RESEARCH
Projects in the Cardiac Research Laboratory have two primary goals:
- Define the underlying cellular and molecular pathophysiology of cardiopulmonary diseases
- Leverage new knowledge in disease pathophysiology to guide clinical decision making for surgical intervention and through the development of new and/or less invasive surgical treatment options
A large focus of the team for the past 16 years has been elucidating the distinct mechanisms governing thoracic aortic disease and specifically leverage a large human aortic tissue and cell bank of >1,000 unique patient specimens.
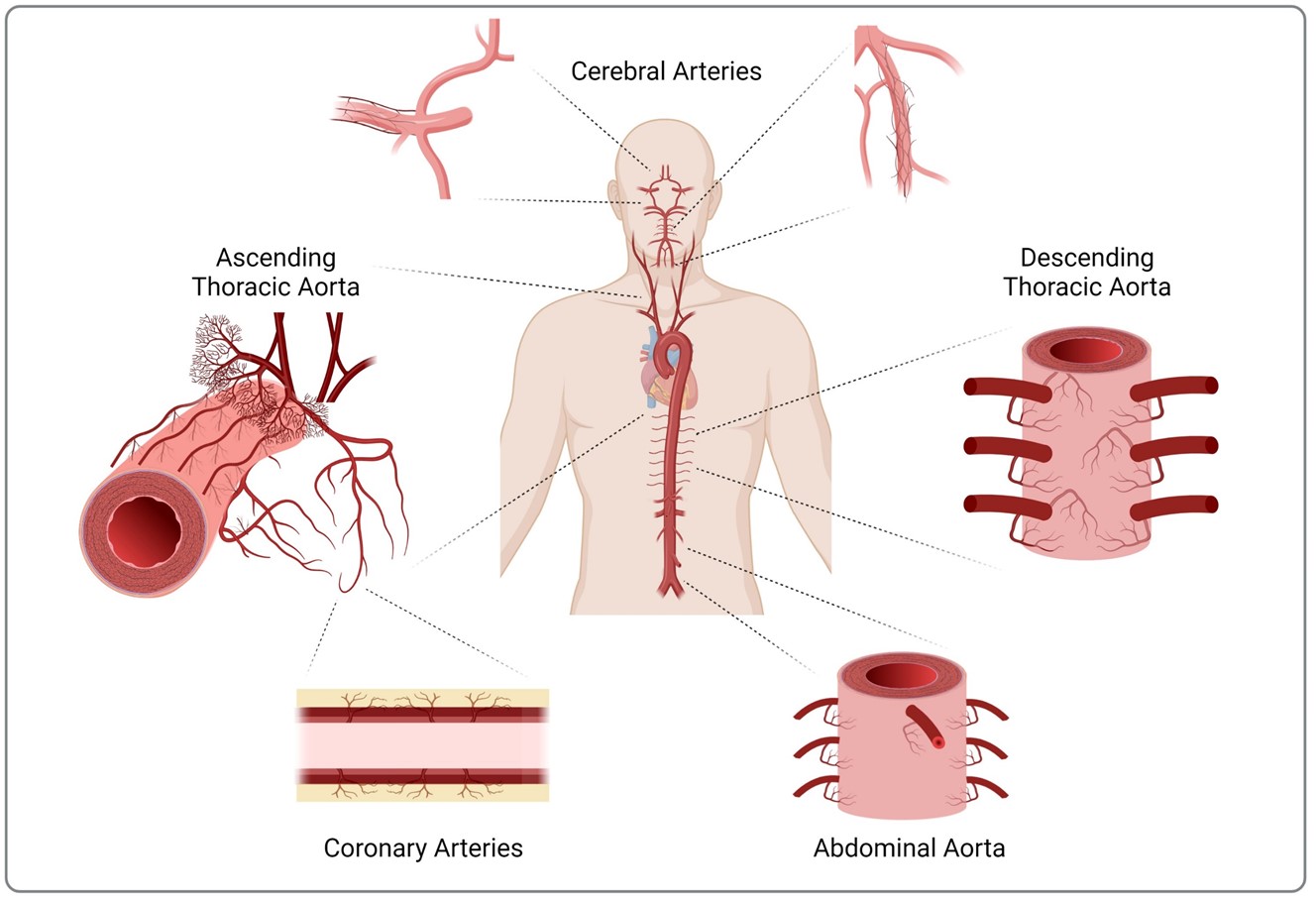
Vasa vasorum comprise a microvascular network that nourishes larger arteries and veins. Source: Phillippi Sci Adv 2022
Microvascular remodeling is an important contributor to vascular disease. Our team is broadly interested in how the extracellular matrix (ECM) affects microvascular function, and we are poised to make important contributions in this area to improve mechanistic understanding of human ascending aortic disease. The long-term goal of our work is to develop better strategies to diagnose and treat the over 2.5 million people affected worldwide by dissection and/or rupture of thoracic aortic aneurysm (TAA). We demonstrated that deficiency in angiogenic factors and collagen immaturity in the adventitial ECM are associated with vasa vasorum remodeling in degenerative and bicuspid aortic valve-associated TAA. These noted adventitial ECM disruptions in TAA helped to shape our central hypothesis that matrix cues in TAA provoke vasa vasorum dysfunction. Preliminary studies by our team revealed that hydrogel bioscaffolds made from adventitial ECM recapitulate native microstructure and retain signaling cues that invoke angiogenesis in vivo and endothelial cell proliferation and formation of branched networks in vitro. We will use human aortic tissue from early stages and multiple etiologies of TAA and porcine arteries to prepare ECM bioscaffolds from these tissues as biomimetic matrices of healthy and disease microenvironments. This project seeks to determine how extracellular matrix of TAA affects remodeling and endothelial function of vasa vasorum; and identify what matrix signals are necessary for regeneration of functional vasa vasorum. Under the first aim, we will comprehensively characterize the adventitial matrix of healthy aorta and various stages of human aneurysmal disease. We use ECM bioscaffolds derived from these human tissues to determine how matrix cues impact remodeling and endothelial function in isolated whole vessels of vasa vasorum and primary cultured human endothelial cells. We will identify what growth factor-dependent and independent matrix cues regenerate functional vasa vasorum by pericytes isolated from TAA using in vitro and in vivo techniques. What we learn about vasa vasorum function in TAA could lead to the development of novel diagnostic approach that measure in vivo microvascular function in patients. Because aortic replacement remains the only treatment for ascending TAA, our work addresses an unmet clinical need to develop better therapies for TAA. Ultimately, the proposed research aspires to yield translational methods to understand, diagnose, and treat TAA, and therefore, improve patient-specific risk mitigation for aortic catastrophe.
Funding: R01HL131632 and R56HL127214 (Phillippi, PI; Gleason, Co-I)
Key Publications:
- Fercana GR*, Yerneni S*, Billaud M, Hill JC, VanRyzin P, Richards TD, Sicari B, Badylak SF, Johnson S, Campbell PG, Gleason TG, and Phillippi JA. Perivascular Extracellular Matrix Hydrogels Mimic Native Matrix Microarchitecture and Promote Angiogenesis via Basic Fibroblast Growth Factor. Biomaterials. 2017 Apr;123: 142-154. *Equal contribution. PubMed PMID: 28167392
- Billaud M, Hill JC, Richards TR, Gleason TG, and Phillippi JA. Medial Hypoxia and Adventitial Vasa Vasorum Remodeling in Human Ascending Aortic Aneurysm. Frontiers in Cardiovascular Medicine. Published online 17 Sept 2018. 5:124. PubMed PMID: 30276199
- Hill JC, Billaud M, Richards TD, Kotlarczyk MP, Shiva S, Phillippi JA* Gleason TG*. Layer Specific Nos3 Expression and Genotypic Distribution in Bicuspid Aortic Valve Aortopathy. European Journal of Cardiothoracic Surgery. European Journal of Cardiothoracic Surgery. 2022 Online ahead of print: 23Apr22. PMID: 34560403. *Co-corresponding authors
- Wintruba KL, Hill JC, Richards TD, Lee YC, Kaczorowski DJ, Sultan I, Badylak SF, Billaud M, Gleason TG, Phillippi JA. Adventitia-Derived Extracellular Matrix Hydrogel Enhances Contractility of Human Vasa Vasorum-Derived Pericytes via α2β1 integrin and TGFβ Receptor. J Biomed Mater Res A. 2022 Dec; 110 (12) 1912-1920. PMCID: 35770946
- Fortunato R, Huckaby LV, Emerel LV, Scholosser V, Phillippi JA, Vorp DA, Maiti S, Gleason TG. The predictive capability of aortic stiffness index for aortic dissection among dilated ascending aortas. JTCVS Accepted 1 Sept 2022 (in press).
- Lee YC, Richards TD, Fantini DA, Kaczorowski DJ, Brown BN, Phillippi JA. Ascending and Descending Aortic ECM Hydrogels for Modeling Aortic Wall Biology. 5 Dec 2022. bioRxiv. 2022. DOI: 10.1101/2022.12.03.518904
Influence of Matrix Biophysical Cues on Pericyte Function in (Patho)Physiological Angiogenesis
The long-term arc of this project is expected to engineer vascularization of tissue deficits and advance treatment of microvascular diseases including myocardial infarction, atherosclerosis, pulmonary arterial hypertension, aneurysm, peripheral artery disease, bone repair, volumetric muscle loss, diabetic wounds, and cancer. To achieve this goal, the project will advance our fundamental understanding of how local fibrous extracellular matrix (ECM) biophysical cues dynamically influence pericytes during neovessel formation. The role of the endothelial cell in vasculogenesis and angiogenesis is well recognized, yet the pericyte’s full scope of work is less understood, despite its known essential role in proper vascular development. We made a surprising observation that pericytes exhibit phenotypic plasticity when they spontaneously assemble into 3D spheroids and reversibly form and adopt endothelial markers when cultured on native and synthetic fibrous biomaterials in vitro, but not on 2D substrates. Akin to vasculogenic blood islands, tip cell-like protrusions sprout and retract from these spheroids comprised of pericytes that transdifferentiate to express endothelial markers.
Funding: R01HL162822 (Phillippi and Nain, MPIs)- PENDING
Other studies by our team revealed that matrix fiber diameter and architecture dictate cell morphology, the mode and rate of cell migration, cell force exertion, protrusion dynamics, and nuclear shape associated with heterochromatic rearrangements. These published and preliminary studies gave rise to a central hypothesis that various matrix biophysical parameters differentially direct neovessel formation by modulating pericyte migration, contraction, protrusion, and phenotypic plasticity. To test this hypothesis, we will further develop a nanofiber cell force sensing platform to mimic (patho)physiological ECMs through exquisite tunable control of ECM fiber biophysical parameters (e.g., diameter, density, alignment). The project’s translational impact will be novel methods of controlling microvascular expansion and regression in diseased, damaged, and engineered tissues.
Key Publications:
- Sharma S, Hill JC, Phillippi JA* and Nain AS*. Fiber Diameter and Architecture Dictate Three-Dimensional Assembly of Pericytes into Spheroids. bioRxiv Epub: 4 Aug 2022 DOI: 10.1101/2022.08.02.502506 *Co-corresponding authors
- Hall A, Chan PG, Sheets K, Apperson M, Delaughter C, Gleason TG, Phillippi JA*, and Nain AS*. Nanonet Force Microscopy for Measuring Forces in Single Smooth Muscle Cells of Human Aorta. Mol Biol Cell. 2017. 28 (14): 1894-1900. *Co-corresponding authors. PubMed PMID: 28450452
BAV-Associated Aortopathy
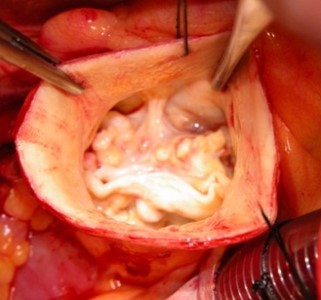
Surgeon’s view of a bicuspid aortic valve.
Ascending aortic aneurysmal disease is a major worldwide health problem. Bicuspid aortic valve (BAV)-associated aortopathy represents the largest subset of affected patients and this congenital anomaly is present in 1-2% of the general population. Current aortic diameter-based guidelines for surgical intervention stem from a non-controlled extrapolation of natural history data that does not reflect patient-specific aortic catastrophe risk rendering under-treatment in some patients and over-treatment in others. This is largely because there is an incomplete understanding of what biological and biomechanical features are unique to BAV-associated aortopathy or other degenerative aneurysms and how these insults potentiate aortic dissection.
During the first five years of NIH support, we uncovered several cellular, tissue architectural, and biomechanical-based features distinguishing BAV-associated aortopathy from that of degenerative aneurysms. We discovered that elevated production of superoxide anion by medial smooth muscle cells, increased oxidative stress-induced cellular damages, and a biomechanical strength profile coupled with an anisotrophic collagen and elastin microarchitecture uniquely define the tissue microenvironment of the BAV aorta. In the next phase of the project, we will elucidate how an interplay of mechanical and oxidative stress mediates ECM remodeling, determine where hypoxia comes into play, and how clinical imaging-derived metrics correspond to cellular and tissue aberrations in the BAV aorta. In a two-aim approach, we will test the central hypothesis that mechanical forces- and local hypoxia-induced oxidative stress invokes differential ECM remodeling in BAV and TAV patients, and these insults can be correlated to patient-specific aortic wall indices that can be imaged, bundled and used to predict disease progression and/or aortic catastrophe. Aim’s approach will employ our established patient-specific 3D culture models to determine how mechanical stretch and low oxygen tension impact antioxidant response, free radical production, cellular oxidative damages, and influence ECM production, microarchitecture, and degradation in BAV aorta-derived smooth muscle cells. In Aim 2, quantification of local hypoxic effects, measures of oxidative cellular damages, ECM microarchitecture, and biochemical ECM composition will be regionally compared and then correlated with patient-specific wall shear stress measurements from 4D flow MRI, aortic wall morphometrics from dynamic ECG-gated CTA, and distensibility metrics from echocardiography to develop a workable patient-specific multi-parameter imaging-based paradigm.
Funding: R01HL109132-08 (Gleason, PI; Phillippi, Co-I) 2012-2018; 2020-present
Completion of this next project phase will generate an aortic bio-map that profiles mechanical and oxidative stress-mediated ECM remodeling in BAV-associated aortopathy and will identify what in vivo bio-imaging endpoints correlate with these tissue insults. A perceived deliverable is a set of building blocks for a workable multi-parameter computational model whose main output will be a patient specific aortic integrity score that more accurately identifies dissection risk for a given patient. This work will also reveal new opportunities for the implementation of PET-based probes to non-invasively detect local aortic vulnerability and identify novel targets for medical therapeutic intervention.
Key Publications:
- Phillippi JA, Klyachko EA, Kenny IV JP, Eskay MA, Gorman RC, and Gleason TG. Basal and Oxidative Stress-Induced Expression of Metallothionein is Decreased in Ascending Aortic Aneurysms of Bicuspid Aortic Valve Patients. Circulation. 2009. 119 (18): 2498-2506. PubMed PMID: 19398671
- Pichamuthu JE*, Phillippi JA*, Cleary DA, Chew DW, Hempel J, Vorp DA, and Gleason TG. Differential Tensile Strength and Collagen Composition in Ascending Aortic Aneurysms by Aortic Valve Phenotype. Ann Thorac Surg. 2013. 96 (6): 2147-54. *Equal contribution. PubMed PMID: 24021768
- Phillippi JA, Green BR, Eskay MA, Kotlarczyk MP, Hill MR, Robertson AM, Watkins SC, Vorp DA, and Gleason TG. Mechanism of Aortic Medial Matrix Remodeling is Distinct in Bicuspid Aortic Valve Patients. J Thorac Cardiovasc Surg 2014. 147(3): 1056-64. PubMed PMID: 23764410
- Tsamis A, Phillippi JA, Koch RG, Krawiec JT, D’Amore A, Watkins SC, Wagner WR, Vorp DA, and Gleason TG. Extracellular Matrix Fiber Microarchitecture is Region- Specific in Bicuspid Aortic Valve-Associated Ascending Aortopathy. J Thorac Cardiovasc Surg. 2016. 151 (6): 1718.-28. PubMed PMID: 26979916.
- Billaud M*, Phillippi JA*, Kotlarczyk MP, Hill JC, Ellis BW, St. Croix CM, Cantu-Medellin N, Kelley EE, and Gleason TG. Elevated Oxidative Stress in the Aortic Media of Bicuspid Aortic Valve Patients. J Thorac Cardiovasc Surg. 2017. 154 (5): 1756-1762. *Equal contribution. PubMed PMID: 28651938.
- Thunes JT, Phillippi, JA, Gleason, TG, Vorp DA, and Maiti, S. Structural Modeling Reveals Microstructure-Strength Relationship for Human Ascending Thoracic Aorta. J Biomech. 2018. 71: 84-93. PubMed PMID: 29544877
- Billaud M, Hill JC, Richards TR, Gleason TG, and Phillippi JA. Medial Hypoxia and Adventitial Vasa Vasorum Remodeling in Human Ascending Aortic Aneurysm. Frontiers in Cardiovascular Medicine. Published online 17 Sept 2018. 5:124. PubMed PMID: 30276199
- Emerel L, Thunes J, Kickliter T, Billaud M, Phillippi JA, Vorp DA, Maiti, S and Gleason TG. Pre-Dissection-Derived Geometric and Distensibility Indices Reveal Increased Peak Longitudinal Stress and Stiffness in Patients Sustaining Acute Type A Aortic Dissection: Implications for Predicting Dissection. J Thorac Cardiovasc Surg. 2019. 158 (2): 355-363. PubMed PMID: 30551966
- Fortunato R, Huckaby LV, Emerel LV, Scholosser V, Phillippi JA, Vorp DA, Maiti S, Gleason TG. The predictive capability of aortic stiffness index for aortic dissection among dilated ascending aortas. JTCVS Accepted 1 Sept 2022 (in press).
Prevalence of Endothelial Progenitor Cells in Lung Failure
The main objective of this research project is to obtain a more complete understanding of how tissue-resident and circulating endothelial progenitor cells (EPCs) are influenced by the extracellular matrix (ECM) in the setting of human lung failure. This study has the potential to generate multiple novel hypotheses to elucidate mechanisms of microvascular expansion in the setting of lung failure and will develop a bioengineered approach for disease modeling and potential therapeutic applications.
Funding: PA Department of Health Commonwealth Universal Research Enrichment (CURE) Program (2021-present)

