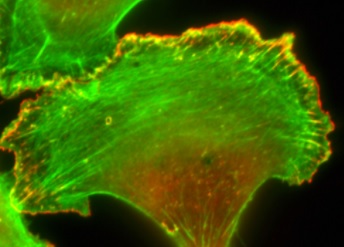
Animals, plants, fungi, and protozoans all have eukaryotic cells, and the cytoskeleton in these systems plays an important role in cell shape, movement, and division. Disruption in this complex network of protein filaments can lead to cell and organ dysfunction.
The Journal of Biological Chemistry (JBC) recently released a special virtual issue compiling a list of papers published in the journal over the last two years that represented the most exciting advances in the eukaryotic cytoskeleton field, and it features research from bioengineers at the University of Pittsburgh Swanson School of Engineering.
McGowan Institute for Regenerative Medicine affiliated faculty member Partha Roy, PhD, Associate Professor of Bioengineering, Cell Biology, and Pathology at Pitt, leads a research group that studies cell migration and applies this fundamental knowledge to pathological conditions, such as cancer metastasis or excessive blood vessel formation. The lab’s article, “The VASP-profilin1 (Pfn1) interaction is critical for efficient cell migration and is regulated by cell-substrate adhesion in a PKA-dependent manner,” was selected for the JBC virtual issue. McGowan Institute faculty member Sanjeev Shroff, PhD, Distinguished Professor of and Gerald E. McGinnis Chair in Bioengineering and Professor of Medicine at the University of Pittsburgh, is also an author on the paper.
“This study looked at the fundamental process of how cells move and the signaling pathway that regulates it,” said David Gau, PhD, postdoctoral associate in the Roy Lab and the first author on the paper.
“For proper cell migration, the actin cytoskeleton has to be efficiently remodeled,” he explained, “and one of the most important parts for remodeling the actin cytoskeleton for cell migration is a process called protrusion.”
In protrusion, a cell attempts to reach out and attach itself to a surface so that it can propel forward. Dr. Roy’s group studied how interaction between two important actin-binding proteins — profilin and VASP — might be regulated during cell movement.
“We proposed a mechanism on how these two proteins interact with each other based on the adhesive state of the cells,” said Dr. Gau. “We showed that when these two proteins are interacting, it promotes actin polymerization, which then allows cells to make protrusions and move.
“When a cell is not in an attached state, there is a decrease in interaction between profilin and VASP,” he continued. “This cyclic adhesion-deadhesion-dependent regulation of profilin-VASP interaction involves tuning of biochemical modification of profilin at a certain site.”
Understanding this fundamental cell process may help reveal ways to reduce aggressive cell movement or impact other cellular dysfunction. Dr. Roy’s lab continues to work in this area by studying how these types of protein interactions promote cancer metastasis or blood vessel formation. Given the importance of these types of actin-binding proteins in cancer, Dr. Gau is also looking at mechanisms to regulate the cellular levels of these proteins.
Illustration: Image of a cell and the actin cytoskeleton fluorescently labeled. (Credit: Roy Lab)
Read more…
University of Pittsburgh Swanson School of Engineering News Release
Abstract (The VASP-profilin1 (Pfn1) interaction is critical for efficient cell migration and is regulated by cell-substrate adhesion in a PKA-dependent manner. David Gau, William Veon, Sanjeev G Shroff, Partha Roy. Journal of Biological Chemistry, 2019 Apr 26;294(17):6972-6985.)
