Roughly one in eight women in the United States will develop invasive breast cancer over the course of her lifetime, and HER2-positive (HER2+) breast cancers represent about 25 percent of all breast cancer cases. Though multiple therapies exist, most patients will develop metastatic disease and resistance to current treatments.
A collaborative research group from the University of Pittsburgh and Harvard Medical School studied the mechanisms behind tumor cell resistance to therapies targeting metastatic HER2+ breast cancer and recently published their work in the Proceedings of the National Academy of Sciences.
The group examined the tumor microenvironment — a collection of cells, molecules, and blood vessels that surround and influence tumor cells — to get the full picture of what drives this resistance. They found that fibroblasts, a cell type important to tissue regeneration, play a large role.
“Our study shows that fibroblasts promote drug resistance through a parallel signaling pathway in a subset of HER2+ breast cancer cells,” said McGowan Institute for Regenerative Medicine affiliated faculty member Ioannis Zervantonakis, PhD, assistant professor of bioengineering at Pitt’s Swanson School of Engineering.
Cancer cells grow uncontrollably, which is partially driven by continuous activation of proteins called kinases. HER2+ breast tumors have high levels of the HER2 receptor kinase that is important for their growth and is a major drug target. Anti-cancer drugs — like lapatinib — work to interfere with kinases, but because of this parallel signaling, fibroblasts are able to protect tumor cells and counteract the drug’s inhibitory effects.
Compare this cell-to-cell signaling process to communication through a radio. The radio antenna is analogous to a receptor in the cell that is activated when an electrical signal is received. Radios can have multiple antennas to respond to different electrical signals.
“By analogy, cancer cells have multiple receptors (antennas) that can be activated by signals in their environment,” explained Dr. Zervantonakis. “In the radio example, transmission of an electrical signal produces a sound. While in cancer cells, fibroblast-derived signals stimulate cancer growth.”
You can produce a series of sounds, and even amplify the volume, by receiving more than one signal at a time through activation of parallel pathways.
“In this scenario, the HER2+ kinase is one signal that is continuously activating sound, and then there is another pathway through which signals emitted by fibroblasts activate sound,” he continued. “Lapatinib only blocks the HER2+ kinase signal, but through another pathway, the fibroblasts are able to transmit signals to keep the cancer cells alive or allow them to grow.”
The findings in this paper are important for restoring sensitivity to breast cancer therapies and developing treatments that are more effective.
“Sensitivity to these drugs can be re-established through a combination of therapies that inhibit critical proteins in the pathway activated by fibroblasts,” said Dr. Zervantonakis. “Particularly, combination therapies with the FDA-approved drug everolimus and investigational agents targeting anti-apoptotic proteins were effective in restoring drug sensitivity in fibroblast-protected cancer cells.”
The next step for Dr. Zervantonakis and his lab is to create predictive mathematical models to identify the fibroblast density range in tumors that will elicit drug resistance. From there, they can develop personalized therapies to improve outcomes in HER2+ breast cancer.
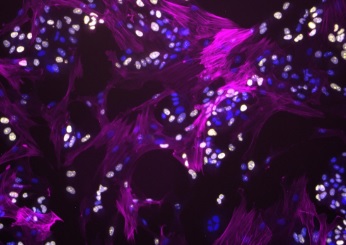
Illustration: Fibroblasts modulate HER2 therapy sensitivity in breast tumor cells. Fibroblasts (magenta) interacting with proliferating breast tumor cells (yellow). Cell nuclei for both tumor and fibroblasts are stained in blue. (Image Courtesy of Alexis Scott)
Read more…
University of Pittsburgh Swanson School of Engineering News Release
Abstract (Fibroblast–tumor cell signaling limits HER2 kinase therapy response via activation of MTOR and antiapoptotic pathways. Ioannis K. Zervantonakis, Matthew D. Poskus, Alexis L. Scott, Laura M. Selfors, Jia-Ren Lin, Deborah A. Dillon, Shailja Pathania, Peter K. Sorger, Gordon B. Mills, and Joan S. Brugge. PNAS first published June 29, 2020.)
