August 2016 | VOL. 15, NO. 8| www.McGowan.pitt.edu
Pitt Researchers Identify Important Mechanism for Biological Scaffold Inductive Abilities

A study from the McGowan Institute for Regenerative Medicine identifies a mechanism by which bioscaffolds used in regenerative medicine influence cellular behavior, a question that has remained unanswered since the technology was first developed several decades ago. The findings were recently published online in Science Advances.
Bioscaffolds composed of extracellular matrix (ECM) derived from pig tissue promote tissue repair and reconstruction. Currently, these bioscaffolds are used to treat a wide variety of illnesses such as hernias and esophageal cancer, as well as to regrow muscle tissue lost in battlefield wounds and other serious injuries.
“Bioscaffolds fulfill an unmet medical need, and have already changed the lives of millions of people,” said lead study investigator Stephen Badylak, DVM, MD, PhD, Professor of surgery at Pitt and Deputy Director of the McGowan Institute, a joint effort of Pitt and UPMC. Other McGowan Institute affiliated faculty members involved in the study include Neill Turner, PhD, Research Assistant Professor in the Department of Surgery at Pitt, and Donna Stolz, PhD, Associate Director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and an Associate Professor in the Departments of Cell Biology and Pathology at Pitt.
Researchers know that ECM is able to instruct the human body to replace injured or missing tissue, but exactly how the ECM material influences cells to cause functional tissue regrowth has remained a fundamental unanswered question in the field of regenerative medicine.
In the new study, Dr. Badylak and his team showed that cellular communication occurs using nanovesicles, extremely tiny fluid-filled sacs that bud off from a cell’s outer surface and allow cells to communicate by transferring proteins, DNA, and other “cargo” from one cell to another.
Exosomes are present in biological fluids such as blood, saliva, and urine, where they influence a variety of cellular behaviors, but researchers had yet to identify them in solid body tissues.
“We always thought exosomes are free floating, but recently wondered if they are also present in the solid ECM and might facilitate the cellular communication that is critical to regenerative processes,” Dr. Badylak said.
To explore this possibility, researchers used specialized proteins to break up the ECM, similar to the process that occurs when a bioscaffold becomes incorporated into the recipient’s tissue.
The research team then exposed two different cell types – immune cells and neuronal stem cells – to isolated matrix bound vesicles, finding that they caused both cell types to mimic their normal regrowth behaviors.
“Sure enough, we found that vesicles are embedded within the ECM. In fact, these bioscaffolds are loaded with these vesicles,” Dr. Badylak said. “This study showed us that the matrix bound vesicles are clearly active, can influence cellular behavior, and are possibly the primary mechanism by which bioscaffolds cause tissue regrowth in the body.”
Researchers also found that vesicles isolated from different source tissues have distinct molecular signatures, and they are now focused on harnessing this new information for both therapeutic and diagnostic purposes.
Illustration: Manual removal of muscle layers from porcine urinary bladder. The Badylak Laboratory.
RESOURCES AT THE MCGOWAN INSTITUTE
September Special at the Histology Lab
Make no Bones about it! The McGowan Histology Core has a Bone to pick FOR YOU!
The Histology lab offers decalcification, processing and embedding, cutting and staining of those difficult boney samples. Our staff has years of experience with bone samples that include special staining and IHC on sometimes difficult to work with, large and small bone samples. We have even developed a technique to insure your sample will not lift from the slide during aggressive antigen retrieval protocols.
Enjoy these beautiful examples, of just some of the special stains we offer at the McGowan Histology Core.
 Alcian Blue |
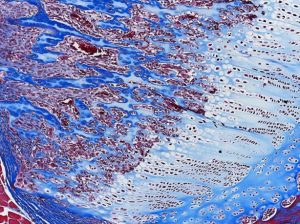 Masson Trichrome |
|---|---|
 Saffron O |
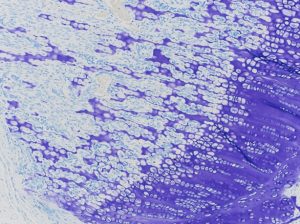 Toloidine Blue |
You’ll receive 25% off bone samples in September when you mention this ad. Contact Lori at the McGowan Core Histology Lab by email: perezl@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Flow Cytometry at the McGowan Institute – Our BDFACSAria cell sorter is going GREEN!
 The Flow Cytometry Laboratory at the McGowan Institute is increasing its performance capabilities to better serve the University of Pittsburgh’s research programs with the installation of a Coherent Sapphire 100mW 552nm Light Green Laser. This laser expands our range of applications while maintaining everything we are currently able to do.
The Flow Cytometry Laboratory at the McGowan Institute is increasing its performance capabilities to better serve the University of Pittsburgh’s research programs with the installation of a Coherent Sapphire 100mW 552nm Light Green Laser. This laser expands our range of applications while maintaining everything we are currently able to do.
New applications and abilities include1:
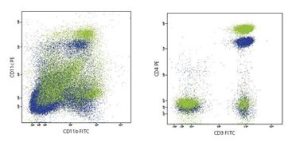 Optimal excitation of classic PE and PE tandem fluorophores as well as excitation of AF594 so dim populations in the PE channels should be more readily resolved.
Optimal excitation of classic PE and PE tandem fluorophores as well as excitation of AF594 so dim populations in the PE channels should be more readily resolved.- Decreased interference by autofluoresence – again making those dim populations more easily seen.
- Less required compensation for PE vs FITC and/or GFP, minimizing problems due to spectral spreading error.
- Optimal excitation of the RFP’s, including dsRed, Kusabira Orange, dTomato, Killer Red, and the frequently requested—mCherry. No more guessing if your transfection actually worked.
- Advanced flow techniques such as:
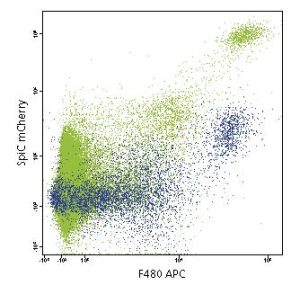 SmartFlare live cell mRNA and miRNA analysis and sorting – analyze and sort cells based on 2 RNA signatures utilizing both Cy3 and Cy5 probes.
SmartFlare live cell mRNA and miRNA analysis and sorting – analyze and sort cells based on 2 RNA signatures utilizing both Cy3 and Cy5 probes.- FUCCI cell cycle model.
- Use of dual emission dyes such as JC-1 to measure mitochondrial potential.
Installation is tentatively scheduled sometime during the week of 8/29/16. Updated analysis and sort worksheets (pdf format) can be downloaded from our website after Labor Day or you can request docx formatted files from Lynda.
A 2-hr Flow Cytometry Laboratory Open House will be held Monday, 9/12/16, 1:30 – 3:30pm for anyone interesting in learning more about how this new laser can be utilized in your research. Contact Lynda Guzik, Flow Cytometry Laboratory Manager, for additional details: 412-648-8660 or guzilj@upmc.edu.
1Advantages of the 561-nm (Yello-Green) Laser on the BD FACSAria III. Catherine McIntyre, Gil Reinin, Dennis Sasaki, and Kevin Weller. BD Biosciences Application Note. April 2010.
UPCOMING EVENTS
Fifth Annual Symposium on Regenerative Rehabilitation

This symposium features renowned researchers and clinicians focused on regenerative rehabilitation treatments and therapies. This emerging field translates discoveries in tissue engineering and cellular therapies into treatments that benefit the functional outcomes of patients. These stimulating innovative approaches are explored with meeting attendees.
The symposium will be held on October 14-16, 2016 at Emory University in Atlanta, GA.
This symposium encourages participation of scientists, clinicians and physical therapists that are in the fields of regeneration and / or physical medicine and rehabilitation. This gathering is a platform for bridging these two areas of expertise in a setting that fosters discussion, interaction, cross discipline pollination intersection and networking.
For more information and to register, please click here.
SCIENTIFIC ADVANCES
Sampling Method Used for New Breast Cancer Tests May Lead to Underestimate of Risk

Not only is breast cancer more than one disease, but a single breast cancer tumor can vary within itself, a finding that University of Pittsburgh Cancer Institute (UPCI) researchers—including McGowan Institute for Regenerative Medicine affiliated faculty member Steffi Oesterreich, PhD, Professor of Pharmacology and Chemical Biology at the UPCI—discovered has the potential to lead to very different patient treatment plans depending on the tumor sample and diagnostic testing used.
The results, reported online and scheduled for an upcoming issue of the journal Clinical Cancer Research, demonstrate that tumor sampling techniques used with newly developed “personalized medicine” gene expression profile tests may need to be refined to ensure that the most appropriate tumor sections are selected for testing.
“These tests are a good thing—they’ve done an incredible job identifying women with breast cancers that have a low risk of recurrence who don’t need chemotherapy, saving them from the toxicity and discomfort of unnecessary treatment,” said Adrian V. Lee, PhD, professor of pharmacology and chemical biology at UPCI, partner with UPMC CancerCenter. “However, as with any new technology, we need to understand how these tests work, and we’re finding that the sampling process, which involves liquefying tumors, loses information that could be important in determining the best treatment plan for patients with more aggressive tumors.”
Gene expression profiling is an increasingly popular type of test that tells doctors what certain genes are doing in a tissue sample, such as causing the cells to actively divide and multiply. Several tests have been developed in recent years to aid oncologists in developing breast cancer treatment plans. They involve taking a small bit of the tumor—or multiple small bits mixed together—and testing it.
The tests can tell oncologists if the cancer has a low, intermediate, or high risk of recurring. The level of risk can help doctors and patients decide whether an aggressive treatment plan involving chemotherapy is beneficial or likely to do more harm than good.
Dr. Lee and his team examined 71 cases of a type of breast cancer called “estrogen-receptor-positive” that was caught early and hadn’t yet spread to other parts of the body. In all cases, the tumor had been removed and samples taken for gene expression profiling. A total of 181 samples were taken from various parts of the tumors, and the researchers measured the expression of 141 different genes from five different types of gene expression profile tests commonly used for breast cancer tumors.
For 25 percent of the patients, their tumors received a different risk of recurrence score depending on which sample was processed.
“This indicates that one part of the tumor is more aggressive than another part. If an oncologist were to know this, he or she would likely recommend a treatment plan tailored to destroy the most aggressive section of the tumor,” said Dr. Lee.
Because the patients in this study were all caught early, their risk of recurrence was low to begin with, and there weren’t enough recurrences to make a meaningful determination on whether they would have done better if more samples were tested from their tumors.
“It would be valuable to repeat this study with a much larger group of breast cancer patients and follow them over time so that we could definitively determine if the way sampling is done with these tests is, indeed, resulting in patients getting cancer recurrences that wouldn’t have happened if the sampling process was changed,” said Dr. Lee.
New Fully Dissolving Heart Stent

UPMC is the first hospital in western Pennsylvania to use Abbott’s fully dissolving Absorb GT1 Biodegradable Vascular Scaffold System (BVS), a first-of-its-kind device recently approved by the Food and Drug Administration (FDA). It functions by opening a blocked artery in the heart, restoring blood flow, and providing relief from symptoms of coronary artery disease (CAD). A 58-year-old woman with severe coronary artery disease was the first patient to receive the absorbable device.
“We’re only at the beginning of understanding the potential benefits of this technology that could have long-lasting impacts for our patients,” said McGowan Institute for Regenerative Medicine affiliated faculty member Catalin Toma, MD, assistant professor, Division of Cardiology, University of Pittsburgh School of Medicine, and director for interventional cardiology research at the UPMC Heart and Vascular Institute and one of the initial medical experts with access to the technology. “By resorbing in time, these implants allow for restoration of the vessel’s natural function without the restriction of the traditional metallic cage of the stent. This may have a positive effect on vessel healing for months and years to come.”
The most common type of heart disease, CAD occurs when arteries that supply blood to the heart become narrowed or blocked due to plaque buildup, leading to chest pain and shortness of breath, as well as an increased risk of heart attack. When patients present with heart attacks or chest discomfort due to plaque buildup, the obstruction is treated with metallic stents. Although this technology has been greatly improved in the past years, potential issues related to the permanent coronary implant may require additional procedures even many years after the initial operation.
Absorb GT1 BVS is made of naturally resorbable material that slowly disappears in about 3 years, leaving behind a restored vessel, free of a permanent implant. Metallic stents permanently restrict vessel movement, limiting future treatment options. Unrestricted vessels with restored function have the potential to flex, pulse, and dilate in response to various demands on the heart, based on people’s lifestyle and activities, and allow for potential treatment options down the road. Similar to current generation drug-eluting stents, the BVS is coated with a drug that prevents the re-narrowing of the vessel over time.
The procedure is similar to regular coronary stenting intervention, which is performed by accessing the artery in the groin or wrist, and threading catheters to the coronary arteries. The blockage is first opened with an angioplasty balloon, followed by balloon-expandable scaffold implantation. While complex and hardened blockages are better served by regular metallic stents at this stage of the technology, the BVS could be beneficial for younger patients with their first CAD presentation, Dr. Toma noted.
UPMC Presbyterian Shadyside is one of the few centers in the U.S. with experience in implanting the scaffolds. The hospital participated in the ABSORB III trial, which led to the recent FDA approval of the device, as well as the ABSORB IV trial, which compares the BVS to the top-performing current metallic stent technology.
The UPMC Heart and Vascular Institute is one of the world’s premier centers for comprehensive care, developing revolutionary devices and new models of treatment that improve the lives of those facing the most complex heart and vascular conditions.
Cumulative Risk for Relatively Safe Medication Like Statins Is Nontrivial

Guidelines on lipid-lowering treatment for primary prevention of cardiovascular disease (CVD) are provided by the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC). The recommendations for lipid-lowering treatment initiation from both guidelines are based on evidence from randomized clinical trials (RCTs) demonstrating the efficacy of statins for primary prevention of CVD. The trial evidence was translated by recommending initiation of treatment for adults with a predicted 10-year risk for CVD exceeding a given threshold. However, global-risk algorithms were never used as an enrollment criterion for RCTs. Therefore, it has been argued that risk-based allocation of statins does not fully reflect the existing evidence. The degree of overlap and discrepancy between U.S. and European guidelines in light of available trial evidence remains unclear. The Netherlands and U.S. authors from a recent paper published in JAMA Cardiology, aimed to compare recommendations from the latest ACC/AHA and ESC prevention guidelines with the evidence from 10 major primary prevention RCTs for statins.
Asked for comment on this study, McGowan Institute for Regenerative Medicine affiliated faculty member Joon Sup Lee, MD, Associate Professor of Medicine at the University of Pittsburgh, Division Chief of Cardiology, and Co-Director Heart and Vascular Institute at the University of Pittsburgh School of Medicine, who was not involved in the work, told Reuters Health by email, “The risk/benefit ratio of primary prevention of cardiovascular disease remains one of the most controversial and difficult areas. The study includes patients as young as 45 years old. Thus, we are talking about exposing a population to a lifelong, daily dose of a medication (which may be 40+ years here) to prevent a disease in a minority of those patients. The cumulative risk for even a relatively safe medication like statins, which have a long and admirable track record, is nontrivial.”
He added, “We are forced to extrapolate a long-term decision based on shorter term studies and evidence. Statins have been available for 30 years and even the best studies follow patients mostly for 5 to 10 years.”
“The paper adds important evidence to this difficult question, but is by no means definitive. In many ways, the identification of 23.6% who are likely not to benefit from statin therapy may be more important than identifying those who do qualify,” Dr. Lee continued. “It’s reasonable to conclude that the patients who qualify by multiple guidelines/studies are more likely to benefit than those whose profile fall into a conflicting recommendation depending on which guideline/study is applied. For the patients in this gray area, the decision remains very personalized.”
Illustration: UPMC.
University of Pittsburgh Licenses Novel Microneedle Patch to Pittsburgh Company

SkinJect, Inc., a Pittsburgh-based company, today announced its completion of a license with the University of Pittsburgh to its novel, minimally invasive treatment for common forms of non-melanoma skin cancer, basal cell and squamous cell carcinoma. Two million new cases of basal cell cancer are reported each year in the United States, and more than half of all patients suffer a recurrence. The new product under development could dramatically change the way these skin cancers are treated.
The SkinJect™ patch is a thumb-sized array of dissolvable microneedles that will deliver a chemotherapeutic agent to kill an existing skin cancer. The SkinJect patch will be applied once a week, for 3 weeks, in the doctor’s office. The microneedles, less than a millimeter long, dissolve within 15 minutes of application.
McGowan Institute for Regenerative Medicine affiliated faculty member Louis D. Falo, MD, PhD, who chairs Pittsburgh’s Department of Dermatology, is a co-inventor of the microneedle patch, along with O. Burak Ozdoganlar, PhD, Professor in Mechanical Engineering and Materials Science and Engineering, Carnegie Mellon University. Both inventors serve as scientific advisors to the company as it develops the array.
“The SkinJect patch is a promising novel approach to combating cancer,” said Dr. Falo. “Right now, a standard of care for basal cell carcinoma is Mohs micrographic surgery, which can be expensive, painful, and disfiguring. The SkinJect patch will offer a cost-effective way to treat an existing cancer and potentially prevent it from coming back again later in life.”
President and CEO of SkinJect Jim Nolan said SkinJect was being watched closely by both the medical and investment communities. “This device has the potential to transform the fields of dermatology and oncology. Its commercial future is extremely promising,” said Nolan.
“We’re excited to have concluded this deal with a company headquartered in Pittsburgh,” said Marc Malandro, Founding Director of the University of Pittsburgh Innovation Institute, who also serves as Chairman of Pennsylvania Bio, the state’s life sciences trade organization. “A number of local biotech entrepreneurs – including Anthony Florence, who played a pivotal role in providing early seed money for this project – have supported SkinJect. The company’s success is an affirmation of our region’s growing biotech community.”
The company plans to file an IND (Investigational New Drug) application, a request to the FDA to begin administering the device to humans, by late 4th quarter 2016 or early 1st quarter 2017.
Skin cancer is the most commonly occurring cancer in the United States. After the age of 65, 50% of the population will develop skin cancer with the majority diagnosed with basal cell carcinoma (BCC). BCC occurs typically on the face, head, or neck and is a highly disfiguring disease. Current therapies involve invasive surgical procedures that are time-consuming, associated with patient recovery/morbidity and are expensive. SkinJect is novel, single use, topical drug delivery patch that, like a ‘Band-Aid’, is applied to the affected skin of those diagnosed with skin cancer. It is based on a proprietary micro-needle array drug delivery platform that uniquely delivers a potent generic chemotherapeutic agent and modifier to kill existing skin cancer and induce a memory immune response to prevent cancer re-occurrence. This project has confirmed high physician and patient acceptance from initial customer feedback.
Illustration: SkinJect.
NIH-Funded Pitt Research Study to Evaluate New Voice Therapy Technique

A voice therapy program that was refined by experts at the UPMC Voice Center and successfully piloted on a small group of patients with voice disorders, will be reaching more patients due to a $300,000 National Institutes of Health (NIH) grant recently awarded to the University of Pittsburgh School of Medicine. McGowan Institute for Regenerative Medicine affiliated faculty member Clark Rosen, MD, Director of the University of Pittsburgh Voice Center and Professor of Otolaryngology in the School of Medicine, is a co-investigator on the 3-year grant (R03 DC015305) awarded by the National Institute on Deafness and other Communication Disorders.
The new voice therapy approach, Conversation Training Therapy (CTT), concentrates on voice training in spontaneous, conversational speech for patients with voice impairment.
“With this approach, we focus on patients becoming aware and efficient in conversation, instead of in voice exercises. Results from our initial trial showed that patients met their voice therapy goals in just three sessions, substantially below the number of sessions typically required in traditional voice therapy programs, which can take up to 12 or even 24 sessions. Patients also reported that they noticed marked improvement in their voice impairment,” said lead researcher Amanda Gillespie, PhD, assistant professor, Department of Otolaryngology, Pitt School of Medicine, and director of Clinical Research, UPMC Voice Center.
Treatment aimed at modifying behaviors that cause or contribute to voice disorders is the standard of care for many people experiencing voice issues. Although voice therapy is effective at treating voice disorders, substantial limitations exist with traditional treatment models. These limitations cause a protracted length of time in required treatment, as well as drop out and voice problem relapse rates approaching 70 percent, which contribute to the high costs associated with treating voice disorders.
CTT was developed by a team of expert voice-specialized speech-language pathologists at voice centers around the U.S. The goal of this study is to determine the effectiveness of CTT in the rehabilitation of patients with two common voice disorders—benign vocal fold lesions and muscle tension dysphonia. Once that is determined, the long term goal is to conduct multi-center trials comparing CTT to traditional voice therapy programs in people with voice disorders.
Pitt’s research study is the first to look at a voice therapy program based in theories of motor learning and neuroplasticity, developed with input from patients with voice disorders and expert, clinical speech-language pathologists.
“Results of the current research have the potential to dramatically change how voice therapy is delivered, including the necessary time spent in treatment, resulting in a potential savings of health care funds and improved quality of life for people with voice disorders,” said Jackie L. Gartner-Schmidt, PhD, co-investigator on the study, co-director of the UPMC Voice Center, and director of Speech-Language Pathology-Voice Division, Pitt School of Medicine.
Researchers will recruit 60 participants to undergo 4 weeks of treatment with a CTT-trained voice therapist. Each will be evaluated prior to starting CTT, before each treatment session, and at 1-week and 3-month intervals after the last CTT session. Outcome measures will include participant-perceived voice handicap, acoustic, aerodynamic, and audio-perceptual voice analyses, and will be compared to matched past patients who previously underwent traditional voice therapy. Participants are compensated for their time.
Drs. Weinbaum, Kameneva, and Waters Receive CMI Grants

The University of Pittsburgh’s Center for Medical Innovation (CMI) awarded grants totaling $140,000 to six research groups through its 2016 Round-1 Pilot Funding Program for Early Stage Medical Technology Research and Development. Two of the six research groups include projects of McGowan Institute for Regenerative Medicine affiliated faculty members—Justin Weinbaum, PhD, Marina Kameneva, PhD, and Jonathan Waters, MD. The six latest funding proposals include developing a novel vascular access system, a shunt for treatment of fetal hydrocephalus in-utero, a system for stroke rehabilitation, a cell therapy for treatment of aortic aneurysm, a method for treatment of sickle cell anemia, and a novel mechanical device for use in general surgery.
The McGowan Institute projects are:
Minimally invasive delivery of therapeutic cells to abdominal aortic aneurysm: Award to develop and perform preclinical testing of a new biological therapy for prevention and treatment of abdominal aortic aneurysm.
Kory Blose
PhD candidate, Department of Bioengineering
Swanson School of EngineeringJustin Weinbaum, PhD
Assistant Professor Bioengineering
Swanson School of EngineeringRyan McEnaney, MD
Division of Vascular Surgery, UPMC
John Curci, MD
Division of Vascular Surgery, UPMC
Reducing alloimmunization and sickle crisis in SCD patients using a novel method of replacing HbS with donor Hb in patient’s RBCs: Continuation award to develop and test a new method for reconditioning the blood of sickle cell patients.
Marina V. Kameneva, PhD
Department of Surgery and Bioengineering
McGowan Institute for Regenerative MedicineJonathan H. Waters, MD
Department of Anesthesiology & Bioengineering
Magee Womens HospitalMark Gartner, PhD
Department of Bioengineering
Swanson School of Engineering
CMI, a University Center housed in Pitt’s Swanson School of Engineering, supports applied technology projects in the early stages of development with “kickstart” funding toward the goal of transitioning the research to clinical adoption. Proposals are evaluated on the basis of scientific merit, technical and clinical relevance, potential health care impact and significance, experience of the investigators, and potential in obtaining further financial investment to translate the particular solution to healthcare.
“This is our fifth year of providing pilot funding, and our leadership team could not be more excited with the breadth and depth of this round’s awardees,” said Alan D. Hirschman, PhD, CMI Executive Director. “This early-stage interdisciplinary research helps to develop highly specific biomedical technologies through a proven strategy of linking UPMC’s clinicians and surgeons with the Swanson School’s engineering faculty.”
AWARDS AND RECOGNITION
Dr. David Whitcomb Receives Henry Lynch Award

McGowan Institute for Regenerative Medicine affiliated faculty member David Whitcomb, MD, PhD, the Giant Eagle Foundation Professor of Cancer Genetics, a Professor of Medicine, Cell Biology and Physiology, and Human Genetics, and the Chief of the Division of Gastroenterology, Hepatology, and Nutrition at the University of Pittsburgh School of Medicine, is the recipient of the Henry Lynch Award by the International Symposium on Inherited Diseases of the Pancreas for his hereditary pancreatitis gene discovery. He was selected by the 9th International Symposium on Inherited Diseases of the Pancreas committee. He received the award during the 2016 PancreasFest held in Pittsburgh.
“I focused on the pancreas mainly because I was told early on that it was a hopeless disease. A hopeless disease needs a champion and I decided to dedicate my career to solving an unsolvable problem and find a way to treat a so-called hopeless condition,” said Dr. Whitcomb.
PancreasFest is an annual meeting of pancreas physicians and translational researchers who convene during the last week of July to find new ways of working together to improve patient care. PancreasFest is an umbrella meeting that supports multiple smaller meetings with very focused goals. These include education programs, discussion sessions for new ideas, learning from other disciplines, investigator meetings, and efforts to help young investigators get started exporing their own ideas.
Drs. Badylak and Matyjaszewski—2016 Most Cited Researchers in Materials Science and Engineering

McGowan Institute for Regenerative Medicine affiliated faculty members Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery at the University of Pittsburgh, a Deputy Director of the McGowan Institute, and Director of the Center for Pre-Clinical Tissue Engineering within the Institute, and Krzysztof Matyjaszewski, PhD, J.C. Warner Professor of Natural Sciences at Carnegie Mellon University, are among the top 300 highly cited researchers or scholars in the field of materials science and engineering. The citation data is based on the Elsevier Scopus database.
MSE Supplies compiled the ranking data and listed the names and institutions of these highly cited authors. The highly cited researchers or scholars are either the corresponding author or the first author of their publications. MSE Supplies is a leading supplier of high quality materials and equipment for advanced materials research and manufacturing.
Congratulations, Drs. Badylak and Matyjaszewski!
Biotech Firm Licensing Pitt Patents Raises $57M

Based upon the work of McGowan Institute for Regenerative Medicine affiliated faculty member Joseph Glorioso III, PhD, Professor in the Department of Microbiology and Molecular Genetics at the University of Pittsburgh, and Paola Grandi, PhD, Assistant Professor, Department of Neurological Surgery at Pitt, Oncorus, Inc. will invest in researching and developing oncolytic viral constructs which will move through preclinical development and ultimately into clinical trials. Currently, the company’s lead candidate is in preclinical development for glioblastoma multiforme (GBM). The company will also expand and improve its technology platform and accelerate the development of pipeline programs in other forms of cancer. Oncorus, Inc. recently raised $57 million in a Series A funding round.
Oncorus, Inc. is an early-stage biotechnology company developing a next-generation immunotherapy platform of oncolytic viruses to treat several types of cancer, including highly malignant and aggressive cancers. A leader in corporate philanthropy, Oncorus has taken a pledge to donate a portion of product sales revenue to fund promising cancer research and to support cancer care in the developing world. The company is located in Kendall Square, Cambridge, Massachusetts.
Badylak Lab Student to Be Awarded TERMIS-AM Recognition

McGowan Institute for Regenerative Medicine is pleased to announce that Jenna Dziki, a doctoral student within the Bioengineering Department at the University of Pittsburgh and a graduate researcher in the Stephen Badylak Laboratory, will be awarded the 2016 Mary Ann Liebert, Inc. Outstanding Student Award during the upcoming 2016 TERMIS-AM Conference. The 2016 TERMIS-AM Conference will be held from December 11-14 in San Diego, California. The TERMIS-AM Mary Ann Liebert, Inc. Outstanding Student award presentation will be conducted on Wednesday, December 14th, after the keynote presentation.
Ms. Dziki’s award-winning manuscript will be published in the journal, Tissue Engineering, Part A. Her research focuses on the characterization of extracellular-matrix-mediated host innate immunomodulation and stem/progenitor cell activation. Specifically, Ms. Dziki is interested in macrophage polarization following ECM treatment in skeletal muscle and gut repair applications and the effects of polarized macrophages upon endogenous stem cell populations.
At the award presentation ceremony, Ms. Dziki will be presented with a plaque recognizing her outstanding research accomplishments within the tissue engineering and regenerative medicine field. She will also receive a complimentary registration to the 2016 TERMIS-AM Conference as well as an honorarium of $500 and reimbursement up to $1,000 to be used towards her travel expenses (airfare, hotel) to attend the conference.
Congratulations, Ms. Dziki!
2016 Wiegand Intern Completes Assignment

The Wiegand Summer Internship provides an opportunity for a high school senior, who resides in Allegheny County and who graduated in the spring of 2016, to spend 5 weeks learning first-hand about regenerative medicine research and scientific investigations in general.
The 2016 Wiegand Intern was Maya Black, a graduate of West Allegheny High School. Maya is now attending Purdue University. Her internship was in the Plastic Surgery Laboratory under the mentorship of McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD, associate professor, Department of Surgery, University of Pittsburgh, director of the Plastic Surgery Laboratory, and co-director of the Adipose Stem Cell Center. Maya worked on a project concerning peripheral nerve injuries in the wrist. The purpose of the project was to help regain pinching motion in human nerve injuries.
The Wiegand Summer Internship is made possible through an endowment from Mr. and Mrs. Bruce Wiegand, friends and supporters of the McGowan Institute for Regenerative Medicine. The endowment is designed to provide an opportunity for a high school senior to experience the opportunities and excitement of a career in science and engineering, with a focus on regenerative medicine.
Illustration: Pictured are (L to R): Gabriella DiBernardo, Maya Black, and Dr. Marra.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#162 –– Dr. Michael Jamieson is the Associate Director of the International Center for Regulatory Sciences, and an Assistant Professor Clinical Pharmacy, School of Pharmacy, University of Southern California. Dr. Jamieson discusses the relationship between industry and academic labs.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
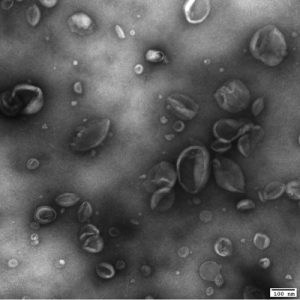
Transmission electron microscopy (TEM) imaging of Matrix-bound nanovesicles (MBVs) identified in proteinase K-treated Urinary bladder matrix (UBM). Scale bar, 100nm.
Similar images were recently published by Dr. Badylak’s group in Science Advances –
Huleihel, L., et al. (2016). “Matrix-bound nanovesicles within ECM bioscaffolds.” Sci Adv 2(6): e1600502.
PUBLICATION OF THE MONTH
Author: Gyanchandani R, Lin Y, Lin HM, Cooper KL, Normolle DP, Brufsky AM, Fastuca M, Crosson W, Oesterreich S, Davidson NE, Bhargava R, Dabbs DJ, Lee AV
Title: Intra-tumor heterogeneity affects gene expression profile test prognostic risk stratification in early breast cancer
Summary: Purpose: To examine the effect of intra-tumor heterogeneity (ITH) on detection of genes within gene expression panels (GEPs), and the subsequent ability to predict prognostic risk. Experimental Design: Multiplexed barcoded RNA analysis was used to measure the expression of 141 genes from five GEPs (Oncotype Dx, MammaPrint, PAM50, EndoPredict, and Breast Cancer Index) in breast cancer tissue sections and tumor-rich cores from 71 estrogen receptor (ER) positive node-negative tumors, on which clinical Oncotype Dx testing was previously performed. If the tumor had foci of high Ki67 (n=26), low/negative PR (n=13), or both (n=5), additional cores were obtained. In total, 181 samples were processed. Oncotype Dx recurrence scores were calculated from NanoString nCounter gene expression data. Results: Hierarchical clustering using all GEP genes showed that majority (61/71) of tumor samples clustered by patient, indicating greater inter-patient heterogeneity (IPH) than ITH. We found a strikingly high correlation between Oncotype Dx recurrence scores obtained from whole sections versus tumor-rich cores (r=0.94). However, high Ki67 and low PR cores had slightly higher but not statistically significant recurrence scores. For 18/71 (25%) patients, scores were divergent between sections and cores and crossed the boundaries for low, intermediate and high risk. Conclusions: Our study indicates that in patients with highly heterogeneous tumors, GEP recurrence scores from a single core could under- or over-estimate prognostic risk. Hence, it may be a useful strategy to assess multiple samples (both representative and atypical cores) to fully account for the ITH-driven variation in risk prediction.
Source: Clinical Cancer Research. 2016 May 16. pii: clincanres.2889.2015. [Epub ahead of print]
GRANT OF THE MONTH
PI: Amanda Gillespie
Co-I: Jackie L. Gartner-Schmidt, Clark Rosen, Jonathan Yabes
Title: Efficacy of Conversation Training Therapy
Description: Voice therapy, the behavioral treatment aimed at modifying behaviors that cause or contribute to voice disorders, is the standard of care for many people with voice disorders. Although voice therapy is effective at treating voice disorders initially; substantial limitations exist with traditional treatment paradigms. These limitations case a protracted length of time in required in treatment, as well as attrition and relapse rates approaching 70%, which contribute to the high costs associated with treating voice disorders. Traditional voice therapy builds hierarchically from breathing and relaxation exercises to production of single sounds, words, phrases, and finally, conversation. Patients report that this final step, transfer of skills to conversation, is the most difficult part of voice therapy. Motor learning theory states that training complex skills as a whole (e.g. conversation) facilitates learning and retention more-so than breaking these complexities down into simple components (e.g. single sounds). It is unknown if eliminating the therapeutic hierarchy and training target voice techniques in conversation, starting in the first session and throughout all sessions, would reduce the time spent in treatment and improve learning and retention, thereby reducing attrition and relapse. To that end, a new voice therapy approach, Conversation Training Therapy (CTT), was developed by a team of expert voice-specialized speech-language pathologists and successfully trialed on a small cohort of five patients with voice disorders. The CTT approach resulted in voice goals met in an average of 3 sessions, substantially below the number of sessions typically required in traditional therapy programs. Further, a clinically meaningful decrease in VHI-10 scores was observed for all patients. The goal of the current application is to determine the efficacy of CTT in the rehabilitation of patients with 2 common voice disorders, benign vocal fold lesions and muscle tension dysphonia. After determining initial efficacy, the long term goal of this research is to conduct multi-center comparative effectiveness trials comparing CTT to traditional voice therapy programs in people with voice disorders. This proposal is an investigation of the first voice therapy program based in theories of motor learning and neuroplasticity, developed without the use of a traditional therapeutic hierarchy, with input from patients with voice disorders and expert, clinical, speech-language pathologists. Results of the current research are potentially paradigm-shifting with regards to how voice therapy is delivered, including the necessary time spent in treatment, resulting in a potential savings of billions of dollars to the healthcare system and improved quality of life for people with voice disorders.
Source: National Institute on Deafness and other Communication Disorders
Term: 03/10/16 – 02/28/19
Source: $149,493 per year
