May 2020 | VOL. 19, NO. 5| www.McGowan.pitt.edu
McGowan-Developed Technology Helps COVID-19 Patients
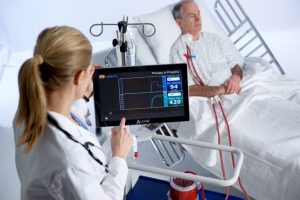
A respiratory dialysis system that employs technologies developed at the McGowan Institute Medical Devices Lab has been used to treat 16 COVID-19 patients, in conjunction with non-invasive or mechanical ventilation. The core technology developed at the Medical Devices Lab, was licensed to the spinout ALung Technologies, and ALung has designed and produced a clinically viable device, which is called the Hemolung® Respiratory Assist System (RAS).
The Hemolung® is a minimally invasive device that does the work of the lungs by removing carbon dioxide directly from the blood, much as a dialysis machine does the work of the kidneys. The Hemolung® can help eliminate damage to the lungs caused by ventilators; with the carbon dioxide removed directly from the blood by the Hemolung, the pressure that the ventilator imposes on the lungs can be reduced, minimizing damage to the lungs.
“Ventilation can cause serious issues in lungs that are already being damaged by the disease itself,” said Dr. Federspiel, Director of the Medical Devices Lab. “The Hemolung® allows the lungs to rest and heal during the ventilation process by allowing for gentler ventilation. It could also prevent certain patients, who have less severe symptoms, from having to go on ventilation in the first place.”
Mechanical ventilation requires patients to be sedated and intubated, and a myriad of complications can arise from the treatment, including collapsed lung, alveolar damage, and ventilator-associated pneumonia. For these more critically ill patients, the Hemolung® could be used to help remove CO2, which would allow the mechanical ventilation process to be done more gently.
The Hemolung® has been in clinical trials in Europe and has received Ce-Mark approval. It is currently in a clinical trial in the U.S., and at this time is not approved for general use in the U.S. However, ALung recently received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration for use of the Hemolung in COVID-19 cases.
Health records from a New York study showed that close to 90 percent of patients who were placed on mechanical ventilation did not survive. Some intensive care units are now considering mechanical ventilation as a last resort because of the complications and side effects associated with the process, and researchers believe the Hemolung device could help. There have been positive outcomes from the 16 COVID-19 patients who were treated with the Hemolung.
Peter DeComo, Chairman and CEO of ALung Technologies, stated, “With published mortality rates as high as 90% for patients receiving invasive mechanical ventilation (IMV), we believe that the Hemolung can be a valuable tool for physicians to be used in conjunction with IMV, by reducing or eliminating the potential of further lung damage caused by high ventilator driving pressures, often referred to as Ventilator Induced Lung Injury. Many of the academic medical centers involved with our clinical trial have already requested the use of the Hemolung RAS for treatment of their COVID-19 patients.”
Created to help chronic obstructive pulmonary disease (COPD) and acute respiratory distress syndrome (ARDS) patients, Hemolung® has already been used on thousands of patients in Europe, where it was approved in 2013, and it is currently in clinical trials in the United States.
“This technology developed by Dr. Federspiel and ALung Technologies is a perfect example of how collaborative research at the McGowan Institute can impact human lives,” said William Wagner, PhD, director of MIRM and professor of surgery, bioengineering and chemical engineering at Pitt. “A clinical viewpoint is necessary, but medical training doesn’t give you an engineer’s perspective of design and manufacturing. You need a solid foot in both camps to make progress.”
Listen to the Regenerative Medicine Podcast, where Dr. Federspiel and Mr. DeComo discuss the Hemolung® and the use of this device in treating COVID-19 patients.
View a Hemolung application in the fight against COVID-19, presented by WPBF News.
View a Hemolung application in the fight against COVID-19, presented by Palm Beach Gardens Medical Center.
View a Hemolung application in the fight against COVID-19, presented by Pittsburgh Technology Council.
Illustration: Hemolung RAS. ALung Technologies, Inc.
RESOURCES AT THE MCGOWAN INSTITUTE
Histology Laboratory Current Procedures
 The histology lab is still able to accept samples for ongoing research.
The histology lab is still able to accept samples for ongoing research.
If you are coming from a building other than Bridgeside Point II: We ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you at the front door to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
If you have access to the Bridgeside Point II Third Floor: We have a cart outside of the laboratory where you can drop off and pick up samples. Please email us if you leave samples on the cart.
An electronic version of our order form can be found at: www.mirm.pitt.edu/badylak/histology.asp. Please feel free to reach out to Julia Hart at hartj5@upmc.edu to discuss your histology needs.
Save the Date!
9th International Symposium on Regenerative Rehabilitation
 With a theme of “Where Applied Biophysics Meets Tissue Engineering & Cellular Therapies”, the Symposium is scheduled for December 3-5, 2020 at the University of Texas at Austin. The program features renowned researchers and clinicians from around the world, focusing on the emerging field of Regenerative Rehabilitation. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
With a theme of “Where Applied Biophysics Meets Tissue Engineering & Cellular Therapies”, the Symposium is scheduled for December 3-5, 2020 at the University of Texas at Austin. The program features renowned researchers and clinicians from around the world, focusing on the emerging field of Regenerative Rehabilitation. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
SCIENTIFIC ADVANCES
Potential Portable Individual Biocontainment Unit to Reduce COVID-19 Transmission
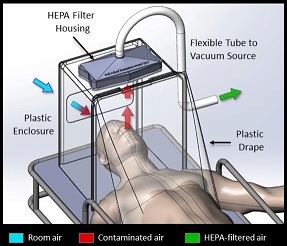
A multidisciplinary team of researchers and clinicians, including McGowan Institute for Regenerative Medicine faculty member Peter Rubin, MD, FACS, Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh, is working on a low-cost negative pressure hood that can be used to protect medical staff during intubations and other situations where aerosol spray is likely from infected patients. Moreover, it can also help prevent spread in environments without a negative pressure room, such as the Emergency Department of a hospital. This apparatus is different and far advanced compared with static protection boxes which are only passive shields. What makes this new hood most remarkable is its active airflow and HEPA filtering.
The device is the brainchild of David Turer, MD, MS, a chief resident in the Department of Plastic Surgery who is also an engineer. The rough prototype is made with hardware store parts and 3D printed parts specifically designed for this application. This device can be manufactured in large numbers very quickly with injection molding and stock parts.
The team is very close to a “field ready” version and McGowan Institute for Regenerative Medicine affiliate faculty member Ron Poropatich, MD, Director of the Center for Military Medicine Research, Health Sciences and Professor of Medicine in the Division of Pulmonary, Allergy, and Critical Care Medicine at the University of Pittsburgh, and a Senior Advisor for Telemedicine, UPMC, is helping with outreach to the U.S. Department of Defense. The military uses are evident, with ability to contain spread of an outbreak in close quarters environments such as an aircraft carrier. Cameron Good, PhD, one of the team members, is a scientist in an army laboratory.
Other notable members of the team include Benjamin Schilling, MS, Robert Turer, MD, MSE, Lucas Dvoracek, MD, Heng Ban, PhD, and Jason Chang, MD.
Illustration: Portable Individual Biocontainment Unit. David Turer, MD, MS.
Using a Smartphone to Diagnose COVID-19 at Home

In Pennsylvania and other U.S. states, one of the keys to safely reopening society amid the COVID-19 pandemic is providing sufficient testing so that new cases of the disease do not overwhelm the public healthcare system. University of Pittsburgh professors—led by McGowan Institute for Regenerative Medicine affiliated faculty member Wei Gao, PhD—are reimagining testing using a device that nearly every American owns — a smartphone.
Using the existing hardware and computing power of commodity smartphones, the team’s project aims to perform non-invasive at-home testing for COVID-19 infection, and it was selected for funding by the National Science Foundation through its RAPID award program in response to the COVID-19 pandemic.
The goal of this work is to provide an easy and low-cost way to monitor and diagnose a large population without the need for special equipment or the involvement of clinicians. It can ultimately be applied to other acute or chronic respiratory diseases, in addition to the novel coronavirus.
“In this project, we will develop new mobile sensing and artificial intelligence techniques for in-home evaluation of COVID-19 in an effort to quickly and effectively identify viral disease carriers,” said Dr. Gao, associate professor of electrical and computer engineering at Pitt’s Swanson School of Engineering.
“We hope this work will also help identify negative cases caused by other diseases with similar symptoms, and therefore, help eliminate unnecessary hospital visits during this pandemic,” he said.
Dr. Gao and his team will build upon smartphones’ microphones and speakers to develop acoustic sensing that can measure changes in human airway mechanics, which are uniquely correlated to COVID-19 infection.
“We will begin by designing new acoustic waveforms to minimize acoustic signal distortion in human airways,” Dr. Gao said. “We will then develop new signal processing techniques for accurate measurements and eventually apply deep learning techniques to create generic models that depict the core characteristics of airway mechanics.”
The system will be implemented as a smartphone app that a user can easily download, install, and operate. Users will need to use a smartphone adapter as a mouthpiece so that the phone’s microphone and speaker can transmit and record acoustic signals from human airways.
If successful, this research will help accurately identify cases of COVID-19 without a visit to the hospital, which could subsequently help contain the spread of the highly contagious virus.
This project is a collaborative effort with Heng Huang, PhD, John A. Jurenko Endowed Professor of Electrical and Computer Engineering at Pitt, and clinicians at the University of Pittsburgh Medical Center Children’s Hospital.
Old Human Cells Rejuvenated with Stem Cell Technology

Old human cells return to a more youthful and vigorous state after being induced to briefly express a panel of proteins involved in embryonic development, according to a new study by researchers at the Stanford University School of Medicine. Study co-author Thomas Rando, MD, PhD, professor of neurology and neurological sciences and the director of Stanford’s Glenn Center for the Biology of Aging, is an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
The researchers also found that elderly mice regained youthful strength after their existing muscle stem cells were subjected to the rejuvenating protein treatment and transplanted back into their bodies.
The proteins, known as Yamanaka factors, are commonly used to transform adult cells into induced pluripotent stem cells, or iPS cells. Induced pluripotent stem cells can become nearly any type of cell in the body, regardless of the cell from which they originated. They’ve become important in regenerative medicine and drug discovery.
The study found that inducing old human cells in a lab dish to briefly express these proteins rewinds many of the molecular hallmarks of aging and renders the treated cells nearly indistinguishable from their younger counterparts.
“When iPS cells are made from adult cells, they become both youthful and pluripotent,” said Vittorio Sebastiano, PhD, assistant professor of obstetrics and gynecology and the Woods Family Faculty Scholar in Pediatric Translational Medicine. “We’ve wondered for some time if it might be possible to simply rewind the aging clock without inducing pluripotency. Now we’ve found that, by tightly controlling the duration of the exposure to these protein factors, we can promote rejuvenation in multiple human cell types.”
Dr. Sebastiano is the senior author of the study, which was published in Nature Communications. Former graduate student Tapash Sarkar, PhD, is the lead author of the article.
“We are very excited about these findings,” said Dr. Rando. “My colleagues and I have been pursuing the rejuvenation of tissues since our studies in the early 2000s revealed that systemic factors can make old tissues younger. In 2012, Howard Chang and I proposed the concept of using reprogramming factors to rejuvenate cells and tissues, and it is gratifying to see evidence of success with this approach.” Howard Chang, MD, PhD, is a professor of dermatology and of genetics at Stanford.
Exposure to proteins
Researchers in Dr. Sebastiano’s laboratory make iPS cells from adult cells, such as those that compose skin, by repeatedly exposing them over a period of about two weeks to a panel of proteins important to early embryonic development. They do so by introducing daily, short-lived RNA messages into the adult cells. The RNA messages encode the instructions for making the Yamanaka proteins. Over time, these proteins rewind the cells’ fate — pushing them backward along the developmental timeline until they resemble the young, embryonic-like pluripotent cells from which they originated.
During this process the cells not only shed any memories of their previous identities, but they revert to a younger state. They accomplish this transformation by wiping their DNA clean of the molecular tags that not only differentiate, say, a skin cell from a heart muscle cell, but of other tags that accumulate as a cell ages.
Recently researchers have begun to wonder whether exposing the adult cells to Yamanaka proteins for days rather than weeks could trigger this youthful reversion without inducing full-on pluripotency. In fact, researchers at the Salk Institute for Biological Studies found in 2016 that briefly expressing the four Yamanaka factors in mice with a form of premature aging extended the animals’ life span by about 20%. But it wasn’t clear whether this approach would work in humans.
Drs. Sarkar and Sebastiano wondered whether old human cells would respond in a similar fashion, and whether the response would be limited to just a few cell types or generalizable for many tissues. They devised a way to use genetic material called messenger RNA to temporarily express six reprogramming factors — the four Yamanaka factors plus two additional proteins — in human skin and blood vessel cells. Messenger RNA rapidly degrades in cells, allowing the researchers to tightly control the duration of the signal.
The researchers then compared the gene-expression patterns of treated cells and control cells, both obtained from elderly adults, with those of untreated cells from younger people. They found that cells from elderly people exhibited signs of aging reversal after just four days of exposure to the reprogramming factors. Whereas untreated elderly cells expressed higher levels of genes associated with known aging pathways, treated elderly cells more closely resembled younger cells in their patterns of gene expression.
When the researchers studied the patterns of aging-associated chemical tags called methyl groups, which serve as an indicator of a cell’s chronological age, they found that the treated cells appeared to be about 1½ to 3½ years younger on average than untreated cells from elderly people, with peaks of 3½ years (in skin cells) and 7½ years (in cells that line blood vessels).
Comparing hallmarks of aging
Next, they compared several hallmarks of aging — including how cells sense nutrients, metabolize compounds to create energy and dispose of cellular trash — among cells from young people, treated cells from old people and untreated cells from old people.
“We saw a dramatic rejuvenation across all hallmarks but one in all the cell types tested,” Dr. Sebastiano said. “But our last and most important experiment was done on muscle stem cells. Although they are naturally endowed with the ability to self-renew, this capacity wanes with age. We wondered, ‘Can we also rejuvenate stem cells and have a long-term effect?’”
When the researchers transplanted old mouse muscle stem cells that had been treated back into elderly mice, the animals regained the muscle strength of younger mice, they found.
Finally, the researchers isolated cells from the cartilage of people with and without osteoarthritis. They found that the temporary exposure of the osteoarthritic cells to the reprogramming factors reduced the secretion of inflammatory molecules and improved the cells’ ability to divide and function.
The researchers are now optimizing the panel of reprogramming proteins needed to rejuvenate human cells and are exploring the possibility of treating cells or tissues without removing them from the body.
“Although much more work needs to be done, we are hopeful that we may one day have the opportunity to reboot entire tissues,” Dr. Sebastiano said. “But first we want to make sure that this is rigorously tested in the lab and found to be safe.”
Let’s Do the Twist
The twisting and bending capabilities of the human muscle system enable a varied and dynamic range of motion, from walking and running to reaching and grasping. Replicating something as seemingly simple as waving a hand in a robot, however, requires a complex series of motors, pumps, actuators and algorithms. Researchers at the University of Pittsburgh and Harvard University have recently designed a polymer known as a liquid crystal elastomer (LCE) that can be “programmed” to both twist and bend in the presence of light.
The research, published in the journal Science Advances, was developed at Pitt’s Swanson School of Engineering by McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical and Petroleum Engineering and John A. Swanson Chair of Engineering; and James Waters, postdoctoral associate and the paper’s first author. Other researchers from Harvard University’s Wyss Institute for Biologically Inspired Engineering and the John A. Paulson School of Engineering include Joanna Aizenberg, PhD, Michael Aizenberg, PhD, Michael Lerch, Shucong Li and Yuxing Yao.
These particular LCEs are achiral: the structure and its mirror image are identical. This is not true for a chiral object, such as a human hand, which is not superimposable with a mirror image of itself. In other words, the right hand cannot be spontaneously converted to a left hand. When the achiral LCE is exposed to light, however, it can controllably and reversibly twist to the right or twist to left, forming both right-handed and left-handed structures.
“The chirality of molecules and materials systems often dictates their properties,” Dr. Balazs explained. “The ability to dynamically and reversibly alter chirality or drive an achiral structure into a chiral one could provide a unique approach for changing the properties of a given system on-the-fly.” To date, however, achieving this level of structural mutability remains a daunting challenge. Hence, these findings are exciting because these LCEs are inherently achiral but can become chiral in the presence of ultraviolet light and revert to achiral when the light is removed.”
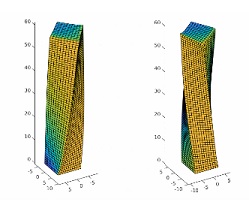
The researchers uncovered this distinctive dynamic behavior through their computer modeling of a microscopic LCE post anchored to a surface in air. Molecules (the mesogens) that extend from the LCE backbone are all aligned at 45 degrees (with respect to the surface) by a magnetic field; in addition, the LCEs are cross-linked with a light-sensitive material. “When we simulated shining a light in one direction, the LCE molecules would become disorganized and the entire LCE post twists to the left; shine it in the opposite direction and it twists to the right,” Dr. Waters described. These modeling results were corroborated by the experimental findings from the Harvard group.
Going a step further, the researchers used their validated computer model to design “chimera” LCE posts where the molecules in the top half of the post are aligned in one direction and are aligned in another direction in the bottom half. With the application of light, these chimera structures can simultaneously bend and twist, mimicking the complex motion enabled by the human muscular system.
“This is much like how a puppeteer controls a marionette, but in this instance the light serves as the strings, and we can create dynamic and reversible movements through coupling chemical, optical, and mechanical energy,” Dr. Balazs said. “Being able to understand how to design artificial systems with this complex integration is fundamental to creating adaptive materials that can respond to changes in the environment. Especially in the field of soft robotics, this is essential for building devices that exhibit controllable, dynamic behavior without the need for complex electronic components.”
Illustration: A simulated LCE micropost with the nematic director oriented at 45 degrees relative to the flat surface. Illuminating one side of the post induces the top of the post to twist relative to the fixed base. Illuminating the opposite face of the post results in a twist in the opposite direction. Color indicates the regions of the post that are illuminated (yellow) or in shadow (blue). (Balazs Lab)
UPMC Close to Immunity Test that Could Help End COVID-19 Lockdown
As reported by David Templeton for the Pittsburgh Post-Gazette, UPMC researchers are close to completing a blood test to determine immunity against the virus that causes COVID-19. Such a test could certify little or no risk of SARS-CoV-2 infection and identify the so-called “super” people who could serve in the health-care battle against COIVD-19 with little risk to themselves.
“We are developing an antibody test based on a European test to see if people were exposed and might be immune,” said Alan Wells, MD, executive vice-chairman of the Section of Laboratory Medicine in the UPMC Department of Pathology and faculty member of the McGowan Institute for Regenerative Medicine. He led the research effort that also involved Drs. Michael Shurin and Sarah Wheeler.
Medical tests already can determine the presence of the three key antibodies that the immune system generates in response to the virus. But their presence alone doesn’t mean the person cannot become ill with COVID-19 and transmit it to others.
“Those [European] tests show that the person was exposed but don’t say in and of themselves if the person is immune,” Dr. Wells said. “There is no clear test for immunity at this point.”
Tests to determine exposure and infection presented their own challenges and aren’t yet 100% determinant, given that a more advanced infection that might be detected only in the lungs rather than nose or throat.
“We need detection to see who’s immune right now, but there are more uncertainties in testing for immunity than there are for testing for the virus,” he said.
Three antibodies are involved in the immune response: Two protective antibodies — IgG and IgA — and, IgM, that reacts to the virus but doesn’t attack it.
“The tests out there now only detect when the person is exposed and not yet immune,” he said. “We are developing an IgG and IgA antibody test developed from a European test, and validated here to determine protection, to see if people exposed to the virus might be immune to it.”
A statement from UPMC explains where they currently are with testing:
UPMC is exploring the use of blood tests to determine if a person has antibodies to SARS-CoV-2, the virus that causes COVID-19. Such a test could indicate if a person already had infection, perhaps without symptoms, and recovered. However, at present there is little guidance on what it means if someone has antibodies. These can be found in people with ongoing disease, and those who have recovered. Furthermore, the current tests do not determine if a person is immune to the disease.
Please rest assured that we understand the value of such antibody-detection tests — for community surveillance, epidemiological tracing and individual diagnosis, among others. These will be more valuable when we learn more about the implications of the positives. We are working hard to find ways to make limited supplies of this test as valuable to our communities as possible.
We do not have the capabilities to test the general public at this time. We are still determining the meaning of the tests. In addition, we expect that supplies will continue to be extremely limited. Once available, it will likely be used in conjunction with various local and national clinical trials that UPMC is organizing or participating in.
Illustration: Coronavirus, COVID-19. CDC.
UPMC Children’s Receives FCC COVID-19 Telehealth Program Grant to Help Immunocompromised Children

The Federal Communications Commission (FCC) has awarded the Hillman Center for Pediatric Transplantation at UPMC Children’s Hospital of Pittsburgh with one of the first six grants from the COVID-19 Telehealth Program Fund — and the first to a children’s hospital.
The grant, totaling $192,500, will provide telehealth services to organ transplant pediatric patients who are immunocompromised and who are at an increased risk for getting COVID-19.
“During this unprecedented time, the only follow-up option for our at-risk and most vulnerable patients are remote visits,” said George Mazariegos, MD, chief of pediatric transplantation, UPMC Children’s, and affiliated faculty member of the McGowan Institute for Regenerative Medicine. “With this grant from the FCC, we can now provide an immediate solution to solving the challenges of the COVID-19 pandemic for the chronic care condition of pre- and post-transplant care.”
There are nearly 1,000 post-transplant patients that UPMC Children’s treats on a regular basis and many are from out-of-state. This can make it very difficult during the COVID-19 pandemic to be able to make on-site follow-up appointments. Ramping up telemedicine services is vital in keeping these patients healthy and safe.
In partnership with Sano Health and RealTime Clinic, Inc., UPMC Children’s will now receive 400 mobile devices with data connectivity, adherence apps and post-transplant educational materials for families.
“The Starzl Network for Pediatric Transplantation and its ability to connect and leverage industry partners really helped the team at UPMC Children’s to rapidly respond to this opportunity and maintain the highest continuity of care for our most vulnerable transplant patients,” added Dr. Mazariegos.
“We are delighted to partner with UPMC Children’s to enable children with organ transplants to receive much needed care through telehealth without the risk of COVID-19 infection,” said Evan Grayer, co-founder of Sano Health. “Positively influencing health outcomes for an at-risk population by leveraging wireless solutions is at the heart of Sano Health’s mission, and we are proud to be associated with the pediatric transplant program at UPMC Children’s.”
“We are thrilled to work with UPMC Children’s to provide a critical telehealth app to share patient educational material and patient-reported measures such as quality-of-life surveys to transplant families,” said Nehal Swami, founder & CEO, RealTime Clinic, Inc. “We look forward to this partnership ensuring continuity of care to at-risk children via telehealth during the COVID-19 crisis.”
The first six grants are just a portion of the $200 million the FCC will distribute through the program through the CARES Act. Other hospitals that received funding include Grady Memorial Hospital, Hudson River HealthCare, Inc., Mount Sinai Health System, Neighborhood Health Care, Inc., and Ochsner Clinic Foundation.
Drs. Alan Wells and Louis Falo Receive Clinical and Translational Science Institute Awards to Support Urgent COVID-19 Research

The Clinical and Translational Science Institute (CTSI) at the University of Pittsburgh has awarded $900,000 to 17 research projects to address different aspects of the COVID-19 pandemic. McGowan Institute for Regenerative Medicine affiliated faculty members Alan Wells, MD, DMSc, and Louis Falo, MD, PhD, will serve as co-principal investigators on two of the funded projects. Their projects and funding are:
- $100,000 Award: SARS-CoV-2 Clinical and Community Serosurveillance, Principal Investigators (co-PIs) Paul Duprex, PhD, Anita McElroy, MD, PhD, and Alan Wells, MD, DMSc
- $50,000 Award: Generation of Transgenic hACE2 Knock-in Mice, co-PIs Andrea Gambotto, MD, Louis Falo, MD, PhD, Mark Shlomchik, MD, PhD, and William Klimstra, PhD
The COVID-19 Pilot Grant Program was launched at the end of March in response to the novel coronavirus pandemic with the intent to support new research initiatives that will make immediate progress toward reducing the harm to individuals, groups and society from COVID-19.
“We need to look at all options to deal with the COVID-19 pandemic, and the response to the call for proposals was overwhelming,” said Steven Reis, MD, director of the CTSI, associate vice chancellor for clinical research, health sciences, and distinguished service professor of medicine at the University of Pittsburgh. “As encouraged as we were with the number, we were even more impressed with the thinking that went into the proposals from so many different parts of the university, and we are optimistic that the impact of research will provide insight and results. We are proud to be part of this thriving research community that has shown how Pittsburgh really steps up when called upon.”
Funding for the projects was provided by the CTSI, the Pitt Office of the Provost, the Pitt Office of the Senior Vice Chancellor for Research and the DSF Charitable Foundation, which contributed $350,000. Support for the health sciences is one of the core funding priorities of the foundation.
“These grants represent the best of research — creative minds working in collaboration with partners to innovate for the benefit to society,” said Rob A. Rutenbar, senior vice chancellor for research. “It’s an honor to help support such vital investigation.”
DSF Charitable Foundation Executive Director Nick Beldecos added: “This obviously time-sensitive research is critically important. We are confident that the broad and deep expertise Pitt is bringing to bear in the fight against COVID-19 will produce significant and timely advances.”
More than 150 proposals were originally submitted from 590 investigators covering 14 schools and 91 departments. Each group submitting a proposal needed at least one team member from Pitt. Forty-six different universities were represented across the teams. The proposals were put through an accelerated and extensive peer-review process before the awardees were selected.
Engineering a New Model for Respiratory Infection Treatment

When a person contracts a respiratory viral infection like COVID-19 or influenza, the immune system responds in a myriad of ways to eliminate the virus. Respiratory viral infections are so dangerous, however, because excessive immune responses may cause extreme lung inflammation. However, new modeling research may help doctors better predict and treat patients who are most at risk to that extreme response.
McGowan Institute for Regenerative Medicine affiliated faculty member Jason Shoemaker, PhD, assistant professor of chemical engineering at the University of Pittsburgh’s Swanson School of Engineering, believes engineering-based mathematical modeling can help clinicians understand why some people’s immune systems react so severely, predicting the risk factors and pinpointing the most effective treatments to reduce inflammation.
The National Science Foundation granted Dr. Shoemaker a CAREER Award for $547,494 over five years to create computational models of the immune response to seasonal, deadly (avian) influenza viruses, which can help identify the best way to suppress immune activity and reduce tissue inflammation. Since this work targets the immune system and not the specific virus, the models are expected to impact many respiratory infections, including COVID-19
“The immune system is a complex, interactive, dynamic system. Its goal is to clear the infection while minimizing collateral damage to the lungs and other organs in the process. But when it comes to respiratory infections, it’s been known that your immune response can do more damage than it should,” says Dr. Shoemaker. “Engineering-based mathematical modeling approaches are ideal for simulating such a complex system and predicting the system’s response to viral infections and treatment.”
Even outside of the current pandemic, respiratory virus infections are a constant threat to public health. Seasonal influenza can result in up to 700,000 hospitalizations and 56,000 deaths in the United States. Dr. Shoemaker’s models will enable researchers to uncover the biochemical markers that lead to excessive immune responses in respiratory infections and will help identify the best method for suppressing immune activity in those cases.
In addition to this research, Dr. Shoemaker and his team will develop virtual reality (VR) games to teach the public about the immune system.
“Our computational work is not tangible, and it’s hard to engage our community with something they can’t see or touch,” says Dr. Shoemaker. “The idea behind our VR games is to create a virtual environment to allow someone to dive in and observe the chemical behaviors of the immune system, seeing up close how they can lead to a dysregulation of the immune system and severe disease.”
The award, titled “CAREER: Enabling Immunomodulatory Treatment of Influenza Infection using Multiscale Modeling,” began on May 1, 2020. The NSF CAREER award program honors “early-career faculty who have the potential to serve as academic role models in research and education and to lead advances in the mission of their department or organization.”
Carnegie Mellon, Pitt Researchers Launch Ventilator Project

Researchers at Carnegie Mellon University (CMU) and the University of Pittsburgh School of Medicine are developing a new, low-cost ventilator they say will address the ventilator shortage, both now and in the future, that has been made evident by the COVID-19 pandemic. The team includes McGowan Institute for Regenerative Medicine affiliated faculty member Keith Cook, PhD, a CMU professor of biomedical engineering. Dr. Cook, of CMU’s Biomedical Engineering Department, has expertise in advanced respiratory support, including the design and development of artificial lungs, liquid ventilation hardware and techniques, and animal models of lung disease.
Dubbed Roboventilator, the device will employ CMU-developed robotic technologies and advanced sensors, filling the gap between the expensive sophisticated mechanical ventilators used in intensive care units and the current low-cost alternatives with limited capabilities being approved emergently by the Food and Drug Administration.
“We’ve already developed robotic and sensor technology that can detect force even as it drives an air pump,” said Howie Choset, PhD, professor of robotics at CMU. “When that is paired with air-management controls developed by Dr. Cook, we believe we can build a closed-loop system that can provide customized and appropriate ventilation to people with respiratory failure from COVID-19.”
Drs. Choset and Cook are working with pulmonary and critical care physician Jason Rose, MD, MBA, an assistant professor of medicine and bioengineering at Pitt, to develop the Roboventilator. Working with CMU’s existing manufacturing partner Foxconn, the team is designing the ventilator with current global supply-chain limits in mind. If there is a significant disruption in the supply of one of the critical device components, the team already has identified alternatives that are available or easily fabricated.
The researchers are pursuing sponsors for the effort and, in the meantime, have begun a crowdfunding campaign to get the project underway.
“All of us are aware that ventilators are essential for treatment of the patients who are most seriously ill from COVID-19, and we know that this pandemic will be with us for a long time, with the potential for several waves of disease,” Dr. Choset said. “We are working to make the Roboventilator available in time to help meet these great needs and prepare for the worst with COVID-19.
“We’re convinced the need for low-cost, easily deployable, fully functional ventilators is not limited to just this moment or just this disease,” he added. “This is a machine that could save lives from emerging pathogens we haven’t even encountered yet or help provide a care option to patients in resource-stretched health systems around the world.”
The Roboventilator employs a modular design with a relatively low number of parts assembled with a high degree of automation. The team estimates it would take about an hour to assemble each unit and the cost could be between $500 and $750 each.
Dr. David Gau Receives National Cancer Center Fellowship for Kidney Cancer Research

According to the American Cancer Society, kidney cancer is among the top ten most common cancers in men and women. More than 73,000 new cases and nearly 15,000 deaths are predicted for in the US for 2020. Clear cell renal cell carcinoma (ccRCC) — the most common subtype of tumor associated with kidney cancer — accounts for more than 75 percent of cases.
David Gau, PhD, a postdoctoral researcher at the University of Pittsburgh, will use a fellowship from the National Cancer Center to study the role of a protein in ccRCC progression. He will work with McGowan Institute for Regenerative Medicine affiliated faculty member Partha Roy, PhD, associate professor of bioengineering in the Swanson School of Engineering, and Walter Storkus, PhD, professor of dermatology and immunology in the School of Medicine. Dr. Gau worked as a Postdoctoral Associate in the Cell Migration Laboratory of Dr. Roy.
“Twenty to thirty percent of patients with clear cell renal cell carcinoma will have cancer metastasis by the time of diagnosis and a third of those treated will have recurrence,” said Dr. Gau. “Our lab wants to look at the underlying mechanisms associated with this disease so that we can help develop more effective treatments.”
A common theme of this type of cancer is the highly vascularized nature of the tumor environment, that is, an abundance of blood vessels in the tumor area. In this project, the research group will look at how to control a process called angiogenesis – the formation of new blood vessels.
“Anti-angiogenic treatments to limit vessel formation in the tumor initially work well in patients, but many will have cancer progression due to innate resistant mechanisms to current anti-angiogenic agents,” explained Dr. Gau. “We want to evaluate the role of the protein Profilin1 in ccRCC progression.”
According to Dr. Gau, current research suggests that increased Profilin1 expression in ccRCC is correlated with poor patient prognosis, and preliminary data suggests that it plays a key role in vessel formation, which would make it a candidate for a potential new therapeutic target.
“Our lab has previously developed Profilin1 inhibitors, which will also be tested as a potential therapy for kidney cancer,” he said. “Completion of this project would demonstrate direct impact of Profilin1 and regulation of vessel formation in clear cell renal cell carcinoma and provide foundational evidence for targeting Profilin1 as a potential treatment for kidney cancer.”
Illustration: University of Pittsburgh Swanson School of Engineering.
AWARDS AND RECOGNITION
Dr. Ivet Bahar Elected to the National Academy of Sciences

McGowan Institute of Regenerative Medicine affiliated faculty member Ivet Bahar, PhD, is among the exceptional scientists elected this year to the National Academy of Sciences (NAS). NAS membership is a widely accepted mark of excellence in science and is considered one of the highest honors that a scientist can receive.
Dr. Bahar is a Distinguished Professor and the founding John K. Vries Chair of the Department of Computational and Systems Biology (CPCB), University of Pittsburgh School of Medicine; and co-founder of an internationally-acclaimed PhD program in Computational Biology, CPCB, jointly offered by the University of Pittsburgh and Carnegie Mellon University.
Dr. Bahar has been elected “in honor of outstanding contributions to computational biology.” She is a pioneer in structural and computational biology, having developed widely used elastic network models for protein dynamics. These models reveal cooperative motions that are intrinsically favored by 3D protein architectures to allow substrate-binding, allosteric regulation, and supramolecular machinery, thus computationally bridging structure and function.
Dr. Bahar adapted fundamental theories and methods of polymer statistical mechanics to biomolecular structure and dynamics. She pioneered a modified version of the classical Rouse model, to examine the collective dynamics of proteins modeled as elastic network models (ENMs). ENMs have three strengths: simplicity, ability to yield a unique solution for each structure, and efficient applicability to supramolecular complexes/assemblies. Her theory and methods have withstood numerous tests since their inception, and established fundamental concepts in molecular biology: the role of entropy-driven fluctuations defined by 3D contact topology in optimizing biomolecular interactions; the evolutionary pressure for robustly maintaining structural dynamics to support flexible mechanisms of actions – not only structure to ensure stability; the ability of proteins to exploit their structure-encoded dynamics to adapt to promiscuous interactions and mutations as demonstrated in numerous applications, including neurotransmitter transporters in recent years. Recent application to chromosomal dynamics provided insights into the physical basis of gene co-expression and regulation events.
NAS is a private, non-profit society of distinguished scholars established in 1863, which aims to provide independent, objective advice to the nation on matters related to science and technology. Approximately 500 current and deceased members of the NAS have won Nobel Prizes. This year’s election of 120 members and 26 international members brings the total number of active NAS members to 2403, and the total number of international members to 501. Members of NAS are elected at the annual meeting in April in recognition of their distinguished and continuing achievements in original research.
Controlled Release Society Elects McGowan Institute’s Steven Little, PhD, to Prestigious College of Fellows

The world’s leading organization for delivery science and technology has recognized McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, with election to its College of Fellows. The Controlled Release Society (CRS) elevated Dr. Little, the William Kepler Whiteford Endowed Professor and Chair of the Department of Chemical and Petroleum Engineering at the University of Pittsburgh’s Swanson School of Engineering, for outstanding and sustained contributions to the field of delivery science and technology over a minimum of ten years.
“This year’s class of CRS Fellows is exceptional. Dr. Little is recognized for his impressive scholarly contributions to the literature, technologies based on his discoveries that are poised to make a clinical impact, and exemplary service to the CRS in building the CRS Focus Groups, and serving as its inaugural director. He is a highly respected leader in the field,” noted Justin Hanes, PhD, CRS President and the Lewis J. Ort Professor and Director of the Center for Nanomedicine at the Wilmer Eye Institute, Johns Hopkins University School of Medicine.
Dr. Little’s novel drug delivery systems mimic the body’s own mechanisms of healing and resolving inflammation, allowing for dosages millions of times smaller than current treatments. These systems need only be applied once and then are released over a period of days or months, depending on the medication. This year, Dr. Little published groundbreaking research revealing a new immunotherapy system that mimics how cancer cells invade the human immune system to reduce the risk of transplant rejection.
Dr. Little has also made advancements to the fundamentals of delivery science with predictive models enabling rational design of drug delivery systems and leading to the founding of Qrono, Inc, a specialty pharma company in Pittsburgh, PA.
“The CRS is an amazing, international organization that is led by many of the world’s leading scientists advancing the field of drug delivery. Election to Fellow in this organization is only possible because of the many contributions by our students, postdoctoral researchers, and our collaborators at the University of Pittsburgh,” said Dr. Little. “It’s extremely humbling to me personally, because the Fellows of the CRS are people that have mentored me, and those who I have admired for my entire career.”
Dr. Little received his PhD in Chemical Engineering from Massachusetts Institute of Technology in 2005, with his thesis winning the American Association for Advancement of Science’s Excellence in Research Award.
Researchers in Dr. Little’s Lab focus upon therapies that are biomimetic and replicate the biological function and interactions of living entities using synthetic systems. Areas of study include bioengineering, chemistry, chemical engineering, ophthalmology, and immunology, and the health issues addressed include autoimmune disease, battlefield wounds, cancer, HIV, ocular diseases, and transplantation. Dr. Little currently has 10 provisional, 2 pending, and 5 issued patents.
Dr. Little has been recognized by national and international awards including the Curtis W. McGraw Research Award from the ASEE, being elected as a fellow of the BMES and AIMBE, a Carnegie Science Award for Research, the Society for Biomaterials’ Young Investigator Award, the University of Pittsburgh’s Chancellor’s Distinguished Research Award, being named a Camille Dreyfus Teacher Scholar, being named an Arnold and Mabel Beckman Young Investigator, and being elected to the Board of Directors of the Society for Biomaterials. In addition, Dr. Little’s exceptional teaching and leadership in education have also been recognized by both the University of Pittsburgh’s Chancellor’s Distinguished Teaching Award and a 2nd Carnegie Science Award for Post-Secondary Education. Dr. Little was also recently named one of Pittsburgh Magazine’s 40 under 40, a “Fast Tracker” by the Pittsburgh Business Times, and also one of only five individuals in Pittsburgh who are “reshaping our world” by Pop City Media.
Congratulations, Dr. Little!
Amrita Sahu, PhD, Receives the Delta Omega Dissertation Award

Amrita Sahu, PhD, is the recipient of this year’s Delta Omega Dissertation Award. This award is presented by the Omicron Chapter of Delta Omega, the National Honors Society in Public Health.
Dr. Sahu graduated with a PhD from the Department of Environmental and Occupational Health, School of Public Health at the University of Pittsburgh under the guidance of McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, MPT, Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine and Rehabilitation at the University of Pittsburgh. During this time, she studied the role of an anti-aging protein, Klotho, in the healthy aging of skeletal muscle. Her goal was to identify this protein as a potential therapeutic target for enhancing skeletal muscle healing capacity of geriatric population by enhancing skeletal muscle mitochondrial health.
Currently, Dr. Sahu is working as a Researcher in the Department of Physical Medicine and Rehabilitation at the University of Pittsburgh. Her primary goal is to develop customizable rehabilitation protocols for individuals based on circulating biomarkers in extracellular vesicles.
Her interests lie in the fields of translational science, quantum biology, regenerative and rehabilitative medicine, biotechnology, tissue engineering, biomaterials science and drug delivery.
Congratulations, Dr. Sahu!
Illustration: Dr. Amrita Sahu.
Dr. Thomas Rando Elected to the American Academy of Arts and Sciences

McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Rando, MD, PhD, Professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, is among the 276 scientists, scholars, artists and leaders elected to the American Academy of Arts and Sciences in 2020.
The academy, one of the country’s most prestigious honorary societies, is a center for independent policy research. Members contribute to academy-led studies in a variety of fields, including science policy, global security, social policy and education.
Dr. Rando is the director of the Glenn Center for the Biology of Aging at Stanford and of the Rehabilitation Research and Development Center of Excellence at the Veterans Affairs Palo Alto Health Care System. His research focuses on understanding the biological signals that activate stem cells in response to injury or other environmental cues, particularly in the context of aging. He is also the deputy director of the Stanford Center on Longevity and is a member of Stanford Bio-X, the Stanford Cardiovascular Institute and the Wu Tsai Neurosciences Institute at Stanford.
Congratulations, Dr. Rando!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#209 –– Dr. William Federspiel and Mr. Pete DeComo discuss how the use of the ALung medical device, Hemolung, has helped CoVid-19 patients as well as their current research.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Michael J Buckenmeyer, Tyler J Meder, Travis A Prest, Bryan N Brown
Title: Decellularization Techniques and Their Applications for the Repair and Regeneration of the Nervous System
Summary: A variety of surgical and non-surgical approaches have been used to address the impacts of nervous system injuries, which can lead to either impairment or a complete loss of function for affected patients. The inherent ability of nervous tissues to repair and/or regenerate is dampened due to irreversible changes that occur within the tissue remodeling microenvironment following injury. Specifically, dysregulation of the extracellular matrix (i.e., scarring) has been suggested as one of the major factors that can directly impair normal cell function and could significantly alter the regenerative potential of these tissues. A number of tissue engineering and regenerative medicine-based approaches have been suggested to intervene in the process of remodeling which occurs following injury. Decellularization has become an increasingly popular technique used to obtain acellular scaffolds, and their derivatives (hydrogels, etc.), which retain tissue-specific components, including critical structural and functional proteins. These advantageous characteristics make this approach an intriguing option for creating materials capable of stimulating the sensitive repair mechanisms associated with nervous system injuries. Over the past decade, several diverse decellularization methods have been implemented specifically for nervous system applications in an attempt to carefully remove cellular content while preserving tissue morphology and composition. Each application-based decellularized ECM product requires carefully designed treatments that preserve the unique biochemical signatures associated within each tissue type to stimulate the repair of brain, spinal cord, and peripheral nerve tissues. Herein, we review the decellularization techniques that have been applied to create biomaterials with the potential to promote the repair and regeneration of tissues within the central and peripheral nervous system.
Source: Methods. 2020 Jan 15;171:41-61. doi: 10.1016/j.ymeth.2019.07.023. Epub 2019 Aug 6.
GRANT OF THE MONTH
PI: Anita McElroy
Co-PIs: Paul Duprex and Alan Wells
Title: SARS-CoV-2 clinical and community serosurveillance
Description: COVID19 no longer requires an introduction since the entire globe is dealing with a worldwide pandemic due to SARSCoV2. Most individuals infected with SARSCoV2 will have mild self-limiting symptoms, and some may even be asymptomatic. This has made control of virus spread quite difficult and has necessitated the drastic measures of social distancing and governmental orders of shelter in place. With testing currently restricted to acute cases and even that being limited due to resource shortages, it is impossible to know how widespread the disease is within the community or within the hospital setting. These data are desperately needed to augment public health efforts to control the spread of the virus and furthermore would hasten our return to normal societal behaviors. In this proposal we seek to develop serologic assays that can quantitate human humoral immune responses to SARSCoV2. These data will inform on how widespread the disease is in the community since these assays will be able to identify individuals who were previously infected but whose symptoms were not severe enough to necessitate hospitalization, or acute phase testing. Importantly, these assays have the potential to speed up our return to normalcy since individuals who have seroconverted would be expected to be immune to re-infection or at least protected from severe disease, and therefore safe to return to work and school settings that would put them in contact with others.
The clinical Immunopathology laboratory has developed a test that can detect IgG and IgA antibodies, and other tests are available that detect IgM/IgG against the virus. However, as these are positive in hospitalized patients with severe disease, they are of limited value in determining immunity, and thus identifying patients able to return to work, and define the necessary ‘herd immunity’.
The following scope of work is being proposed:
- Define an enzyme linked immunosorbent assay (ELISA) to detect neutralizing antibodies against SARSCoV2. We need a test that identifies neutralizing antibodies. Several different antigenic targets will be assessed- three different forms of the viral surface protein (known as the spike) as well as the viral nucleoprotein which is the most abundantly produced protein and likely to be the most immunogenic target of the immune system. Purified proteins and whole cell lysates will be assessed in these assays. Positive and negative control serum will be derived from known clinical cases (provided by Dr. Wells). This is a traditional approach that will use an indirect detection method. These types of assays are commonly performed in clinical labs around the world and would be easily translatable into any clinical setting.
- Develop and validate a microneutralization assay to determine whether these antibodies are neutralizing antibodies against the SARSCoV2 virus. The development of neutralizing antibodies is a classic correlate of immune mediated protection from infection. Using live SARSCoV2 in the Regional Biocontainment lab BSL-3, we will develop an assay that can quantitate the neutralizing antibodies that are present in the serum of patients and controls. This assay will also have utility in the convalescent serum passive immunization and future vaccine trials, as these trials will seek to establish a correlate of vaccine mediated protection and it will be critical to compare vaccine mediated immunity with naturally acquired immunity.
- Define the kinetics of the serological response from acute infection into convalescence in patients with COVD19. Serial samples (provided by Dr. Wells) will be tested in the SARSCoV2 ELISA and microneutralization assays to determine when in the course of disease SARSCoV2 specific antibody responses can be detected and will quantitate the magnitude of these responses.
- Define the level of community seroconversion. Using SARSCoV2 ELISA assays and microneutralization assays we will screen normal healthy community members for evidence of prior disease. These individuals will be recruited under a normal healthy phlebotomy protocol that is currently undergoing IRB review.
Source: Clinical and Translational Science Institute
Term: 1 year
Amount: $100,000
