July 2019 | VOL. 18, NO. 07| www.McGowan.pitt.edu
Dr. Stephen Badylak to Receive BioMed SA’s 2019 Award for Innovation in Healthcare and Bioscience

McGowan Institute for Regenerative Medicine Deputy Director Stephen Badylak, DVM, PhD, MD, a nationally recognized pioneer in stem cell and tissue regeneration, will receive BioMed SA’s 2019 Award for Innovation in Healthcare and Bioscience in San Antonio, Texas, September 19, 2019.
The award is given annually to an innovator who has made significant contributions to the biomedical industry by putting novel ideas into action with transformational results. The innovations of Dr. Badylak, a Professor of Surgery and Bioengineering at the University of Pittsburgh, have benefitted more than 10 million patients, including wounded warriors, through his collaborations with the U.S. Army Institute for Surgical Research and other San Antonio-based organizations.
BioMed SA is a non-profit organization founded by former Mayor Henry Cisneros and other San Antonio community and industry leaders in 2005 to promote and grow the city’s healthcare and bioscience sector. This is the city’s leading industry, employing more than 1 of every 6 members of the local workforce.
Dr. Badylak was nominated for this year’s award by Sy Griffey, PhD, Chief Operating Officer of the StemBioSys in San Antonio. In his nomination, Dr. Griffey called Dr. Badylak a prolific inventor of novel technologies with hundreds of scientific manuscripts published in peer-reviewed journals, plus more than 60 US patents and 300 patents worldwide. Dr. Griffey’s nomination package also described Dr. Badylak’s transformational achievements in developing and commercializing novel biomaterials in the field of regenerative medicine. Dr. Badylak has also founded and advised multiple biotech companies and received numerous awards for science and innovation.
Congratulations, Dr. Badylak!
RESOURCES AT THE MCGOWAN INSTITUTE
August Histology Special
There are two ways that a cell can die: necrosis and apoptosis. Necrosis occurs when a cell is damaged by an external force, such as poison, a bodily injury, an infection or getting cut off from the blood supply (which might occur during a heart attack or stroke).
Apoptosis, on the other hand, is when a cell commits suicide. It’s sometimes referred to as programmed cell death.
Apoptosis is a form of cell death that eliminates compromised or superfluous cells. It is controlled by multiple signaling and effector pathways that mediate active responses to external growth, survival, or death factors.
Our method for examining apoptosis via DNA fragmentation is by the TUNEL assay. This technique can detect early-stage apoptosis in systems where chromatin condensation has begun, and strand breaks are fewer, even before the nucleus undergoes major morphological changes.
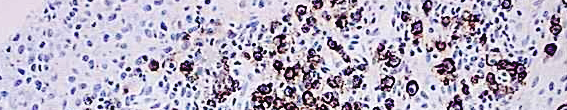
The McGowan Institute Histology Core Laboratory is offering 30% off Tunel staining for the month of August, when mentioning this ad. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology work with the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
Dr. Freddie Fu Weighs In On Unproven Stem Cell Treatments

Liz Szabo of Kaiser Health News recently reported in Time the controversary surrounding the hope vs. hype of stem cell treatments for the relief of arthritic conditions. She notes the benefits of stem cells are hotly debated in the medical community, and federal regulators have warned the public to beware of clinics that peddle unapproved injections as a cure-all. Many doctors and ethicists say they fear the public is being misled about how well stem cells work—and whether the procedures save their money or waste it.
“This definitely is not a high-quality, proven treatment,” says McGowan Institute for Regenerative Medicine faculty member Freddie Fu, MD, chairman of orthopedic surgery at the University of Pittsburgh Medical Center.
Regenexx, previously known as Regenerative Sciences, is one of the oldest stem cell companies in the U.S. When it opened its doors in 2005, it had only a handful of competitors. Today, there are more than 1,000 stem clinics in the U.S., says Leigh Turner, PhD, an associate professor at the University of Minnesota’s Center for Bioethics, who has published a series of articles describing the stem cell market.
A Regenexx marketing booklet says 70% of orthopedic surgeries “can be completely avoided with a Regenexx procedure”—a claim Dr. Fu calls “silly.”
“There is zero evidence that you can replace 70% of surgeries with stem cells,” he says.
Recent research suggests stem cells and platelets may work no better than placebos, Dr. Fu adds. In a recent analysis, over 80% of patients with knee arthritis experienced a noticeable improvement in pain after receiving simple salt-water injections, writes Benjamin Rothrauff, PhD, a postdoctoral fellow who works with Dr. Fu at the University of Pittsburgh.
There’s also no definitive evidence stem cells and platelets can regrow lost cartilage, Dr. Fu says. A 2018 review concluded platelets have “marginal effectiveness,” and experts note that most published studies are so small or poorly designed that their results aren’t reliable.
Dr. Fu says that relatively few people with joint pain undergo surgery, which doctors typically view as a last resort for patients who have exhausted all other treatment options. Although 14 million Americans have knee arthritis, the Arthritis Foundation estimates that doctors perform only about 757,000 knee replacements each year.
Before recommending joint replacement, doctors often tell patients to try exercise, physical therapy, weight loss, supportive shoe inserts or steroid injections, says Henry Garlich, director of health care value solutions and enhanced clinical programs at Blue Shield of California. Physical therapy, in particular, helps many patients, says Dr. Fu.
Unproven Stem Cell Therapies Leave Patients Having to Sort Out Hope from Hype

Pittsburgh has been on the cutting edge of what has been regenerative medicine for the better part of 40 years, turning early leadership in organ transplantation into other researched therapies. Because stem cell therapy clinics have begun springing up in our region and across the country, patients are now put in the position of having to sort out the hope from the hype with these therapies. The focus of an early 2019 program organized by the McGowan Institute for Regenerative Medicine helped to distinguish what’s good and what’s bad.
More recently, on the WPXI program, Our Region’s Business, William Wagner, PhD, Director of the McGowan Institute for Regenerative Medicine as well as a Professor of Surgery, Bioengineering and Chemical Engineering at the University of Pittsburgh, and J. Peter Rubin, MD, FACS, Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh, sat down with host Bill Flanagan to discuss the importance of knowing the difference between unproven therapy claims and those therapy procedures that are clinically safe and effective.
As Dr. Wagner notes, “A lot of these therapies are not quite established yet. There is hope, but there’s a lot of hype,” and unfortunately patients are left to figure out the difference. Dr. Wagner points to the International Society for Stem Cell Research as a professional organization that has a great website which can help patients learn how to distinguish what is real and what is fake.
Dr. Rubin adds, “I want to start by making the point that stem cell research, cell therapy research or regenerative medicine is a field that is really growing. It is growing based on phenomenal science right in our region. In Pittsburgh we have an incredible array of investigators funded by the National Institutes of Health and the Department of Defense for example. There are a lot of new developments coming from this. However, when we look at the broad landscape of cell therapies that are being offered in many different settings, we see that the claims being made are just really getting ahead of the science. And that’s the root of the problem.”
Illustration: Mr. Bill Flanagan, Dr. William Wagner, and Dr. Peter Rubin (left to right). Source: Erin Hare, UPMC.
Big Data Reveals Hidden Subtypes of Sepsis

Much like cancer, sepsis isn’t simply one condition but rather many conditions that could benefit from different treatments, according to the results of a University of Pittsburgh School of Medicine study involving more than 60,000 patients. McGowan Institute for Regenerative Medicine affiliated faculty members who are co-authors on the study include:
- Derek Angus, MD, MPH, Chair of the Department of Critical Care Medicine of both the University of Pittsburgh School of Medicine and the UPMC Healthcare System. At the University, he holds the rank of Distinguished Professor and the Mitchell P. Fink Endowed Chair in Critical Care Medicine with secondary appointments in Medicine, Health Policy and Management, and Clinical and Translational Science and he directs the CRISMA (Clinical Research, Investigation, and Systems Modeling of Acute Illnesses) Center. He also co-directs the UPMC ICU Service Center, responsible for the provision of ICU services across the 20-plus hospital system.
- Gilles Clermont, MD, Professor of Critical Care Medicine, of Industrial Engineering, and of Mathematics at the University of Pittsburgh. He is also the Medical Director of the Center for Inflammation and Regenerative Modeling, University of Pittsburgh. Dr. Gilles also serves on the Critical Care Medicine Attending Staff at hospitals of the University of Pittsburgh Medical Center and the Veterans Affairs Medical Center, Pittsburgh.
- John Kellum, MD, FACP, FCCM, Professor in the Departments of Critical Care Medicine (primary), Medicine, Bioengineering, and Clinical and Translational Science at the University of Pittsburgh. He is also the Director of the Center for Critical Care Nephrology and the Vice-Chair for Research, both appointments in the Department of Critical Care Medicine, and Associate Director for Acute Illness in the Institute for Personalized Medicine at Pitt. Dr. Kellum also serves as an Intensivist at UPMC.
- Yoram Vodovotz, PhD, Professor in the Department of Surgery with secondary appointments in the Department of Computational & Systems Biology, the Department of Bioengineering, the Department of Immunology, the Department of Communication Science and Disorders (of the School of Health and Rehabilitation Science), and the Clinical and Translational Science Institute. He also is the Director of the Center for Inflammation and Regenerative Modeling at the McGowan Institute.
These findings, announced in JAMA and presented at the American Thoracic Society’s Annual Meeting, could explain why several recent clinical trials of treatments for sepsis, the No. 1 killer of hospitalized patients, have failed. Sepsis is a life-threatening condition that arises when the body’s response to an infection injures its own tissues and organs.
“For over a decade, there have been no major breakthroughs in the treatment of sepsis; the largest improvements we’ve seen involve the enforcing of ‘one-size fits all’ protocols for prompt treatment,” said lead author Christopher Seymour, MD, MSc, associate professor in Pitt’s Department of Critical Care Medicine and member of Pitt’s Clinical Research Investigation and Systems Modeling of Acute Illness Center. “But these protocols ignore that sepsis patients are not all the same. For a condition that kills more than 6 million people annually, that’s unacceptable. Hopefully, by seeing sepsis as several distinct conditions with varying clinical characteristics, we can discover and test therapies precisely tailored to the type of sepsis each patient has.”
In the “Sepsis ENdotyping in Emergency Care” (SENECA) project, funded by the National Institutes of Health (NIH), Dr. Seymour and his team used computer algorithms to analyze 29 clinical variables found in the electronic health records of more than 20,000 UPMC patients recognized to have sepsis within six hours of hospital arrival from 2010 to 2012.
The team then studied the electronic health records of another 43,000 UPMC sepsis patients from 2013 to 2014. The findings held. And they held again when the team studied rich clinical data and immune response biomarkers from nearly 500 pneumonia patients enrolled at 28 hospitals in the U.S.
In the next part of the study, Dr. Seymour and his team applied their findings to several recently completed international clinical trials that tested different promising therapies for sepsis—all of which had ended with unremarkable results.
When trial participants were classified by the four sepsis types, some trials might not have been failures. For example, early goal-directed therapy (EGDT), an aggressive resuscitation protocol that includes placing a catheter to monitor blood pressure and oxygen levels, delivery of drugs, fluids and blood transfusions was found in 2014 to have no benefit following a five-year, $8.4 million study. But when Dr. Seymour’s team re-examined the results, they found that EGDT was beneficial for the Alpha type of sepsis patients. Conversely, it resulted in worse outcomes for the Delta subtype.
“Intuitively, this makes sense—you wouldn’t give all breast cancer patients the same treatment. Some breast cancers are more invasive and must be treated aggressively. Some are positive or negative for different biomarkers and respond to different medications,” said senior author Dr. Angus. “The next step is to do the same for sepsis that we have for cancer—find therapies that apply to the specific types of sepsis and then design new clinical trials to test them.”
Additional authors on this research publication are Jason N. Kennedy, MS, Shu Wang, MS, Chung-Chou H. Chang, PhD, Zhongying Xu, MS, Hernando Gomez, MD, MPH, David Huang, MD, Qi Mi, PhD, Victor Talisa, MS, Shyam Visweswaran, MD, PhD, and Donald M. Yealy, MD, all of Pitt; Corrine F. Elliott, MS, and Scott Berry, PhD, both of Berry Consultants in Texas; Steven M. Opal, MD, of Rhode Island Hospital; Tom van der Poll, MD, PhD, of Pitt and the University of Amsterdam; Jeremy C. Weiss, MD, PhD, of Carnegie Mellon University; and Sachin Yende, MD, MS, of Pitt and the VA Pittsburgh Healthcare System.
Illustration: New findings from the University of Pittsburgh School of Medicine reveal that sepsis is not just one disease. Credit: Speed Drawing Video.
Following Injury, A New Approach Could Help Rebuild Muscle
McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Rando, MD, PhD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, and California and Florida researchers recently developed a two-pronged method that makes mouse leg muscles regrow better. Their research was recently published in Communications Biology.
As reported by Mandy Erickson for Scope, after a traumatic injury to arms and legs, surgeons will first repair bone and nerves, because without them, you can’t move your limbs. They worry about the muscle later: Muscle can regenerate to some degree, and even with diminished muscle, you can still move.
“Muscle is lower down on the totem pole,” said Ngan Huang, PhD, an assistant professor in cardiothoracic surgery and a principal investigator at Veterans Affairs Palo Alto Health Care System. In her work at the VA, Dr. Huang has seen how muscle injuries from war trauma can cripple veterans: “The patients still have some muscle — they can use the limb — but it’s much weaker.”
The only option currently available is to transplant muscle from another part of a patient’s body. It doesn’t always work, but even when it does, it weakens the muscle that acted as a donor. Dr. Huang and her team wondered: What if we are able to give injured muscle a boost?
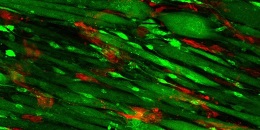
The recipe for enticing muscle to regenerate involves laying strips of collagen — a fibrous protein found in the skin and connective tissues, among other areas — in neat parallel rows in a petri dish. Then, researchers add endothelial cells, a type of cell found on the insides of blood vessels. After allowing the cells to multiply for nine days, researchers implanted the endothelium-enriched scaffolding into the injured muscle.
The photo shows the scaffolding on day nine; endothelial cells appear red, and muscle cells appear green.
For comparison, the researchers also implanted scaffolding without endothelial cells as well as scaffolding that wasn’t arranged in rows. The muscles with parallel scaffolding plus endothelial cells functioned far better than the control muscles: They contracted in a more synchronized manner when they were stimulated electrically, and they contained more blood vessels; the muscle cells were also longer.
“We didn’t think the combination of aligned scaffolding and endothelial cells would be so effective,” Dr. Huang said, but she has a clue why it worked: The neat rows coaxed the muscle progenitor cells to form parallel bundles of muscle fibers, the way they are normally organized in the body.
“Regeneration is a complex process,” she said. “The scaffolding orients the cells; it provides spatial cues. The endothelial cells are promoting integration with the vessels of the host. Both are equally important for muscle regeneration to happen in an effective way.”
The researchers plan to continue studying the technique and, if it appears successful, eventually try it out in patients.
Illustration: Scaffolding on day nine; endothelial cells appear red, and muscle cells appear green. Courtesy of Karina Nakayama.
Shedding Light on ‘Black Box’ of Inpatient Opioid Use

People who receive opioids for the first time while hospitalized have double the risk of continuing to receive opioids for months after discharge compared with their hospitalized peers who are not given opioids, according to research led by scientists at the University of Pittsburgh Graduate School of Public Health.
McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, chair of Pitt’s Department of Critical Care Medicine, is senior author on this research. The findings, published in the Annals of Internal Medicine, are among the first to shed light on the little-studied causes and consequences of inpatient opioid prescribing.
“I was surprised by the level of opioid prescribing to patients without a history of opioid use,” said lead author Julie Donohue, PhD, professor in Pitt Public Health’s Department of Health Policy and Management. “About half of the people admitted to the hospital for a wide variety of medical conditions were given opioids. The stability of this prescribing also was surprising. Nationally and regionally, as people have become more aware of how addictive opioids can be, we’ve seen declines in outpatient opioid prescribing. But we didn’t see that in inpatient prescribing.”
Previous studies have shown that some surgical and medical patients who fill opioid prescriptions immediately after leaving the hospital go on to have chronic opioid use. Until this study, however, little was known about how and if those patients were being introduced to the opioids while in the hospital.
Dr. Donohue and her colleagues reviewed the electronic health records of 191,249 hospital admissions of patients who had not been prescribed opioids in the prior year and were admitted to a community or academic hospital in Pennsylvania between 2010 and 2014.
Opioids were prescribed in 48% of the admissions, with those patients being given opioids for a little more than two-thirds of their hospital stay, on average.
Almost 6% of patients receiving opioids during their hospital stay were still being prescribed opioids three months later, compared with 3% of those without inpatient opioid use. And 7.5% of patients who received opioids less than 12 hours before discharge were still receiving opioids 90 days later, compared with 3.9% of their peers who were free of opioids for at least 24 hours prior to discharge.
Additionally, non-opioid painkillers and anti-inflammatory medications, such as ibuprofen, aspirin or naproxen, were rarely tried before an opioid was administered—as little as 7.9% of the time for some conditions.
“Inpatient opioid use has been something of a black box,” Dr. Donohue said. “And, while our study could not assess the appropriateness of opioid administration, we identified several practices—low use of non-opioid painkillers, continuous use of opioids while hospitalized, opioid use shortly before discharge—which may be opportunities to reduce risk of outpatient opioid use and warrant further study.”
Creating Lungs “From Scratch”

Erica Comber, a PhD student in Carnegie Mellon University’s (CMU’s) Department of Biomedical Engineering (BME), and her colleagues in Carnegie Mellon University’s Bioengineered Organs Initiative, are working to help people with lung disease by making them new ones.
Ms. Comber and her adviser Keith Cook, PhD, professor of biomedical engineering at CMU and an affiliated faculty member at the McGowan Institute for Regenerative Medicine, recently outlined several different approaches to creating human lungs from scratch in a first-of-its-kind paper.
“There’s a huge divergence of approaches in this field, and no one approach is necessarily more valid than another,” Ms. Comber said. “It’s about using different techniques to try to accomplish the same goal and then learning from each other.”
By identifying important parameters to consider and describing different approaches, the paper, published in Translational Research, will act as a guide to future researchers looking to create human organs de novo, or “from scratch.”
Approaches span from using existing biological organs as a starting point — by removing cells from existing organs and recellularizing them with the patient’s own cells — to generating completely artificial organs.
Ms. Comber’s work is a hybrid of those two approaches. Using natural materials such as collagen type 1, Ms. Comber makes artificial lungs that can be housed within the chest and designed in geometries that optimize how much oxygen and carbon dioxide can be cycled in and out of the circulatory system.
Existing artificial lungs are largely stopgap measures, and a plethora of complications can arise from their use. The average duration of use is about a week, and a patient’s chance of surviving the therapy shrinks the longer they’ve been supported. Artificial lungs made from polymer also can cause blood to form clots on the surface, which is why they fail and must be frequently replaced. Drugs used to slow blood clots also can cause bleeding, and each time artificial lungs are replaced, the patient can be exposed to a risk of infection.
De novo lung biofabrication could be the key to solving these issues. By designing artificial lungs that can be permanently attached to the circulatory system, and that can be created in geometries that approximate lung geometries but optimize for gas exchange, researchers could remedy the blood clotting and bleeding risk associated with existing artificial lungs.
“We have a long way to go, but we expect to see these de novo organs commercially available in our lifetime,” Ms. Comber said.
Ms. Comber is co-advised by Dr. Cook and Adam Feinberg, PhD, associate professor of biomedical engineering and materials science and engineering at CMU and an affiliated faculty member at the McGowan Institute of Regenerative Medicine.
Illustration: Carnegie Mellon University.
Using Hep C Infected Organs in Heart Transplant Operations

Hepatitis C is a liver disease caused by the hepatitis C virus (HCV), which is carried in the blood of infected people. According to various estimates, anywhere from 3 to 10 million people in the United States are carriers of the virus. One reason for this is because the virus wasn’t even diagnosed until the late 1980s. In fact, a majority of carriers are still unaware of their HCV status.
Hepatitis C is serious for some people, but not for others. Most people who get hepatitis C carry the virus for the rest of their lives. The majority will experience some liver damage but may not feel sick from the disease. Some people with liver damage, due to hepatitis C, may develop cirrhosis (scarring) of the liver and liver failure, which may take many years to develop.
But today there’s good news about Hep C: Thanks to a new generation of antiviral drugs, the cure rate for those with the virus is 95 percent. And progressive hospitals like UPMC, are taking the first steps in using this breakthrough to access a larger pool of donor hearts with the hope of narrowing the chronic and life-threatening shortage of organs that plagues transplantation.
“It’s a case of balancing risks,” says McGowan Institute for Regenerative Medicine affiliated faculty member Christopher Sciortino, MD, PhD, Assistant Professor of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine and Surgical Director of the UPMC Advanced Heart Failure Center. “With the new drugs, the risk of dying from Hep C is very small, and you balance that against a heart patient’s risk of dying from heart disease, which can be much, much higher.”
“I think this is an important thing we’re doing,” Dr. Sciortino says. “That’s why UPMC has established a conservative research protocol that helps us do it in the safest way possible. I want to provide my patients with whatever advantage they can get as long as it’s safe.”
UPMC, where organ transplantation was greatly advanced in the 1980s by the pioneering research of Dr. Thomas E. Starzl, is one of a handful of hospitals with the resources and experience to perform this kind of transplant operation. Dr. Sciortino expects the research opportunities and the quality of the patient experience to take another giant step with the completion of the UPMC Heart and Transplant Hospital in 2020.
“As in any hospital, the people are what make this a special place,” he says. “The new facility will have a whole new care environment that will add on top of that.”
The New Wave of Brain-Computer Interface Technology
Researchers have made groundbreaking strides in brain-computer interface (BCI) research, allowing paralyzed individuals to connect mind to machine and control robotic devices with their brains. The Defense Advanced Research Projects Agency (DARPA) wants to tap into this breakthrough technology and develop a nonsurgical option that provides a new way for able-bodied individuals to interact with machines.
Through the Next-Generation Nonsurgical Neurotechnology (N3) program, the agency selected Battelle and Carnegie Mellon University (CMU) to lead projects and awarded each institution funding totaling nearly $20 million over four years. Both projects include the University of Pittsburgh’s Douglas Weber, PhD, Robert Gaunt, PhD, and Jennifer Collinger, PhD. Dr. Weber is an affiliated faculty member of the McGowan Institute for Regenerative Medicine and associate professor in the Department of Bioengineering at the University of Pittsburgh with secondary appointments in the Department of Physical Medicine and Rehabilitation, the Department of Rehabilitation Science and Technology, and the Center for the Neural Basis of Cognition.
The most effective neural interfaces require surgery to implant electrodes into the brain, but DARPA’s N3 program aims to develop a high-resolution, portable neural interface system that is either completely noninvasive or only minutely invasive, making the technology accessible to a wider population of potential users. Most current BCI technology helps individuals with disabilities perform everyday tasks, but DARPA wants to progress the technology to able-bodied individuals, starting with military service members.
Dr. Weber directs the Rehab Neural Engineering Labs where his group has developed systems that enable individuals to control and feel prosthetic limbs through direct connections to the nervous system. He will lead the preclinical safety and efficacy studies for designs from Battelle and CMU.
“The goal of this program is to create and demonstrate new, noninvasive technologies for interfacing with the brain at high resolution,” said Dr. Weber. “The Battelle and CMU investigators are working on unique technologies that may suit this purpose, and I will work with those teams to validate and refine the technology in animal and human BCI studies.”
The CMU team is led by Pulkit Grover, PhD, associate professor of electrical and computer engineering (ECE), along with Maysam Chamanzar, PhD, assistant professor of ECE, and Jana Kainerstorfer, PhD, assistant professor of biomedical engineering. The group will apply novel concepts in physics, biology, and engineering to fight dispersion of waves as they enter the head. McGowan Institute for Regenerative Medicine affiliated faculty member Shawn Kelly, PhD, Senior Systems Scientist at the Institute for Complex Engineered Systems at CMU with courtesy faculty appointments in the Electrical and Computer Engineering and Biomedical Engineering Departments, is also a member of this team.
“Our team has taken on the ambitious goal of completely noninvasive sensing and stimulation at unprecedented spatiotemporal resolution,” said Dr. Grover. “The noninvasive aspect will make our solutions widely applicable, but it is also what makes our goal extremely challenging. Fundamentally, all waves – light, ultrasound, electrical currents – disperse in the head due to presence of a thick skull. To compensate for this, we are leveraging two completely new technologies being developed at CMU. In Drs. Weber, Gaunt, and Collinger at Pitt, we have the ideal collaborators to validate and improve these technologies and bring them that much closer to practice.”
Battelle is the prime on a second N3 program. Gaurav Sharma, PhD, a senior research scientist in the Medical Devices and Neuromodulation group, and his team have created a concept for the N3 program called BrainSTORMS (Brain System to Transmit Or Receive Magnetoelectric Signals). This technology involves the development of a novel nanotransducer that would be delivered through an intravenous injection and then targeted to a specific area of the brain. When the task is complete, the nanotransducer will be magnetically guided out of the brain for clearance out of the body.
“This is one of the most exciting and challenging projects I have worked on,” said Dr. Sharma in a prepared statement. “With BrainSTORMS, we will again be pushing the limits of engineering and physics. If successful, this technology would not only provide a safe and efficient way to facilitate human-machine interactions but also has the potential to revolutionize how the nervous system is probed and studied.”
Fellow RNEL Lab members Drs. Gaunt and Collinger, assistant professors of physical medicine and rehabilitation, will help to validate these technologies in first-in-human trials. All three researchers have experience with BCI technology and neuroprosthetics; they aim to better understand how humans use sensory information to regulate actions and apply that knowledge to prosthetic devices. They will use their expertise in this field to run the human trials of the developed N3 program technologies.
Illustration: Rehab Neural Engineering Labs.
Pitt, CMU to Create an Autonomous Robotic Trauma Care System
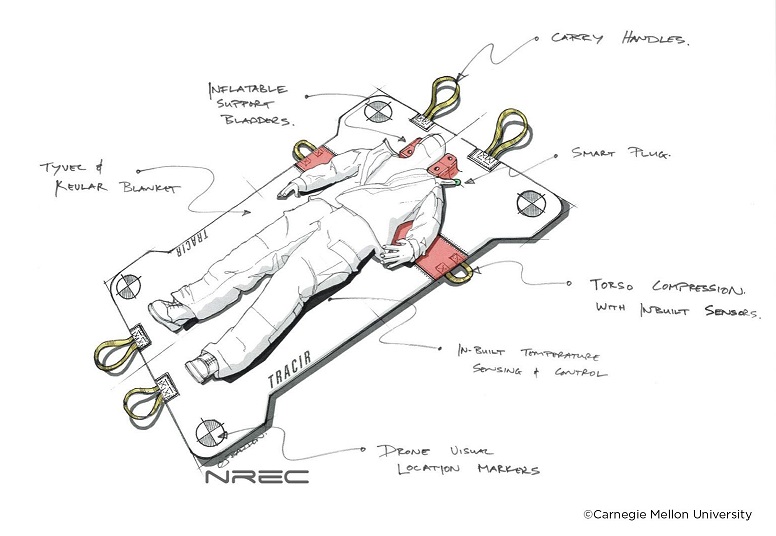
The University of Pittsburgh School of Medicine and Carnegie Mellon University each have been awarded four-year contracts totaling more than $7.2 million from the U.S. Department of Defense to create an autonomous trauma care system that fits in a backpack and can treat and stabilize soldiers injured in remote locations.
The goal of “TRAuma Care In a Rucksack: TRACIR” is to develop artificial intelligence (AI) technologies enabling medical interventions that extend the “golden hour” for treating combat casualties and ensure an injured person’s survival for long medical evacuations.
A multidisciplinary team of Pitt researchers and clinicians from emergency medicine, surgery, critical care and pulmonary fields will provide a wealth of real-world trauma data and medical algorithms that CMU roboticists and computer scientists will incorporate in the creation of a “hard and soft robotic suit” into which an injured person can be placed. Monitors embedded in the suit will assess the injury, and AI algorithms will guide the appropriate critical care interventions and robotically apply stabilizing treatments, such as intravenous fluids and medications.
McGowan Institute for Regenerative Medicine affiliated faculty member Ron Poropatich, MD, retired U.S. Army colonel, director of Pitt’s Center for Military Medicine Research and professor in Pitt’s Division of Pulmonary, Allergy and Critical Care Medicine, is overall principal investigator on the $3.71 million Pitt contract, with McGowan Institute affiliated faculty member Michael Pinsky, MD, professor in Pitt’s Department of Critical Care Medicine, as its scientific principal investigator. Artur Dubrawski, PhD, research professor at CMU’s Robotics Institute, is principal investigator on the $3.5 million CMU contract.
“Battlefields are becoming increasingly remote, making medical evacuations more difficult,” said Dr. Poropatich. “By fusing data captured from multiple sensors and applying machine learning, we are developing more predictive cardio-pulmonary resuscitation opportunities, which hopefully will conserve an injured soldier’s strength. Our goal with TRACIR is to treat and stabilize soldiers in the battlefield, even during periods of prolonged field care, when evacuation is not possible.”
Much technology still needs to be developed to enable robots to reliably and safely perform tasks, such as inserting IV needles or placing a chest tube in the field, Dr. Dubrawski said. Initially, the research will be “a series of baby steps,” demonstrating the practicality of individual components the system will eventually require.
“Everybody has a slightly different vision of what the final system will look like,” Dr. Dubrawski added. “But we see this as being an autonomous or nearly autonomous system—a backpack containing an inflatable vest or perhaps a collapsed stretcher that you might toss toward a wounded soldier. It would then open up, inflate, position itself and begin stabilizing the patient. Whatever human assistance it might need could be provided by someone without medical training.”
With a digital library of detailed physiologic data collected from more than 5,000 UPMC trauma patients, Drs. Pinsky and Dubrawski previously created algorithms that could allow a computer program to “learn” the signals that an injured patient’s health is deteriorating before damage is irreversible and tell the robotic system to administer the best treatments and therapies to save that person’s life.
“Pittsburgh has the three components you need for a project like this—world-class expertise in critical care medicine, artificial intelligence and robotics,” Dr. Dubrawski said. “That’s why Pittsburgh is unique and is the one place for this project.”
While the immediate goal of the project is to carry forward the U.S. military’s principle of “leave no man behind,” and treat soldiers on the battlefield, there are numerous potential civilian applications, said Dr. Poropatich.
“TRACIR could be deployed by drone to hikers or mountain climbers injured in the wilderness; it could be used by people in submarines or boats; it could give trauma care capabilities to rural health clinics; or be used by aid workers responding to natural disasters,” he said. “And, someday, it could even be used by astronauts on Mars.”
Illustration: Title: TRACIR. Caption: This artistic rendering shows what TRAuma Care In a Rucksack: TRACIR, an autonomous trauma care system being created by the University of Pittsburgh and Carnegie Mellon University, could look like. Credit: National Robotics Engineering Center and Carnegie Mellon University.
Winners of First RoosterBio Inc. hMSC Development Grant Announced

RoosterBio, a regenerative medicine manufacturing technology leader, announced four winners of the first RoosterBio Human Mesenchymal Stem/Stromal Cell (hMSCs) Development Grant aimed at facilitating investigators who share the mission of RoosterBio, Inc to accelerate the path to clinical translation for adult cell-based therapeutics.
Two of the four award recipients/projects included efforts from McGowan Institute for Regenerative Medicine affiliated faculty members. They are:
- Ipsita Banerjee, PhD, Associate Professor, Department of Chemical and Petroleum Engineering, University of Pittsburgh: “Towards a biometric islet organoid engineering for applications in diabetes therapy and drug discovery”
- David Vorp, PhD, Associate Dean for Research, Swanson School of Engineering, University of Pittsburgh, the John A. Swanson Professor of Bioengineering, with secondary appointments in the Departments of Cardiothoracic Surgery, Surgery, and the Clinical & Translational Sciences Institute at the University of Pittsburgh, a Co-Director of the Center for Medical Innovation, and the Director of the Vascular Bioengineering Laboratory: “Vascular regeneration driven by adipose-derived hMSC”
The award winners receive RoosterBio’s cell and media products totaling up to $25,000 each and an additional $500 RoosterBio Travel Grant to support trainees presenting a poster or talk at an eligible conference.
RoosterBio launched the hMSC Development Grant in 2018 to supply standardized, highly engineered hMSC bioprocess systems to streamline product development and manufacturing process design for projects in early stage development, focused on proof of concept and/or understanding the impact of donor variability or manufacturing scale-up on final product quality attributes. Priority was given for projects identified in focus areas including gene editing, exosomes or extracellular vesicles (EVs), tissue engineering and bioreactor expansion; however, applications for all emerging areas were encouraged. Grant applicants were required to be affiliated with a research and development (R&D) lab at a university or industry setting with access to lab space.
“MSCs are a critical raw material for many regenerative medicine (RM) products which until now have faced a significant bottleneck in RM product development,” said RoosterBio CEO Margot Connor. “Breaking down those barriers requires process innovations that lead to a paradigm shift in adult stem cell product development, clinical translation and commercial-scale manufacturing. We are pleased to support each of the winners with our systems and watch their research move forward.”
Congratulations, Drs. Banerjee and Vorp!
Boninger Lab Student Receives NIH F30 Award

University of Pittsburgh graduate student Stephanie Rigot, DPT, received an F30 Individual Predoctoral NRSA Fellowship from the National Institutes of Health. The award provides funding for students who are matriculated in a combined dual-doctoral degree training program and who intend to pursue careers as physician-scientists or other clinician-scientists.
Dr. Rigot is a member of the inaugural class of the Doctor of Physical Therapy/PhD in Bioengineering (DPT-PhD) dual-degree program, a unique offering that integrates clinical and research experiences in the School of Health and Rehabilitation Sciences and the Swanson School of Engineering.
“This program combines the outstanding evidence-based physical therapy education and innovative bioengineering research training that already exists at the university and builds upon synergies between faculty members of the nationally-ranked Departments of Bioengineering and Physical Therapy,” said Patrick Sparto, associate professor of physical therapy and co-director of the DPT-PhD program.
Dr. Rigot works in the lab of McGowan Institute for Regenerative Medicine affiliated faculty member Michael Boninger, MD, Professor and UPMC Endowed Vice Chair for Research in the Department of Physical Medicine & Rehabilitation, where she aims to develop a new measure of impairment after spinal cord injury using leg movements measured by activity monitors.
“Current testing, which is primarily measured by brute tests of strength and sensation, may not be sensitive enough to provide an accurate representation of an individual’s impairment and functional abilities,” explained Dr. Rigot. “Our new measure could be used to track an individual’s recovery over time, as well as provide a novel method to predict an individual’s long-term mobility potential using data collected soon after their spinal cord injury.”
The length of stay in inpatient rehabilitation after a spinal cord injury is decreasing, which forces clinicians to quickly make critical decisions about where to focus time in therapy to maximize an individual’s functional mobility.
Dr. Rigot plans to develop a new clinical prediction rule that would provide clinicians, individuals with spinal cord injuries, and their families with a more accurate and descriptive estimation of the individual’s future mobility. This strategy will allow patients to tailor their therapy and focus on the ideal interventions.
“If we can develop a tool to assist clinicians in determining the optimal interventions during therapy early after an injury, then we can hopefully improve the participation, quality of life, pain, and other outcomes for many of the nearly 18,000 people in the United States that experience a new spinal cord injury each year,” said Dr. Rigot.
Illustration: University of Pittsburgh Swanson School of Engineering.
NSF Awards $500,000 to Pitt Researchers to Create Neuromorphic Vision System Mimicking Human Sight

Self-driving cars rely on their ability to accurately “see” the road ahead and make adjustments based on what they see. They need to, for instance, react to a pedestrian who steps out from between parked cars, or know to not turn down a road that is unexpectedly closed for construction. As such technology becomes more ubiquitous, there’s a growing need for a better, more efficient way for machines to process visual information.
New research from the University of Pittsburgh will develop a neuromorphic vision system that takes a new approach to capturing visual information that is based on the human brain, benefitting everything from self-driving vehicles to neural prosthetics.
McGowan Institute for Regenerative Medicine affiliated faculty member Ryad Benosman, PhD, professor of ophthalmology at the University of Pittsburgh School of Medicine who holds appointments in electrical engineering and bioengineering, and Feng Xiong, PhD, assistant professor of electrical and computer engineering at the Swanson School of Engineering, received $500,000 from the National Science Foundation (NSF) to conduct this research.
Conventional image sensors record information frame-by-frame, which stores a great deal of redundant data along with that which is useful. This excess data storage occurs because most pixels do not change from frame to frame, like stationary buildings in the background. Inspired by the human brain, the team will develop a neuromorphic vision system driven by the timings of changes in the dynamics of the input signal, instead of the conventional image-based system.
“With existing neuromorphic camera systems, the communication between the camera and the computing system is limited by how much data it is trying to push through, which negates the benefits of the large bandwidth and low power consumption that this camera provides,” says Dr. Xiong. “We will use a spiking neural network with realistic dynamic synapses that will enhance computational abilities, develop brain-inspired machine learning to understand the input, and connect it to a neuromorphic event-based silicon retina for real-time operating vision.”
This system will work more efficiently than existing technology, with orders of magnitude better energy efficiency and bandwidth.
“We believe this work will lead to transformative advances in bio-inspired neuromorphic processing architectures, sensing, with major applications in self-driving vehicles, neural prosthetics, robotics and general artificial intelligence,” says Dr. Benosman.
The grant will run July 1, 2019 to June 30, 2022.
AWARDS AND RECOGNITION

Dr. Garrett Coyan Receives the 2019 Resident Academic Leadership Award
Garrett Coyan, MD, MS, resident in the Six-Year Integrated Cardiothoracic Surgery Residency Program was awarded the 2019 Resident Academic Leadership Award by a faculty committee in the Department of Cardiothoracic Surgery, University of Pittsburgh. This award was announced at the Chairman’s Dinner and Resident Graduation Event on June 28, 2019, at the Fox Chapel Golf Club. Dr. Coyan’s research in the laboratory of McGowan Institute for Regenerative Medicine director William Wagner, PhD, Professor of Surgery, Bioengineering and Chemical Engineering at the University of Pittsburgh, is focused on tissue engineered heart valves.
Congratulations, Dr. Coyan!
Dr. Anthony Delitto Appointed Member of National Advisory Council

McGowan Institute for Regenerative Medicine affiliated faculty member Anthony Delitto, PhD, professor and dean of the School of Health and Rehabilitation Sciences and professor in the Department of Physical Therapy at the University of Pittsburgh, was recently appointed as a member of the National Advisory Council for Complementary and Integrative Health.
The council is responsible for advising, consulting with and making recommendations to the director of the National Center for Complementary and Integrative Health on matters relating to the research activities and functions of the center.
Dr. Delitto treats people with painful musculoskeletal disorders, and his current research is focused on implementing classification and treatment effectiveness studies into quality improvement initiatives. He is also conducting trials in exercise interventions for people with Parkinson’s disease.
Congratulations, Dr. Delitto!
Brown Lab Student/sciVelo Alumna Spotlight on Elizabeth (Abby) C. Stahl, PhD

Elizabeth (Abby) Stahl, PhD, former student in the lab of McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, associate professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh, is one of many examples of how sciVelo rounds out mentoring with academic entrepreneurship experiences. Dr. Stahl is now a postdoctoral scholar with Jennifer Doudna, PhD, CRISPR-Cas9 inventor, at University of California, Berkeley.
Dr. Stahl’s graduate work was focused on characterizing phenotypic and functional changes in tissue-specific macrophage subsets during aging and applying these findings to modulate age-associated inflammation in the liver. Her PhD work also led to deeper understanding of mechanisms associated with various pathologies, including muscular dystrophy and cancer. As a result, she published 14 research articles in peer-reviewed journals and is the co-inventor of two invention disclosures filed through Pitt’s Innovation Institute.
sciVelo’s Ceren Tuzmen, PhD, reports that during her time at sciVelo as a Commercial Translation Architect, Dr. Stahl worked on translational research projects in immunotherapy, transplantation and molecular biology. She tackled diverse projects, including aiding investigators with customer discovery for the NIH NIDCR Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center, early-stage investment pitches for the Center for Commercial Applications of Healthcare Data of the Pittsburgh Health Data Alliance and helped to construct translational research proposals and invention disclosures for the UPMC Immune Transplant and Therapy Center, all with the goal of advancing translational research at the University of Pittsburgh. Although she successfully coordinated these projects and developed valuable skills, such as project prioritization, project management and team science, she admits it was challenging at times. “The most challenging thing about working with sciVelo is the high bar that sciVelo sets for its team members. But this was also one of the most productive experiences thanks to having great mentors to learn from.”
Dr. Stahl continues, “sciVelo fostered my career development in several ways. Most importantly to me, sciVelo helped to expand the way I communicated science by simplifying complex ideas into succinct concepts to highlight a commercial application such as a drug or a molecular diagnostic. I gained these skills from both editing invention disclosures and fine tuning translational-research-funding pitch slide decks. As a result, I felt more confident at communicating my perspectives to senior leadership, which I think was a very valuable skill!”
Illustration: sciVelo (Dr. Stahl). McGowan Institute (Dr. Brown).
Former Little Lab Student Candidate for 2019 NCAA Woman of the Year

The Atlantic Coast Conference (ACC) has selected Gillian Schriever, distance runner and 2019 graduate of the University of Pittsburgh’s Swanson School of Engineering, as one of the two ACC candidates for 2019 NCAA Woman of the Year. Duke University golfer Virginia Elena Carta also received the ACC’s nomination.
The NCAA Woman of the Year honors female student-athletes whose performance in academic achievement, athletics excellence, service and leadership stands out throughout their collegiate careers.
Ms. Schriever, who is originally from Tuckerton, NJ, earned a bachelor’s degree in chemical engineering from the Swanson School of Engineering and currently works as a Business Technology Analyst at Deloitte.
During her undergraduate work at Swanson, she researched polymer microspheres as a method for treating dry eye disease under Steven Little, PhD, Chairman of the Department of Chemical and Petroleum Engineering and the William Kepler Whiteford Endowed Professor in the Departments of Chemical and Petroleum Engineering, Bioengineering, Immunology, and Ophthalmology.
“Gillian was both a dedicated student and an exceptional athlete,” says Dr. Little. “She is a great role model for women in STEM, proving that with hard work and perseverance, they can achieve their academic and professional goals and excel in what they’re passionate about.”
Ms. Schriever later interned with the Bettis Naval Nuclear Laboratory in West Mifflin, PA, in her junior year.
NCAA member colleges and universities nominated a record-breaking 585 female student-athletes for 2019 NCAA Woman of the Year. The top 10 candidates from each division will be selected in September, and the selection committee will choose top three from each to make up the nine finalists. The national winner will be announced on Oct. 20, 2019, at the 2019 NCAA Woman of the Year Awards in Indianapolis.
Illustration: University of Pittsburgh Swanson School of Engineering.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#199 –– Dr. Themis Kyriakides discusses his research in implanted biomaterials and tissues and in the development of biodegradable polymers. He also discusses his role in the Journal of Immunology and Regenerative Medicine..
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Zamora R, Barclay D, Yin J, Alonso EM, Leonis MA, Mi Q, Billiar TR, Simmons RL, Squires RH, Vodovotz Y
Title: HMGB1 is a Central Driver of Dynamic Pro-inflammatory Networks in Pediatric Acute Liver Failure induced by Acetaminophen
Summary: Acetaminophen (APAP) overdose (APAPo) is predominant in the NIH Pediatric Acute Liver Failure (PALF) Study. We assayed multiple inflammatory mediators in serial serum samples from 13 PALF survivors with APAPo + N-acetylcysteine (NAC, the frontline therapy for APAPo), 8 non-APAPo + NAC, 40 non-APAPo non-NAC, and 12 non-survivors. High Mobility Group Box 1 (HMGB1) was a dominant mediator in dynamic inflammation networks in all sub-groups, associated with a threshold network complexity event at d1-2 following enrollment that was exceeded in non-survivors vs. survivors. We thus hypothesized that differential HMGB1 network connectivity after day 2 is related to the putative threshold event in non-survivors. DyNA showed that HMGB1 is most connected in non-survivors on day 2-3, while no connections were observed in APAPo + NAC and non-APAPo + NAC survivors. Inflammatory dynamic networks, and in particular HMGB1 connectivity, were associated with the use of NAC in the context of APAPo. To recapitulate hepatocyte (HC) damage in vitro, primary C57BL/6 HC and HC-specific HMGB1-null HC were treated with APAP + NAC. Network phenotypes of survivors were recapitulated in C57BL/6 mouse HC and were greatly altered in HMGB1-null HC. HC HMGB1 may thus coordinate a pro-inflammatory program in PALF non-survivors (which is antagonized by NAC), while driving an anti-inflammatory/repair program in survivors.
Source: Scientific Reports. 2019 Apr 12;9(1):5971.
GRANT OF THE MONTH
PI: Ryad Benosman
Co-PI: Feng Xiong
Title: FET: Small: Neuromorphic Spiking Neural Networks with Dynamic Graphene Synapses for Event-based Computation
Description: With the emergence of social media and high-definition video streaming, there is a growing need for a more efficient way to process streams of visual information in terms of both bandwidth and energy. Currently, conventional image sensors record visual information frame by frame, unnecessarily acquiring huge amounts of redundant data since most pixels often may not change from one frame to the next. Inspired by the human brain, this project will develop a neuromorphic vision system, which is driven by the timings of changes in the dynamics of the input signal instead of the conventional image-based stroboscopic acquisition. This work will lead to transformative advances in bio-inspired neuromorphic processing architectures, sensing, with major applications in self-driving vehicles, neural prosthetics, robotics, and general artificial intelligence. The project team will work closely with local communities to encourage participation by students from all backgrounds including underrepresented group in computing careers by fostering interest in neuromorphic computing and artificial intelligence through outreach activities including lab demonstrations, summer internships, and career workshops.
The objective of this project is to build a brain-inspired vision system by integrating a neuromorphic, event-based silicon retina with a spiking neural network (SNN). In most existing neuromorphic vision systems, the communication between the event-based camera and the computing system is still limited by the memory bottleneck, largely negating the benefits of the large bandwidth and low power consumption of the neuromorphic camera. This project will: (1) build a spiking neural network with realistic graphene-based dynamic synapses allowing advanced computational capabilities; (2) develop a brain-inspired machine learning and general computation capabilities; (3) connect the developed hardware with a neuromorphic event-based silicon retina to demonstrate real-time operating vision system with orders of magnitude better energy efficiency and bandwidth.
Source: National Science Foundation
Term: July 1, 2019 – June 30, 2022 (Estimated)
Amount: $500,000
