According to the American Cancer Society, kidney cancer is among the top ten most common cancers in men and women, and clear cell renal cell carcinoma (ccRCC) – the most common subtype of tumor associated with kidney cancer – accounts for more than 75 percent of cases.
McGowan Institute for Regenerative Medicine affiliated faculty members will serve as co-investigators on a $603,807 award from the Department of Defense to identify novel regulators that can potentially target ccRCC progression. The collaborators are Partha Roy, PhD, Associate Professor of Bioengineering, Cell Biology, and Pathology at the University of Pittsburgh, and Michael Lotze, MD, Professor of Surgery and Bioengineering, University of Pittsburgh; Vice Chair of Research, Department of Surgery, University of Pittsburgh School of Medicine; and Assistant Vice Chancellor Sponsored Training Grants, University of Pittsburgh Schools of the Health Sciences.
“The five-year survival of patients with advanced ccRCC is still only 10 percent,” said Dr. Roy. “A distinguishing hallmark of ccRCC is the highly vascular (angiogenic) tumor microenvironment due to genetic loss of VHL, a major negative regulator of angiogenesis – the process in which a network of blood vessels is developed.”
Through this network of blood vessels, oxygen and nutrients can be supplied to the tumor microenvironment, allowing it to grow and spread.
“The tumor microenvironment is a collection of cells, molecules, and blood vessels that surround a tumor,” explained Dr. Roy, “and studying the close relationship and interactions between the tumor and its microenvironment may help researchers better understand the growth and development of cancer.
“Given my lab’s research interests in angiogenesis and cancer, we would like to identify novel regulators of tumor blood vessel formation in the kidney that contribute to disease progression when dysregulated and therefore, be potentially targeted to slow down tumor progression.”
Dr. Roy directs the Cell Migration Laboratory in the Swanson School of Engineering, where they have developed a better understanding of the role of actin-binding protein profilin1 (Pfn1) in physiological and pathological processes and want to examine its role in kidney cancer.
“With clear evidence of a clinical association between high Pfn1 level and poor disease outcome in ccRCC, we plan to use this study to determine whether Pfn1 contributes to ccRCC progression and investigate whether Pfn1 inhibition by novel small molecules is an effective strategy to control the tumor microenvironment and slow down the progression of RCC.”
The underlying hypothesis of this study is that Pfn1 stimulates tumor angiogenesis and limits the immune responses that otherwise would help slow cancer progression. In the first part of the study, the group will address whether Pfn1 dysregulation in vascular cells effects the tumor microenvironment and ccRCC progression. They will also determine if Pfn1 expression has any correlation with the responsiveness of ccRCC patients to immunotherapy.
In the second part of the study, they will determine if novel investigative compounds targeting Pfn1 function can be used to suppress tumor angiogenesis, improve immune response, and inhibit ccRCC progression.
“The results of this study could establish Pfn1 as a novel regulator of ccRCC progression and a biomarker for predicting the therapeutic response of ccRCC patients,” said Dr. Roy. “This could pave the way for future development of improved classes of Pfn1 inhibitor as a possible novel therapeutic direction that might benefit patients who are resistant to standard VEGF-targeted anti-angiogenic therapy.”
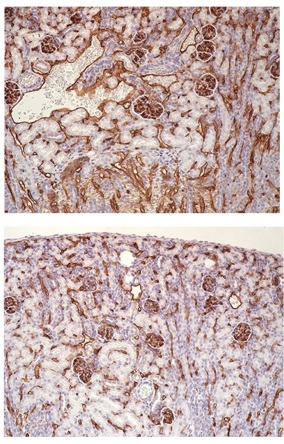
Illustration: Renal microvasculature in wild-type (top) vs conditional profilin1 knockout (bottom) mice showing profilin1-dependency for blood vessel generation in the kidney (courtesy: Roy lab).
Read more…
University of Pittsburgh Swanson School of Engineering News Release
