratory failure requiring CO2 removal. To date, the Hemolung has been utilized in the treatment of greater than 120 patients suffering from severe COVID-19 respiratory failure. In its EUA approval letter to ALung, the FDA stated that it believes the Hemolung has the potential to treat lung failure as an adjunct to noninvasive or invasive mechanical ventilation, to reduce hypercapnia and hypercapnic acidosis due to COVID 19, and/or to maintain normalized levels of partial pressure of carbon dioxide (PCO2) and pH in patients suffering from acute, reversible respiratory failure due to COVID-19 for whom ventilation of CO2 cannot be adequately, safely, or tolerably achieved and, in turn, may provide clinical benefit, and that there is no adequate, approved and available alternative to the emergency use of the Hemolung RAS to treat lung failure caused by COVID 19.
ALung Technologies, Inc. is a privately held Pittsburgh-based developer and manufacturer of innovative lung assist devices. Founded in 1997 as a spin-out of the University of Pittsburgh, ALung has developed the Hemolung as a dialysis-like alternative or supplement to mechanical ventilation. ALung is backed by Philips, UPMC Enterprises, Abiomed, The Accelerator Fund, Allos Ventures, Birchmere Ventures, Blue Tree Ventures, Eagle Ventures, Riverfront Ventures, West Capital Advisors, and other individual investors.
For more information about ALung and the Hemolung RAS, visit www.alung.com.
For more information on the VENT-AVOID trial, and a list of enrolling sites, please visit www.clinicaltrials.gov.
For more information on the use of the Hemolung RAS for COVID-19 patients, please visit www.alung.com/covid-19.
To listen to a Regenerative Medicine Today podcast featuring Dr. Federspiel and Mr. DeComo, please visit http://regenerativemedicinetoday.com/?s=209.
Illustration: Hemolung RAS (ALung)/Federspiel (McGowan Institute for Regenerative Medicine)
As We Celebrate Thanksgiving, Let Us Extend Our Thanks…
![]()
- To the 240+ scientists, engineers and clinicians that are the teams of professionals who are committed to the development and translation of regenerative medicine and medical device-based therapies;
- To the dedicated support staffs who make it possible for the scientists, engineers and clinicians to pursue the goals and objectives of the Institute;
- To the hundreds of student trainees who are learning the fundamentals and developing the applications that continue to make regenerative medicine the promise of the future in improved health care and quality of life;
- To the many agencies, foundations, companies, and individual donors without whose fiscal support and encouragement the outcomes that we give thanks for would not have been possible, and;
- To the many patients who have trusted our teams. These individuals are the true pioneers and the heart of the achievements we recognize.
Since the formation of the McGowan Center for Artificial Organ Development (1992) and the McGowan Institute for Regenerative Medicine (2001) there have been many accomplishments advancing the science and clinical achievements in regenerative medicine-based therapies. We see progress in the areas of medical devices, tissue engineering and cell-based therapies, and the prospects for the future are many. Thanks for the opportunities and outcomes created by all who participate in or support the initiatives of the McGowan Institute. Best wishes for another successful year!
RESOURCES AT THE MCGOWAN INSTITUTE
December Histology Special – Frozen Sections
Let it snow! At the McGowan Histology Core, we are cutting frozen sections and offering 25% off your entire frozen project bill.
We offer a high degree of technical expertise and can accommodate even the more specialized service needs.
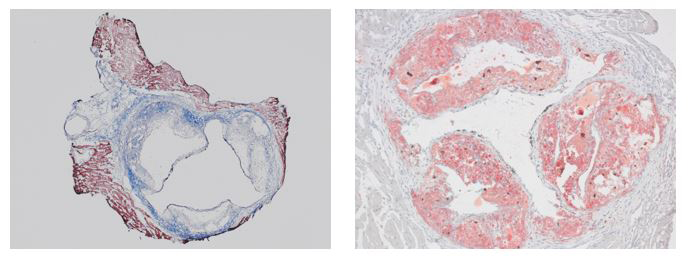
Mouse aortic root valve stained with Masson’s Trichrome (left) and Oil Red O (right).
You’ll receive 25% off your frozen project in December when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
UPCOMING EVENTS
Pediatric Device Innovators Forum

A great need currently exists for medical devices designed specifically for children.
Pediatric Device Innovators Forum (PDIF) is a recurring collaborative educational experience designed to connect and foster synergy among innovators across the technology development ecosystem who are interested in pediatric medical device development.
A collaboration between the FDA and the five Pediatric Device Consortia (PDC):
- Pennsylvania Pediatric Medical Device Consortium, Robert Levy, MD
- National Capital Consortium for Pediatric Device Innovation 2.0, Kolaleh Eskandanian, PD, MBA
- Southwest National Pediatric Device Innovation Consortium, Chester Koh, MD
- University of California San Francisco-Stanford Pediatric Device Consortium, Michael Harrison, MD
- The West Coast Consortium for Technology & Innovation in Pediatrics, Juan Espinoza, MD
Thank you for helping us reach more people by the details of our upcoming Pediatric Device Innovators Forum! Below are several templates you can use across any number of platforms. You can click on the links in the Table of Contents below to jump to the specific template you want.
Questions? Contact southwestpdc@bcm.edu.
SCIENTIFIC ADVANCES
Newly Patented Device Anchored in 50+ Years of Musculoskeletal Research
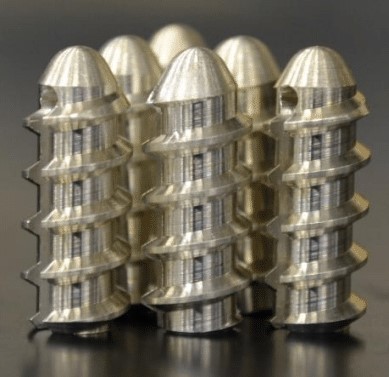
Soft tissue injuries — like rotator cuff tears or hip impingement — are fairly common, particularly among athletes and older populations. These injuries frequently require surgery where multiple suture anchors are inserted into the bone, providing a strong base through which a suture can be threaded to reattach tendons and ligaments.
Much like anchors used to hang a heavy object on a wall, suture anchors need to be durable and remain fixed in place. A newly patented magnesium-based suture anchor from University of Pittsburgh Professor Savio L-Y. Woo’s laboratory, addresses the shortcomings of the metallic and polymer devices currently available on the market. Dr. Woo is a distinguished university professor emeritus of bioengineering at Pitt’s Swanson School of Engineering and a former McGowan Institute for Regenerative Medicine affiliated faculty member.
“Our design improves device performance by mitigating common failures in polymer suture anchors, including device breakage, eyelet failure, and low pullout strength,” said Dr. Woo.
Current metallic devices are non-degradable in the body and interfere with magnetic resonance imaging (MRI), while polymer devices degrade very slowly and are associated with osteolysis — the destruction of bone tissue.
“By switching to biodegradable and bioresorbable magnesium materials, the suture anchors can be replaced by newly formed bone. Magnesium is also ductile; its mechanical properties are closer to bone, making it better than other metallic materials. Ultimately, magnesium suture anchors will alleviate pain and improve the quality of life for patients who receive soft tissue repair surgery,” added Dr. Woo, who is also the founding director of Pitt’s Musculoskeletal Research Center (MSRC).
The device was developed in collaboration with Kwang Kim, PhD, and Jonquil Flowers Mau, PhD, former bioengineering graduate students in the MSRC. The work was sponsored by the National Science Foundation (NSF) – Engineering Research Center (ERC) for Revolutionizing Metallic Biomaterials grant in collaboration with North Carolina Agricultural and Technical State University (NCA&T) as well as the University of Cincinnati.
Illustration: University of Pittsburgh Swanson School of Engineering.
A New Breakthrough for Treatment of Male Infertility

With global rates of male infertility continuing to rise, a new study in spermatogonial stem cell research led by researchers at the University of Georgia (UGA) provides hope for future clinical therapies. McGowan Institute for Regenerative Medicine affiliated faculty members Kyle Orwig, PhD, Professor, OB/GYN and Reproductive Sciences, University of Pittsburgh School of Medicine, with secondary appointments in Microbiology and Molecular Genetics, Developmental Biology, and the Clinical and Translational Science Institute, and the founding Director of the Fertility Preservation Program of UPMC and the UPMC Magee Center for Reproduction and Transplantation, and Gerald Schatten, PhD, Director of the Pittsburgh Development Center, Professor of Obstetrics, Gynecology and Reproductive Sciences, Cell Biology, Bioengineering and Director of the Division of Developmental and Regenerative Medicine, are collaborators on this work.
The study, which was published recently in Fertility and Sterility Science, is the first to show that functional sperm cells can be made in a dish using primate embryonic stem cells.
“This is a major breakthrough towards producing stem cell-based therapies to treat male infertility in cases where the men do not produce any viable sperm cells,” said lead researcher Charles Easley, PhD, an associate professor in UGA’s College of Public Health.
Researchers used embryonic stem cells from rhesus macaque monkeys to generate immature sperm cells known as round spermatids, which they showed to be capable of fertilizing a rhesus macaque egg.
Scientists have been able to produce sperm-like cells using mouse stem cells, said Dr. Easley, but rodent sperm production is distinctly different than humans. Until this work, it wasn’t clear that this technology could ever work in humans.
“This is the first step that shows this technology is potentially translatable. We’re using a species that’s more relevant to us, and we’re having success in making healthy embryos,” said Dr. Easley.
Rhesus macaques share similar reproductive mechanisms to humans, making them an “ideal and necessary model for exploring stem cell-based therapies for male infertility,” the authors write.
Using a novel method, the researchers differentiated the cells into immature sperm cells known as round spermatids. Like immature spermatids in vivo, fertilization with in vitro spermatids requires activating the egg and the addition of other factors to enable the fertilized egg to develop into a healthy embryo.
This fall, the researchers plan to take the next critical step of implanting these embryos into a surrogate rhesus macaque to examine whether these embryos from in vitro spermatids can produce a healthy baby.
If that step is successful, the team will carry out the same process using spermatid-like cells derived from macaque skin cells.
Cardiothoracic Surgery Research Training Program Receives $1.6 Million Funding

Over $1.6 million has been received from the NIH’s National Heart, Lung, and Blood Institute (NHLBI) for the 5-year Cardiothoracic Surgery Research Training Program. This research training grant is focused on the next generation of academic cardiac and thoracic surgeons and will be led by the following team which includes four McGowan Institute for Regenerative Medicine affiliated faculty members:
Program Director: James Luketich, MD
Co-PDs: Julie Phillippi, PhD, and David Vorp, PhD
Associate Directors: Vera Donnenberg, PhD, and Matthew Schuchert, MD
In the long-term, this program will increase the number of academic cardiothoracic surgeons and will help to promote and sustain a diverse workforce capable of accomplishing the NHLBI’s mission.
The project summary follows:
This application is for a research training grant focused on the next generation of academic cardiac and thoracic surgeons. Given the current and future shortage of cardiothoracic surgeons, the extremely diverse clinical activities, and broad array of conditions treated by cardiac and thoracic surgeons, an NIH T32 training program in cardiothoracic surgery is an emergent unmet need to improve the academic cardiothoracic surgical workforce. The necessary resources to support this mission is largely dictated by the capacity for early research training mid-way through the residency-fellowship training continuum. The proposed program leverages the experience and framework of our successful ACGME accredited six-year Integrated Thoracic Residency Program, the research and mentoring expertise of our Department of Cardiothoracic Surgery faculty, and the infrastructure of the University of Pittsburgh School of Medicine (UPSOM) and UPMC Health System, to create a new T32 program in cardiothoracic surgery training. The proposed T32 program will provide a strong foundation of training in research, serving leadership, and collaborative partnerships during residency training, which will help to establish and promote successful independent career paths in academic cardiothoracic surgery. The proposed T32 program is expected to increase the pool of investigators who will be competitive for independent K- and R-awards as faculty.
This program will achieve the following specific goals: 1) Provide an early, stable, and supportive infrastructure of research training for academic cardiothoracic surgeons; 2) Recruit and select promising scholars and monitor their initial, ongoing, and long-term successes; 3) Coordinate faculty mentoring and training of cardiothoracic surgeons to provide hands-on research training coupled with a trainee-specific didactic curriculum that addresses the unique unmet needs of cardiothoracic surgery; 4) Leverage internal UPSOM, UPMC, and other institutional resources in trainee-specific training plans; 5) Promote an inclusive culture at all levels through diversity training and community outreach to recruit under-represented minority candidates to cardiothoracic surgery; 6) Provide structured training in leadership, partnership, and research program establishment during and beyond the period of support to position trainees for launching successful independent academic careers. In the long-term, the proposed T32 program will increase the number of academic cardiothoracic surgeons and will help to promote and sustain a diverse workforce capable of accomplishing the NHLBI’s mission.
Testicular Tissue Cryopreservation Shown to Be a Safe and Feasible Option of Male Fertility Preservation

When a child has cancer, their parents are rightfully focused on a cure. But that cure can come at a future cost: infertility.
Kien Tran is a PhD candidate in the Molecular Genetics and Developmental Biology Graduate Program at the University of Pittsburgh School of Medicine who is working in the laboratory of McGowan Institute for Regenerative Medicine affiliated faculty member Kyle Orwig, PhD, at the Magee-Womens Research Institute which focuses on reproductive biology. Dr. Orwig is a Professor, OB/GYN and Reproductive Sciences, University of Pittsburgh School of Medicine with secondary appointments in Microbiology and Molecular Genetics, Developmental Biology, and the Clinical and Translational Science Institute and the founding Director of the Fertility Preservation Program of UPMC and the UPMC Magee Center for Reproduction and Transplantation. Ms. Tran is participating in the UPMC Science Writing Mentorship Program and authored the article in Inside UPMC.
In her research, Ms. Tran has learned that patients and their guardians are willing to pursue an experimental fertility preservation procedure when no alternatives are available. Sperm freezing is not possible for prepubescent boys, who are at risk for infertility due to medical treatments for cancers and other diseases, because they are not yet producing sperm. The only option to preserve their future fertility is testicular tissue cryopreservation (TTC).
Recently, Ms. Tran presented at the American Society for Reproductive Medicine’s annual meeting in Baltimore on her research regarding reproductive potential of frozen-and-thawed pre-pubertal testicular tissues. Studies that demonstrate the safety and feasibility of the testicular tissue cryopreservation protocol can help clinicians accurately counsel patients and their families. For patients and parents who are new to this, Ms. Tran has put together a primer.
Some medical treatments cause male infertility
Radiation and chemotherapy used to treat cancers or benign diseases, including sickle cell disease and beta-thalassemia, can lead to permanent infertility. Prior to these treatments, patients should discuss fertility preservation options with their medical team if they wish to have biological children in the future.
Fertility preservation options for men and boys
Ms. Tran’s research focuses on male fertility. Adult males have the option to cryopreserve, meaning freezing in liquid nitrogen, their semen samples that contain viable sperm. A previous study demonstrated that sperm frozen up to 40 years were still able to produce healthy offspring. However, boys who are not yet producing sperm are not able to use the sperm-freezing approach. But more than 50% of cancer survivors wish to become parents in the future. What option do they have?
The stem cell pool, named spermatogonial stem cells (SSCs), in the testes emerge shortly after birth. SSCs balance self-renewing and differentiating divisions to maintain the stem cell pool in the testes, as well as producing sperm throughout a man’s life. Before puberty, SSCs do not undergo the sperm production process. Pre-pubertal boys who are not producing sperm have the option to freeze their testicular biopsy prior to their medical treatments if they desire to have biological children in the future. Each of these biopsies would be small, but they do contain SSCs that could be used for fertility restoration purposes. Upon thawing, the SSCs that exist in these tissue pieces can be matured to produce functional sperm capable of generating offspring. Many studies in mammals, including mice, sheep, pigs, cows, and even monkeys, have produced sperm from frozen-and-thawed pre-pubertal testicular tissues.
World-wide coordinated network for testicular cryopreservation as a fertility preservation option for pre-pubertal male patients
Centers around the world have joined an international effort to cryopreserve testicular biopsies for pre-pubertal patients who are at risk of permanent infertility due to side-effects of their medical treatments.
Dr. Orwig’s lab established the Fertility Preservation Program in Pittsburgh in 2010 when he gained approval to begin cryopreserving ovarian tissues and testicular tissues. The Fertility Preservation Program now provides a comprehensive menu of standard and experimental fertility preservation options for adults and children. In addition to the Western Pennsylvania region, the program provides centralized processing of reproductive tissues for a coordinated network of more than 30 centers in the United States. The lab also collaborates with two centers in Israel. Related to Ms. Tran’s project, the Pittsburgh center has cryopreserved testicular tissues for almost 500 patients. The average patient age was 7.6 years, ranging from 3 months old to 34 years old. The diagnoses of the patient cohort include cancers (63.2%), myeloablative conditioning prior to bone marrow transplant (28.4%), gender dysphoria (3.9%), and others (4.5%).
Most of the patients donated 25% of their testicular biopsy to research. This tremendous gift allows researchers to develop next-generation stem-cell-based technologies for male fertility preservation and restoration.
Does long-term cryogenic storage impact cell function?
Since freezing testicular tissues for a decade now, the team wonders whether the long-term cryogenic storage has a negative impact on cell quality. They performed an in vivo assay – an experiment conducted using a live body – by transplanting human testicular cells, isolated from frozen-and-thawed testicular tissues, into mouse testes.
The data revealed no difference among samples frozen for less than two years, two to four years, four to six years, or eight to 10 years. This gives patients assurance that their testicular tissues frozen up to a decade ago still contain functional reproductive cells that they may use to have biological children in the future when stem-cell-based technologies are fully developed.
Does prior exposure to chemotherapy compromise reproductive function of the testes?
Even though it is best to cryopreserve testicular tissues prior to cancer treatments, more than 40% of the patients studied had initiated their chemotherapy and/or radiation prior to testicular tissue cryopreservation. The team is curious whether this exposure compromised reproductive cell or tissue quality in these patients’ biopsies. The result showed no difference in the reproductive cell number between the chemo-treated group versus the no-chemo control group. However, the in vivo transplantation assay, as described above, showed a decrease in functional SSCs in the chemo-treated group compared to the no-chemo control group. While SSC numbers were reduced in the chemo-treated group, researchers were able to recover functional SSCs from all chemo-treated and no-chemo control samples. Therefore, these findings may help assure patients that, while it is best to cryopreserve tissues prior to the initiation of medical treatments, even testicular samples that have undergone early-stage chemotherapy still possess some reproductive function.
Illustration: UPMC/Tran and McGowan Institute for Regenerative Medicine/Orwig.
Surviving COVID: Comorbidities Don’t Tell the Full Story

Advanced age, being a man, and several types of preexisting health conditions have been associated with poor outcomes among hospitalized patients with COVID, but a new study suggests that other biological factors may be more important in determining prognosis and survival than these factors.
Improved understanding of the biological underpinnings of different responses to SARS-CoV-2 infection could improve treatment for patients.
Most studies seeking to identify risk factors for COVID have grouped patients by age, sex, and preexisting conditions such as obesity, diabetes, and heart failure. “But that can potentially obscure contributions from underlying factors and obscure the potential that a single comorbidity can be associated with different outcomes,” says Soojin Park, MD, the study’s senior author, associate professor of neurology at Columbia University Vagelos College of Physicians and Surgeons, and a neurocritical care physician at Columbia University Irving Medical Center/NewYork-Presbyterian. Three McGowan Institute for Regenerative Medicine affiliated faculty members are co-authors of this study:
- Michael Pinsky, MD, professor of critical care medicine, bioengineering, cardiovascular diseases, clinical and translational science, and anesthesiology at the University of Pittsburgh
- Gilles Clermont, MD, a professor of critical care medicine, industrial engineering, and mathematics, and the medical director of the Center for Inflammation and Regenerative Modeling, University of Pittsburgh
- Yoram Vodovotz, PhD, professor of surgery, computational and systems biology, bioengineering, immunology, communication science and disorders (of the School of Health and Rehabilitation Science), clinical and translational science, and the director of the Center for Inflammation and Regeneration Modeling
To identify underlying factors that predict outcome, Dr. Park and her team used machine learning to analyze patient laboratory data collected throughout the hospitalizations of 528 patients treated at a single hospital during the first few months of the pandemic in 2020.
The analysis identified four unique groups of patients that had vastly different outcomes not expected based on classic risk factors such as age, sex, and preexisting conditions.
The group with the highest number of comorbidities, for example, did not have the worst outcomes and had a relatively low mortality rate, indicating that comorbidities alone are not responsible for variable COVID clinical courses.
The group of patients with the worst outcomes and most deaths had fewer comorbidities than expected but had higher levels of circulating inflammatory markers than the other three groups.
“Having certain high- or low-risk conditions does not guarantee a certain outcome,” says Dr. Park. “Rather, this highlights that healthy patients can still have poor COVID outcomes and patients with numerous chronic diseases can still have good outcomes.”
“Given the risk of poor outcome in a group of patients who were previously quite healthy, vaccination remains essential for everyone,” say Benjamin Ranard, MD, the study’s first author and a pulmonary and critical care fellow at Columbia.
Further examination of the different groups has potential to yield clinical and pathobiological insight into what is driving the vastly different clinical courses experienced by patients with COVID.
Molecular Atlas of Senescent Cells Could Chart Way to Therapies for Age-Related Diseases and Cancer

Most cells throughout the body can divide and multiply to replace old cells and repair damaged tissue, but in response to certain stresses, cells can lose their ability to proliferate. These so-called senescent cells accumulate with age and may contribute to cancer and age-related disorders, such as chronic lung disease, cardiovascular disease, frailty, and dementia, by pumping out signals that damage neighboring tissues.
But because these rare cells are tiny islands in the vast world of the human body, the molecular landscape of senescence has remained relatively uncharted.
With the goal of building an atlas of cellular senescence to understand how and why senescent cells develop and set the course for new therapies for age-related diseases, the National Institutes of Health’s Common Fund established the Cellular Senescence Network (SenNet) Program. The program announced it will award $125 million to 16 teams that form the new SenNet Consortium. Two of the projects are led by University of Pittsburgh and UPMC researchers, who will receive a combined $31 million over five years.
In collaboration with researchers at Carnegie Mellon University, The Ohio State University, and the University of Rochester, Toren Finkel, MD, PhD, distinguished professor of cardiology and director of the Aging Institute at Pitt and UPMC, is leading the TriState SenNet Tissue Mapping Center. It will contribute to this atlas by developing maps of senescence in heart and lung cells. Like molecular cartographers, the researchers will map gene expression and protein composition in senescent cells from human tissue slices and lab-grown mini organs, or organoids. They’ll compare different types of senescent cells from across the lifespan to characterize signposts, or biomarkers, of senescence. McGowan Institute for Regenerative Medicine affiliated faculty member Mauricio Rojas, MD, professor of internal medicine and the associate vice chair of research, Department of Internal Medicine, in the Division of Pulmonary, Critical Care, & Sleep Medicine of the Davis Heart & Lung Research Institute, at The Ohio State University, is a co-investigator on this project.
“We don’t know if cellular senescence is one thing or many things,” said Dr. Finkel. “An analogy is cancer: Lung cancer, pancreatic cancer and lymphomas are all very different, even though we call them all cancer. We want to understand how senescent cells triggered by different stresses and occurring in different tissues are similar and how they are different.”
According to Melanie Königshoff, MD, PhD, professor of medicine in the division of Pulmonary, Allergy and Critical Care Medicine (PACCM) at Pitt, answering these questions is important for understanding the molecular mechanisms of senescence, which could point to new therapies called senolytics that seek and destroy senescent cells involved in age-related diseases.
“In cancer, there are certain genetic changes in tumor cells, which allow you to develop targeted therapies,” said Dr. Königshoff. “Similarly, if we can define what is different between a senescent cell and adjacent normal cells, we might be able to devise therapies that eliminate these cells based on their molecular signatures.”
These maps of senescent heart and lung cells combined with maps from different organs created by other teams in the consortium will contribute to a global atlas of cellular senescence spanning many tissues of the human body.
To create an organized and navigable atlas, Jonathan Silverstein, MD, professor in the Department of Biomedical Informatics at Pitt and chief research informatics officer at Pitt and UPMC’s Institute for Precision Medicine, and researchers from Carnegie Mellon University and the Pittsburgh Supercomputing Center—building on previous success in managing The Human BioMolecular Atlas Program (HubMAP), a similar program that aims to map healthy human tissues at a cellular level—are leading the SenNet Consortium Organization and Data Coordination Center (CODCC).
The CODCC will act like a library for the consortium, developing something like the Dewey Decimal System to annotate and organize the vast collection of maps produced by the other teams.
Eventually, the atlas of cellular senescence will be published online in an open-source repository so that other researchers can explore these data to make new discoveries about senescent cells and how they contribute to human health.
“I think that the science of senescent cells is tremendously exciting because of their potential impact on a whole variety of diseases,” said Dr. Silverstein. “By doing the basic science of collecting all of this information and presenting it through SenNet, there is incredible potential to learn more about the role these cells play in disease and develop pharmaceuticals that target them.”
The principal investigators of the TriState SenNET (Lung and Heart) Tissue Map and Atlas consortium (U54AG075931) are Drs. Finkel and Königshoff, Pitt; Irfan Rahman, PhD, University of Rochester; and Ana Mora, MD, The Ohio State University. Other co-investigators on the project are Oliver Eickelberg, MD, FERS, ATSF, Pitt; Dr. Rojas, The Ohio State University; and Ziv Bar-Joseph, PhD, Carnegie Mellon University.
The principal investigators of the Cellular Senescence Network (SenNet) Consortium Organization and Data Coordinating Center (U24CA268108) are Dr. Silverstein, Pitt; Dr. Bar-Joseph, Carnegie Mellon University, and Philip Blood, PhD, Pittsburgh Supercomputing Center.
Illustration: The Ohio State University.
2021 Pitt Innovation Challenge Winners Named

The Pitt Innovation Challenge (PInCh®) is a program supported by the Clinical and Translational Science Institute (CTSI) and designed to generate innovative solutions to challenging health problems by mitigating risk and providing financial and administrative support to move ideas forward. The PInCh program stimulates the translation of novel problem-focused research into the community by giving researchers a venue to be creative, develop new ideas, and work with people beyond their usual sphere of collaborators. PInCh provides funding and project management to help develop high-quality health science research that serves people and communities outside of the university. In 2021, the PInCH competition looked for answers to the following: What is your bold solution to a challenging health problem?
This year’s winners that include McGowan Institute for Regenerative Medicine affiliated faculty team members include:
$100,000 Winner + additional $15,000 health disparity bonus award
NextGenET
Description: A simplified endotracheal tube that prevents ventilator-associated pneumonia.
Team: Carl Snyderman, MD, MBA, Garett Coyan, MD, MS, Ross Beresford, MS, Lauren Grice, Garett Craig.
NextGenET is a simplified, low-cost-disposable endotracheal tube designed to prevent ventilator-associated pneumonia at its root. Our unique design uses a layer of panels that passively seal the airway, consistently outperforming current inflatable cuffs without requiring constant monitoring. While specialized tubes exist, they are simply too expensive for most hospital systems to implement consistently. NextGenET produces leak-proof seals, is affordable, and is simple for healthcare specialists to use and maintain. While our tube is currently designed to be single-use in modern US hospital ICUs, we believe it can be re-sterilization for repeated use without degrading effectiveness (as happens with current inflatable tubes) in underserved developing regions. NextGenET has the potential to substantially reduce ventilator-associated pneumonia in the ICU, saving thousands of lives and billions of dollars in the US alone.
Elevator Pitch Competition Awardees
Platelet-Be-Gone-Stent
Description: A novel vascular stent coating process to prevent thrombosis and perfusion.
Team: John Pacella, MD, MS, Ellen Gawalt, PhD, Jared Romeo, DO.
This project will develop a heart stent, coated with an FDA-approved drug, which will prevent blood clots from forming at the site of the stent. This will eliminate the need for a patient to take a lifetime of blood-thinning medications. This coating also can be applied to any blood-contacting surfaces to prevent clots, i.e., intravenous catheters, heart valves, and dialysis catheters.
Congratulations to all!
Illustration: University of Pittsburgh Clinical and Translational Science Institute PInCH logo.
FLASH Registry Interim Data Demonstrating Benefits of Lytic-Free Mechanical Thrombectomy

Positive acute and long-term interim results from the first 500 pulmonary embolism (“PE”) patients enrolled in the FlowTriever Outcomes Registry (“FLASH”) were presented at TCT 2021 by Principal Investigator, Catalin Toma, MD, an Interventional Cardiologist at University of Pittsburgh Medical Center (UPMC) and an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
FLASH is a prospective, multicenter, single-arm registry evaluating real-world patient outcomes after treatment of PE with FlowTriever, developed by Inari Medical, Inc., a medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases.
At 48 hours post procedure, the major adverse event rate and mortality rate were low, at 1.4% and 0.2%, respectively. None of the deaths or major adverse events were device related. Collectively, these PE patients experienced substantial on-table improvements in hemodynamics and symptoms, which translated to 6-month improvements in cardiac function, functional status, and quality of life measures. The outcomes were achieved while limiting utilization of hospital resources, with less than 4% of patients receiving adjunctive therapy and a median of 0 days in the ICU post procedure.
“These interim results reinforce the strong safety profile of the FlowTriever System in real-world PE patients, with substantial on-table clinical improvements and immediate symptom relief,” said Dr. Toma. “We believe these 6-month follow-up data suggest that removal of clot burden without the risks of lytics has potential positive long-term implications for PE patients, including strikingly low rates of hospital readmissions, dyspnea, CTED, and CTEPH. These data suggest that treatment with FlowTriever may fundamentally improve the natural course of the disease, and that is tremendously exciting.”
“FlowTriever is quickly becoming the frontline therapy for intermediate and high-risk PE. FLASH, already the largest prospective interventional data set in the field of PE, reinforces the excellent safety, functional improvement, and long-term outcomes of this approach,” said Thomas Tu, MD, Chief Medical Officer of Inari Medical. “We believe these data continue to raise the bar to which existing and new treatments will be held. We remain committed to venous thromboembolism patients and to advancing the treatment of this disease through clinical research and the continued development of purpose-built devices.”
AWARDS AND RECOGNITION
Honoring Veterans in Research

From the author of the GI Bill of Rights to a former Army Surgeon General, veterans have shaped the trajectory of the University of Pittsburgh — and the world. As Pitt honored those who have served this Veterans Day, the Office of Veterans Services and Archives and Special Collections highlighted veterans from the 20th and 21st centuries who have made a major difference in Pitt’s history. Three of those veterans are McGowan Institute for Regenerative Medicine affiliated faculty members.
Military career: Dr. Poropatich served 30 years on active duty in the U.S. Army with extended assignments at the Walter Reed National Military Medical Center from 1985-2012. His last assignment was at the U.S. Army Medical Research and Materiel Command at Fort Detrick, serving as deputy director of the Telemedicine and Advanced Technology Research Center from 2006-12. He managed sizeable medical research programs and developed novel research and technologies in medical informatics and telemedicine. He retired in 2012 at the rank of colonel.
At Pitt: Dr. Poropatich is the current director for Pitt’s Center for Military Medicine Research and a professor of Medicine in the Division of Pulmonary, Allergy, and Critical Care Medicine. He is also a professor of Sports Medicine and Nutrition in the Department of Sports Medicine and Nutrition Science. His research interests include the role of AI in health care, telemedicine, medical informatics, and pre-hospital care in remote environments. He is the UPMC senior advisor for telemedicine and currently has more than $10 million awarded in grant funding to various projects.
Military career: Dr. Cooper enlisted in the U.S. Army in 1976. In 1980, he was serving in Germany when a bicycle accident left him paralyzed from the waist down. Since then, Dr. Cooper has used technology to positively influence the lives of veterans and others who are disabled.
At Pitt: The renowned biomedical engineer joined Pitt in 1994. He currently serves as the FISA/PVA Distinguished Professor in the Department of Rehabilitation Science and Technology and professor of bioengineering, physical medicine and rehabilitation, and orthopaedic surgery.
Dr. Cooper founded the Human Engineering Research Laboratories, a VA Rehabilitation R&D Center of Excellence partnership with Pitt, and has been the driving force behind innovative wheelchair designs and other assistive technology and equipment. HERL’s Experiential Learning for Veterans in Assistive Technology and Engineering (ELeVATE) program is working to increase the enrollment, retention, graduation, and career success of wounded, injured and ill veterans in engineering and technology.
Dr. Cooper has authored or co-authored more than 300 peer-reviewed journal publications and has 20 patents awarded or pending. He is the author of two books, “Rehabilitation Engineering Applied to Mobility and Manipulation” and “Wheelchair Selection and Configuration,” and is co-editor of “An Introduction to Rehabilitation Engineering,” “Warrior Transition Leader: Medical Rehabilitation Handbook,” and the award-winning book, “Care of the Combat Amputee.”
Among many other feats, he also survived a near-fatal handcycle crash in 2019.
Military career: Prior to coming to the University of Pittsburgh, Dr. Nindl worked for over 20 years as a government scientist working for the US Army Research Institute of Environmental Medicine within the US Army Medical Research and Materiel Command and the Army Institute of Public Health within the US Army Public Health Command.
Dr. Nindl received a BS in biology from Clarkson University in 1989, a MS in physiology of exercise from Springfield College in 1993, a PhD in physiology from The Pennsylvania State University in 1999, and a Master of Strategic Studies from the US Army War College in 2012. His research interests span human performance optimization/injury prevention domains with a focus on adaptations of the neuromuscular and endocrine systems (growth hormone/insulin-like growth factor-I axis) to both exercise and military operational stress. Dr. Nindl is internationally recognized for his work in these areas and was co-chair of the 3rd International Congress on Soldiers’ Physical Performance in 2014 and has performed research sabbaticals at the University of Jyvaskyla in Finland (2009) and the University of Wollongong in Australia (2014).
Dr. Nindl is also an Army Reservist (LTC(P)) having been deployed in 2004-2005 in Mosul, Iraq where he was awarded a Bronze Star and the Combat Action Badge.
At Pitt: Dr. Nindl is director of the Neuromuscular Research Laboratory/Warrior Human Performance Research Center at the University of Pittsburgh. The Warrior Human Performance Research Center has a very specific focus: Helping military members succeed. Its researchers work closely with service members in the U.S. Navy, Army, Air Force and Marine Corps to develop highly technical biological markers of injury risk as well as tools to keep troops healthy and safe during training and combat. Led by Dr. Nindl, the center has more than six studies underway at military sites across the nation. “It is an exciting time for our center, and the University of Pittsburgh plays a pivotal role in our success,” says Dr. Nindl, who serves as vice chair for research in the Department of Sports Medicine and Nutrition in Pitt’s School of Health and Rehabilitation Sciences. “Our researchers are uniquely positioned to excel, and this is because we have all the right ingredients: a culture that drives innovation and collaboration, a world-class core of biomedical expertise, and remarkable partnerships with the United States military.”
Thank you each for your military service and continued research efforts in support of all soldiers.
Dr. Michael Lotze Honored with Lifetime Achievement Award from Society for Immunotherapy of Cancer

McGowan Institute for Regenerative Medicine affiliated faculty member Michael Lotze, MD, was honored with the 2021 Society for Immunotherapy of Cancer (SITC) Lifetime Achievement Award at the SITC 36th Annual Meeting, Nov. 10-14, in Washington D.C. SITC is a member-driven organization dedicated to improving cancer patient outcomes by advancing the science and application of cancer immunotherapy through educational programs that foster scientific exchange and collaboration.
Dr. Lotze is currently professor of surgery, immunology and bioengineering at the University of Pittsburgh School of Medicine and Chief Cellular Therapy Officer of Nurix Therapeutics. He is widely regarded as the leader in exploring cancer as a disorder of cell death and is devising novel strategies to approach the disease in this context. He initiated the first approved gene therapy protocols at the National Institutes of Health and has treated more than 100 patients on gene therapy protocols at the University of Pittsburgh. He is the co-inventor of 10 patents in dendritic cell vaccines and antigen discovery, and author of more than 500 scientific papers and chapters in basic and applied tumor immunology and cytokine biology.
Dr. Lotze also leads the 14 members of the Alliance for Cancer Gene Therapy (ACGT) Scientific Advisory Council Chair Scientific Advisory Council in rigorously reviewing and monitoring the research selected for funding by ACGT. The importance and value that the Council contributes to the ACGT funding process distinguishes ACGT from many other funding agencies. Council members are among the most accomplished thought-leaders in the field of cancer cell and gene therapy. They are experienced scientists whose decades of research and patient care have elevated them into important leadership positions at top institutions across the U.S. and in Canada.
Congratulations, Dr. Lotze!
Dr. Peter Rubin Elected President of the American Society of Plastic Surgeons

The American Society of Plastic Surgeons (ASPS), the world’s largest group of board-certified plastic surgeons, named McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, MBA, FACS, as its new president. Dr. Rubin took office in Atlanta at Plastic Surgery, The Meeting, the Society’s annual scientific meeting, and will serve for one year.
“It’s truly an honor to lead the world’s largest organization of board-certified plastic surgeons at a pivotal time in the history of organized medicine,” Dr. Rubin said. “Throughout history, plastic surgeons have evolved as innovators in medicine. That inventive spirit continues – combined with a strong mission of service and contribution – and is applied to finding solutions that improve patient care across the United States and around the globe.”
Dr. Rubin is chair of the University of Pittsburgh Medical Center Department of Plastic Surgery and the UPMC Endowed Professor of Plastic Surgery, as well as being a professor of bioengineering and director of UPMC wound healing services. He has been a faculty member at the University of Pittsburgh since 2002.
Before being elected president, Dr. Rubin served as the ASPS Vice President of Finance and Treasurer. He is past president of the International Federation for Adipose Therapeutics and Science, for which Dr. Rubin led an international group of scientists worldwide in studying the use of fat stem cells for treating numerous disorders. He served as chair of the Plastic Surgery Research Council, as well as chair of a joint-society task force on the use of stem cells and co-chair of a multi-society task force on safety in gluteal fat grafting. He is a member of several domestic and international medical societies, including serving on the International Society of Plastic Regenerative Surgeons Board of Directors.
Dr. Rubin published a textbook on plastic surgery after weight loss and is lead editor of a multi-author textbook project on body contouring surgery. He has published extensively in the medical literature and received several awards for his research, notably the Presidential Early Career Award for Science and Engineering for his National Institutes of Health-funded work with fat-derived stem cells.
After earning his undergraduate degree in biology from Grinnell College, Dr. Rubin earned his medical degree from Tufts University School of Medicine. He completed a categorical residency training program in general surgery at Boston University/Boston City Hospital. He took time away from the clinic to pursue a two-year fellowship in surgical basic science at Massachusetts General Hospital/Harvard Medical School. After graduating from general surgery residency, he completed a three-year residency in plastic surgery at Harvard Medical School. He is a fellow of the American College of Surgeons.
Congratulations, Dr. Rubin!
Dr. Burhan Gharaibeh Awarded the Discipline-based Science Education Research Leader Award

Eighteen faculty members in the natural sciences departments in the University of Pittsburgh’s Dietrich School of Arts & Sciences were recently awarded the Discipline-based Science Education Research (dB-SERC) Leader Award. McGowan Institute for Regenerative Medicine affiliated faculty member Burhan Gharaibeh, PhD, faculty member in the Department of Biological Sciences and an adjunct faculty member in the Department of Bioengineering at the University of Pittsburgh, is one of the awardees.
This yearly award recognizes and celebrates the contributions of the dB-SERC faculty learning community and their active participation in many dB-SERC events during the last academic year. These faculty members have played a key role in the dB-SERC weekly lunch discussions focusing on innovative approaches to teaching and learning in the natural sciences.
The dB-SERC promotes and supports evidence-based approaches to teaching and learning in the natural sciences. In addition to workshops and special events, the dB-SERC faculty learning community gives the faculty members opportunity to share their course transformation projects involving evidence-based approaches. More information about dB-SERC can be found here.
Congratulations, Dr. Gharaibeh!
Badylak Lab Student Wins Outstanding Student Award from TERMIS-AA

Maddie Cramer, a doctoral student in the Bioengineering Department at the University of Pittsburgh and a team member in the laboratory of Stephen Badylak, DVM, PhD, MD, will receive the Outstanding Student Award from TERMIS-AA. Dr. Badylak is a deputy director of the McGowan Institute for Regenerative Medicine, professor in the Department of Surgery, and director of the Center for Pre-Clinical Tissue Engineering within the Institute.
This honor includes publication of her paper in Tissue Engineering Part A, a $500 honorarium, and an oral presentation of her work at their December Webinar Series. She will receive $1000 travel toward the annual meeting in Toronto next July and be recognized on stage in person for her award.
The title of Ms. Cramer’s paper is “The Influence of Matrix Bound Nanovesicle Associated Interleukin-33 on Macrophage Phenotype.” The abstract follows:
The innate immune response, particularly the phenotype of responding macrophages, has significant clinical implications in the remodeling outcome following implantation of biomaterials and engineered tissues. In general, facilitation of an anti-inflammatory (M2-like) phenotype is associated with tissue repair and favorable outcomes, while pro-inflammatory (M1-like) activation can contribute to chronic inflammation and a classic foreign body response. Biologic scaffolds composed of extracellular matrix (ECM) and, more recently, matrix bound nanovesicles (MBV) embedded within the ECM are known to direct macrophages toward an anti-inflammatory phenotype and stimulate a constructive remodeling outcome. The mechanisms of MBV-mediated macrophage activation are not fully understood, but interleukin-33 (IL-33) cargo within the MBV is required for M2-like activation. Previous work has shown that IL-33 is encapsulated within the lumen of MBV and stimulates phenotypical changes in macrophages independent of its canonical surface receptor ST2. In the present study we used next generation RNA-sequencing to determine the gene signature of macrophages following exposure to MBV with and without IL-33 cargo. MBV-associated IL-33 instructed an anti-inflammatory phenotype in both wildtype and st2-/- macrophages by downregulating M2-like and upregulating M1-like genes. The repertoire of genes regulated by ST2-independent IL-33 signaling were broadly related to the inflammatory response and crosstalk between cells of both the innate and adaptive immune systems. These results signify the importance of the MBV cargo protein IL-33 in stimulating a pro-remodeling M2-like phenotype in macrophages and provides guidance for the designing of next generation biomaterials and tissue engineering strategies.
Congratulations, Ms. Cramer!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#227 –– Dr. George Hussey discusses his research in clinical translation of extracellular matrix (ECM)-based materials for functional tissue reconstruction.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Judy D. Day, Soojin Park, Benjamin L. Ranard, Harinder Singh, Carson C. Chow, and Yoram Vodovotz
Title: Divergent COVID-19 Disease Trajectories Predicted by a DAMP-Centered Immune Network Model
Summary: COVID-19 presentations range from mild to moderate through severe disease but also manifest with persistent illness or viral recrudescence. We hypothesized that the spectrum of COVID-19 disease manifestations was a consequence of SARS-CoV-2-mediated delay in the pathogen-associated molecular pattern (PAMP) response, including dampened type I interferon signaling, thereby shifting the balance of the immune response to be dominated by damage-associated molecular pattern (DAMP) signaling. To test the hypothesis, we constructed a parsimonious mechanistic mathematical model. After calibration of the model for initial viral load and then by varying a few key parameters, we show that the core model generates four distinct viral load, immune response and associated disease trajectories termed “patient archetypes”, whose temporal dynamics are reflected in clinical data from hospitalized COVID-19 patients. The model also accounts for responses to corticosteroid therapy and predicts that vaccine-induced neutralizing antibodies and cellular memory will be protective, including from severe COVID-19 disease. This generalizable modeling framework could be used to analyze protective and pathogenic immune responses to diverse viral infections.
Source: Frontiers in Immunology. 28 October 2021.
GRANT OF THE MONTH
Program Director: James Luketich
Co-PDs: Julie Phillippi and David Vorp
Title: Cardiothoracic Surgery Research Training Program
Description: This application is for a research training grant focused on training the next generation of academic cardiac and thoracic surgeons. Given the current and future shortage of cardiothoracic surgeons, the extremely diverse clinical activities, and broad array of conditions treated by cardiac and thoracic surgeons, an NIH T32 training program in cardiothoracic surgery is an emergent unmet need to improve the cardiothoracic surgical workforce. The necessary resources to support this mission is largely dictated by the capacity for initial research training mid-way through the residency-fellowship training continuum. The proposed program leverages the experience and framework of our successful ACGME accredited six year Integrated Thoracic Training Program, the research and mentoring expertise of our Department of Cardiothoracic Surgery faculty, and the infrastructure of the University of Pittsburgh School of Medicine (UPSOM) and UPMC Health System, to create a new T32 program in cardiothoracic surgery. The proposed T32 program will provide a strong foundation of training in research, serving leadership, and collaborative partnerships during residency training, which will help to establish and promote successful independent career paths in academic cardiothoracic surgery. The proposed T32 program will increase the pool of investigators who will be competitive for independent K- and R- awards as faculty. This program will achieve the following specific goals: 1) Provide a stable and supportive infrastructure of research training for academic cardiothoracic surgeons; 2) Recruit and select promising scholars and monitor their initial, ongoing, and long-term successes; 3) Coordinate faculty mentoring and training of cardiothoracic surgeons to provide a curated and individualized didactic curriculum and hands-on training that specifically addresses the unique concerns of cardiothoracic surgery; 4) Leverage internal UPSOM, UPMC, and other institutional resources in trainee-specific training plans; 5) Promote an inclusive culture at all levels through diversity training and community outreach to recruit under-represented minority candidates to cardiothoracic surgery; 6) Provide structured training in leadership, partnership and research program establishment during and beyond the period of support to position trainees for launching successful independent academic careers. In the long-term, the proposed T32 program will increase the number of academic cardiothoracic surgeons and will help to promote a diverse workforce capable of accomplishing the NHLBI’s mission.
Source: NIH/NHLBI
Term: 2021-2026
Amount: $1,642,437
