Projects
 Dr. Antonio D’Amore’s research in cardiovascular tissue engineering combines cellular and advanced biomaterials approaches to replace or restore the compromised function of cardiovascular tissue and organs. Dr D’Amore‘s research activity focuses on three major applications:
Dr. Antonio D’Amore’s research in cardiovascular tissue engineering combines cellular and advanced biomaterials approaches to replace or restore the compromised function of cardiovascular tissue and organs. Dr D’Amore‘s research activity focuses on three major applications:
1. Tissue engineered heart valve (TEHV): to develop effective tissue surrogates and biomedical devices for heart valve replacement and repair, (in collaboration with Dr M. Sacks, University of Texas at Austin and Dr J. Mayer, Harvard University)
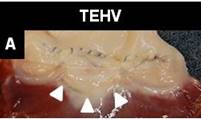 Figure A shows 16 week explant of electrospun, anisotropic valve leaflet in pulmonary position in ovine model, arrows identify the engineered leaflet, note the low thrombogenicity of the scaffold [A. S Bayoumi, et al. Unseeded Engineered Valve Leaflets Retain Function and Remodel After Implant in Sheep Right Pulmonary Outflow Tract. Circulation, 2012 Vol. 126, (21): A19201].
Figure A shows 16 week explant of electrospun, anisotropic valve leaflet in pulmonary position in ovine model, arrows identify the engineered leaflet, note the low thrombogenicity of the scaffold [A. S Bayoumi, et al. Unseeded Engineered Valve Leaflets Retain Function and Remodel After Implant in Sheep Right Pulmonary Outflow Tract. Circulation, 2012 Vol. 126, (21): A19201].
 2. Tissue engineered cardiac patch (TECP): to develop effective cardiac restraint devices for post myocardial infarction patients
2. Tissue engineered cardiac patch (TECP): to develop effective cardiac restraint devices for post myocardial infarction patients
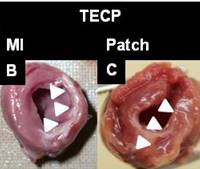
Figures B-C show comparison between the infarction control and the patch treated left ventricle at 8 week on rat model. Arrows identify the ischemic region and an ECM-polymer patch treated area showing a mitigated left ventricle thinning at 8 week on a rat model [A. D’Amore, et al. Bi-layred polyurethane-extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model. Biomaterials 2016(107), 1–14].
3. Tissue engineered vascular graft (TEVG): to develop effective engineered blood vessels for coronary artery bypass graft (in collaboration with Dr D. Vorp).
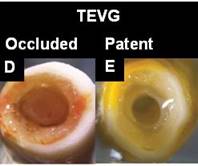 Figure D and E offer a comparison between an occluded (control) and patent graft produced by electrospinning, TIPS and cell seeding, 8 week explant on rat model [J. Krawiec, et al. In Vivo Functional Evaluation of Tissue Engineered Vascular Grafts Fabricated Using Human Adipose-Derived Stem Cells from High-Cardiovascular Risk Populations. Tissue Engineering Part A, 2016 22 (9-10), 765-775].
Figure D and E offer a comparison between an occluded (control) and patent graft produced by electrospinning, TIPS and cell seeding, 8 week explant on rat model [J. Krawiec, et al. In Vivo Functional Evaluation of Tissue Engineered Vascular Grafts Fabricated Using Human Adipose-Derived Stem Cells from High-Cardiovascular Risk Populations. Tissue Engineering Part A, 2016 22 (9-10), 765-775].
These research efforts will advance bioengineering strategies for ischemic heart failure, vascular and valvular diseases which have the potential to be translated to the bedside reducing patients’ morbidity and mortality.
Dr. Sang-Ho Ye’s research is focused on improvement of blood-biocompatibility on cardiovascular devices and tissue engineered scaffolds. Since there are no cardiovascular devices or tissue engineered scaffolds that have sufficient blood compatibility and have not negative side effects to the patients, improvement of blood-biocompatibility is particularly needed for the patents are at risk for thromboembolism and must take anticoagulants or anti-platelet therapy. Dr. Ye’s recent works are summarized as follows:
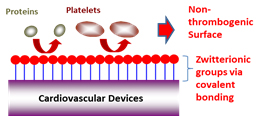 1. Improving blood compatibility cardiovascular devices: Dr. Ye seeks to develop technologies that reduce the thrombogenicity on cardiovascular device surfaces (e.g. ventricular assistant devices (VAD), artificial lung, stents, heart valves) by generating a stable non-thrombogenic interface. The current work has focused on covalent attachment of zwitterionic phosphorylcholine (PC) or sulfobetaine (SB) functional block copolymers which have previously been shown an excellent blood biocompatibility.
1. Improving blood compatibility cardiovascular devices: Dr. Ye seeks to develop technologies that reduce the thrombogenicity on cardiovascular device surfaces (e.g. ventricular assistant devices (VAD), artificial lung, stents, heart valves) by generating a stable non-thrombogenic interface. The current work has focused on covalent attachment of zwitterionic phosphorylcholine (PC) or sulfobetaine (SB) functional block copolymers which have previously been shown an excellent blood biocompatibility.
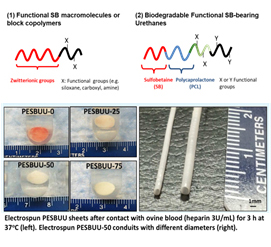 2. Synthesis of functional polymers for non-thrombogenic biomaterials: His study is also focusing on that synthesis of novel surface modifiers (block copolymers) or biodegradable elastomers composing zwitterionic PC or SB groups as well as other functional groups (e.g. alkoxy silane, carboxyl, amine, or thiol groups). He has studied related a small diameter biodegradable vascular graft which has improved patency with the anti-thrombogenic property in vivo evaluations. Recently developed totally degradable non-thrombogenic and drug-eluting elastomers could be used as small diameter tissue engineered vascular grafts as well as coating materials for biodegradable magnesium alloy stent.
2. Synthesis of functional polymers for non-thrombogenic biomaterials: His study is also focusing on that synthesis of novel surface modifiers (block copolymers) or biodegradable elastomers composing zwitterionic PC or SB groups as well as other functional groups (e.g. alkoxy silane, carboxyl, amine, or thiol groups). He has studied related a small diameter biodegradable vascular graft which has improved patency with the anti-thrombogenic property in vivo evaluations. Recently developed totally degradable non-thrombogenic and drug-eluting elastomers could be used as small diameter tissue engineered vascular grafts as well as coating materials for biodegradable magnesium alloy stent.
 3. Blood-biocompatibility analysis: Except developing of the surface modification and non-thrombogenic biomaterials, Dr. Ye is also researching in vitro blood-biocompatibility analysis related to quantitative studies of protein adsorption, platelet deposition and clotting time analysis on various biomaterials. His work also includes analysis of platelet and leukocyte activation using a flow cytometric analysis that could monitor the biocompatibility during the in vivo study of developing cardiovascular devices (e.g. pediatric VADs, ambulatory compact artificial lung device) to assist collaborative researches.
3. Blood-biocompatibility analysis: Except developing of the surface modification and non-thrombogenic biomaterials, Dr. Ye is also researching in vitro blood-biocompatibility analysis related to quantitative studies of protein adsorption, platelet deposition and clotting time analysis on various biomaterials. His work also includes analysis of platelet and leukocyte activation using a flow cytometric analysis that could monitor the biocompatibility during the in vivo study of developing cardiovascular devices (e.g. pediatric VADs, ambulatory compact artificial lung device) to assist collaborative researches.

