October 2017 | VOL. 16, NO. 10| www.McGowan.pitt.edu
An Engineer’s Guide to the Embryo

In roughly 48 hours, the single cell of the fertilized frog egg will undergo dramatic change to develop vital body parts like muscles, a skeleton, eyes, a heart, and a tadpole tail. Scientists have been studying this process to better understand human development, birth defects, and cancer and to advance technologies like organoid generation and cell replacement therapy. Scientists can disrupt embryo development, pause it, and accelerate it; however, they can’t exactly explain how development works. Supported by the National Institutes of Health (NIH), bioengineers at the University of Pittsburgh are taking a crack at understanding what is going on inside the egg.
The NIH Department of Health and Human Services awarded McGowan Institute for Regenerative Medicine affiliated faculty member Lance Davidson, PhD, professor of bioengineering at Pitt’s Swanson School of Engineering, $1,327,207 for his study “Biomechanics of Morphogenesis.” Dr. Davidson, who directs the MechMorpho Lab at the University of Pittsburgh, aims to take a structural engineer’s approach to the biomechanics of developing embryos.
The Pitt researchers are reverse-engineering the mechanical processes that shape the basic body plan and organ development in embryos using tests, techniques, and tools more likely to be found in a mechanical engineering lab than a molecular genetics lab.
“If you saw a bridge for the first time, how would you figure out how it worked?” Dr. Davidson asks. “A geneticist might blast it into pieces and analyze how each piece works, but an engineer would look at the ensemble, taking measurements of force and movement. They would put more weight on it and see when it breaks. We are applying these structural analysis principals to understanding embryos.”
In the surrounding labs, researchers work with mice, fruit flies, zebrafish, and rats. In Dr. Davidson’s lab, there is Xenopus—a frog native to sub-Saharan Africa. Frogs are ideally suited for Dr. Davidson’s research because their embryos and tissues are incredibly tolerant of lab conditions and resilient to an engineer’s ‘touch.’ Even after removing them from their protective shells, inducing genetic defects, or injecting fluorescent protein tracers, these frogs won’t croak.
“We use frogs because you can extract tissues very easily, and they will continue to grow correctly,” Dr. Davidson says. “A frog’s eye or brain can be isolated and will continue to grow in a petri dish. That won’t happen with a mouse or fish. When the outer layer of a non-amphibian embryo is cut, the embryo won’t maintain its structure. Frog embryos are more like Play-doh, you can cut and paste tissues and reshape them, although Play-doh is still much stiffer than these embryos.”
The frog eggs start out about the size of a pencil tip. In a field of study that’s used to accommodating steel beams or reinforced concrete measurements, Dr. Davidson’s group has to get creative with the tools they use.
“To perform microsurgery on the frog embryos, we use a scalpel made of human eyebrow hair and a hair loop made from baby hair,” says Dr. Davidson. “The embryos are tiny, wet, and soft; however, they still obey the same shape principles of steel or wood.”
“A civil or mechanical engineer might regularly perform tests applying ten million pascals of stress,” he continues. Ten million pascals is about the amount of water pressure coming out of a pressure washer, and one pascal is about how much pressure a single piece of paper exerts on a tabletop. “We have to design special tools that can both apply and measure stress between five to 20 pascals. You can’t just order something like from Amazon, so we improvise in our lab to design and fabricate custom equipment for our needs.”
By studying the mechanics of morphogenesis—the process of an embryo changing shape—Dr. Davidson hopes to develop a tool that will provide bioengineers with a much greater understanding of and control over tissue self-assembly.
“Many engineering fields have some kind of software or simulation tool that can take the guess work out their designs before they actually start building. We are developing something similar for tissue engineers so they don’t have to rely on trial and error all the time,” explains Dr. Davidson.
Creep tests, strain maps, and micro-aspiration are all engineering techniques employed by Dr. Davidson’s team to understand the underlying mechanics of morphogenesis. These frogs might not be turning into princes any time soon, but from a tiny ball of cells, the embryo can shape itself into a structurally complex tadpole with working organs.
“In the course of one study, quite by accident, we observed two sets of eggs, one set starting about twice the size of the other. We watched the embryos develop side-by-side. Because of the initial size difference, we expected to see lots of structural deformities or at least for the tadpoles to come out twice as big. To our surprise many of the ‘big egg’ embryos survived and their tadpoles grew to the same size as the ‘little egg’ tadpoles, somehow managing to self-correct while they developed,” Dr. Davidson says.
At a time when tissue engineering is becoming increasingly useful in regenerative medicine therapies, Dr. Davidson estimates there are only about five or six other groups in the world making material property measurements in the living tissue of vertebrates like frogs. Building on his research and combining it with results from a 2016 NIH-funded study “Mechanical Control of Mesenchymal-to-Epithelial Transition,” he will continue to flesh out the mechanics of growing tissue.
Illustration: Xenopus tadpoles are excellent test subjects because their transparent bodies allow for unobstructed views into their internal anatomy. Credit: MechMorpho Lab/Lance Davidson.
RESOURCES AT THE MCGOWAN INSTITUTE
November Histology Special
Reticular fibers, or reticulin is a type of fiber in connective tissue, composed of type III collagen secreted by reticular cells. Reticular fibers crosslink to form a fine meshwork (reticulin). This network acts as a supporting mesh in soft tissues such as liver, bone marrow, and the tissues and organs of the lymphatic system.
You will receive 25% off your Jones Silver Stain in November when you mention this ad.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Determining Apoptosis in Normal, Metaplastic, and Neoplastic Esophageal Epithelial Cells Treated with Solubilized Extracellular Matrix
Lindsey Saldin, bioengineering PhD student, in the laboratory of McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor in the Department of Surgery and director of the Center for Pre-Clinical Tissue Engineering within the Institute, recently used flow cytometry to aid in her research towards tissue engineering treatments for esophageal adenocarcinoma. Her experience follows:
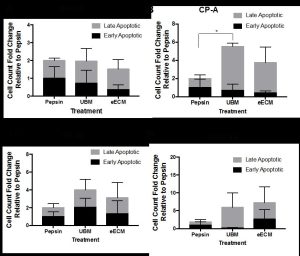
Figure 1. The fold change of early and late apoptotic cells with pepsin-solubilized urinary bladder matrix (UBM) or esophageal mucosa (eECM) treatment compared to pepsin negative control is shown for normal Het-1A (A), cancer pre-cursor Barrett’s CP-A (B), and two types of esophageal adenocarcinoma OE33 (C) and SK-GT-4 (D) esophageal epithelial cell lines. Early/late apoptosis was determined using Propidium Iodide/Annexin V staining.
The incidence of esophageal adenocarcinoma (EAC) is increasing at a faster rate than any other cancer type in the United States [1]. The standard of care is esophagectomy, a procedure with a mortality rate of 1-18% [2-5] and complication rate as high as 64% [3]. In 2011, the Badylak laboratory showed that long segment mucosal resection of EAC confined to the mucosa (i.e., stage T1a) followed by placement with an extracellular matrix (ECM) bioscaffold was shown to minimize or prevent subsequent stricture, promote the replacement of a functional esophageal mucosa, and perhaps most importantly, was not associated with recurrence of EAC in 5 patients [6]. Since the 2011 report, 9 additional patients have been treated with similar results (unreported data).The ECM scaffolds had completely degraded within two weeks of placement, showing the bioinductive cues of the extracellular matrix changed the default esophageal healing response in an area of resected cancer. The inductive cues ECM provides to direct cell behavior may be explained by the concept of “dynamic reciprocity,” the bidirectional crosstalk between a cell and its surrounding matrix to dictate cell behavior. Hence, the rationale for ECM as a material post cancer-resection is that restoring normal microenvironmental cues by supplying “normal” ECM to the site of resected cancer may provide an early inductive cue and reprogram the cells toward normalcy.
The objective of the present study was to expose pepsin-solubilized degradation products of the extracellular matrix to normal, Barrett’s metaplastic cells (the esophageal precursor cancer state), and EAC cells, and then determine changes in cell phenotype, functions associated with tumorigenesis, and cancer signaling pathways. Two types of ECM were used, homologous urinary bladder matrix (UBM) and heterologous esophageal mucosa ECM (eECM). It was hypothesized the ECM would downregulate key neoplastic functions and cancer cell signaling pathways, and that eECM would provide a more pronounced effect to modulate EAC behavior as the homologous ECM source.
One of the cell functions investigated was the degree of early and late apoptosis. This study was conducted in collaboration with Lynda Guzik at the McGowan Flow Cytometry Core. Resistance to apoptosis is a hallmark of neoplastic cells [7]. Normal (Het-1A), Barrett’s (CP-A) and EAC (OE33 and SK-GT-4) cell lines were exposed to pepsin-solubilized UBM, eECM, and pepsin negative control for 24h and then evaluated using flow cytometry with Annexin V/Propidium Iodide (PI) staining. Results showed the metaplastic (CP-A) cells increased late apoptosis compared to pepsin control. No other significant differences were found, however, trends toward increased early/late apoptosis for OE33 and SK-GT-4 cancer cells with UBM and eECM treatment were noted. We are grateful for Ms. Guzik’s expertise in 1) developing the experimental design and optimization of experimental conditions; 2) the performance of the experiment while accommodating our schedule; and 3) her help with the analyses of results. We look forward to collaborating with the flow cytometry core in future studies.
References
- Pohl, H. and H.G. Welch, The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst, 2005. 97(2): p. 142-6.
- Luketich, J.D., et al., Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg, 2003. 238(4): p. 486-94; discussion 494-5.
- Atkins, B.Z., et al., Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg, 2004. 78(4): p. 1170-6; discussion 1170-6.
- Swisher, S.G., et al., Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg, 2000. 119(6): p. 1126-32.
- Metzger, R., et al., High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus, 2004. 17(4): p. 310-4.
- Badylak, S.F., et al., Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A, 2011. 17(11-12): p. 1643-50.
- Hanahan, D. and R.A. Weinberg, Hallmarks of cancer: the next generation. Cell, 2011. 144(5): p. 646-74.
If you have a research project where flow cytometry may benefit your efforts, please feel free to contact Lynda Guzik for more information.
SCIENTIFIC ADVANCES
ALung Receives U.S. FDA Approval to Conduct Hemolung RAS Trial

ALung Technologies, Inc., was founded in 1997 by McGowan Institute for Regenerative Medicine faculty members William Federspiel, PhD, Professor of Bioengineering at the University of Pittsburgh, and the late Brack Hattler, MD, a renowned cardiothoracic surgeon. Drs. Federspiel and Hattler and the team from the McGowan Institute Medical Devices Laboratory developed the original Hemolung technology which was subsequently licensed by ALung for commercial development. The Hemolung Respiratory Assist System (RAS) has been approved outside of the United States since 2013 and is commercially available in major European markets.
ALung recently announced the U.S. Food and Drug Administration (FDA) approval of its Investigational Device Exemption (IDE) to conduct a pivotal clinical trial of the Hemolung RAS for the treatment of adults with severe acute exacerbation of chronic obstructive pulmonary disease (COPD). The FDA’s approval of the IDE makes ALung’s VENT-AVOID Trial the first pivotal trial of extracorporeal carbon dioxide removal (ECCO2R) for treating patients with COPD exacerbations.
“The achievement of FDA approval for initiation of the VENT-AVOID Trial is an important milestone towards making the Hemolung RAS and ECCO2R therapy available to US patients and their physicians,” said Peter DeComo, Chairman and CEO of ALung. “We believe that there is great potential for the Hemolung technology to facilitate ventilator avoidance, resulting in improved clinical outcomes and a lower cost of care through a reduction in length of stay in the intensive care unit.”
The VENT-AVOID Trial is a prospective, multi-center, randomized, controlled, pivotal trial to validate the safety and efficacy of the Hemolung RAS for COPD patients experiencing an acute exacerbation requiring ventilatory support. Forty hospitals will enroll up to 800 patients in the trial. The study protocol is built around a state of the art adaptive statistical plan which will allow for PMA submission when early success criteria are reached, potentially with as few as 300 patients enrolled. COPD patients suffering severe exacerbations will be eligible for the study if they are either
- failing non-invasive ventilation and presenting a high risk of being intubated and mechanically ventilated, or
- have required intubation and invasive mechanical ventilation due to acute respiratory failure.
Serving as the study principal investigator is Nicholas Hill, MD, Chief, Division of Pulmonary, Critical Care and Sleep Medicine at Tufts Medical Center. Dr. Hill is an international leader in pulmonary critical care medicine, having led studies which established non-invasive ventilation as the standard of care for COPD exacerbations.
In addition to the US-based VENT-AVOID study, The Hemolung RAS is also being studied in a landmark pivotal study for patients with acute respiratory distress syndrome (ARDS) as part of the 1,120-patient REST Trial in the United Kingdom. “Our commitment to clinical science runs very deep,” added Mr. DeComo. “We will soon be the only company participating in not just one, but two major pivotal trials validating the safety and efficacy of extracorporeal carbon dioxide removal therapy provided by the Hemolung RAS.”
ALung worked collaboratively with the FDA under its Expedited Access Pathway (EAP) program to obtain IDE approval. The Expedited Access Pathway is a new FDA program aimed to facilitate more rapid patient access to breakthrough technologies intended to treat or diagnose life-threatening or irreversibly debilitating diseases or conditions. ALung will continue to collaborate with the FDA during study enrollment and through the PMA process.
COPD affects 30 million Americans and is the third leading cause of death in the United States behind cancer and heart disease. Acute exacerbations, defined as a sudden worsening of COPD symptoms, are a major cause of morbidity and mortality in COPD patients. For patients with severe exacerbations, high levels of carbon dioxide can result in respiratory failure and the need for intubation and mechanical ventilation as life saving measures. Unfortunately, mechanical ventilation is associated with many side effects, and in-hospital mortality remains as high as 30%. ECCO2R therapy with the Hemolung RAS allows carbon dioxide to be removed from the blood independently of the lungs with the aim of facilitating the avoidance or reduction of intubation and invasive mechanical ventilation.
Illustration: ALung Technologies, Inc.
Wearable Artificial Lung to Deliver Greater Mobility to Patients

McGowan Institute for Regenerative Medicine faculty member William Federspiel, PhD, William Kepler Whiteford Professor in the Department of Bioengineering, Chemical Engineering, and Critical Care Medicine and the Director of the Medical Devices Laboratory at the McGowan Institute, recently spoke to Pittsburgh’s NPR News Station WESA about current research and pre-clinical trial efforts towards the development of a wearable artificial lung for patients suffering from lung failure. The new device promises to deliver greater mobility and increased odds for survival following severe lung damage.
“What distinguishes this technology is that we’ve combined the blood pump and the lung unit into one compact unit so that patients would be able to ambulate around in the clinical setting,” Dr. Federspiel said. “It’s pretty well established in the literature that if you can get these patients up and moving around, they do better. Whether it’s recovering from their illness or doing better with their post-transplant outcomes.”
Those suffering from lung failure have limited options. After being placed on the organ transplant list, it normally takes several months for a patient to receive a lung transplant. During this time, patients are usually confined to the hospital bed, sedated, and hooked up to costly and cumbersome heart-lung machines that function to replace their damaged lungs.
The new unit takes less blood out of the patient to fill the tubing and the device. Dr. Federspiel said by doing that, one device could be used in three types of patients: adults who need a low-flow CO2 removal device, adults who need a higher-flow oxygenation device, and pediatric patients who need a high-flow device.
While there are only about 200 pediatric patients in the U.S. each year who need such devices, there are between 10,000 and 50,000 adult patients who need the higher-flow assistance, according to Dr. Federspiel. He said there are an additional 100,000 to 300,000 patients in the U.S. who need the CO2 removal devices.
Dr. Federspiel said making a device only for pediatric needs might not be commercially viable, but building a multi-use device that can be used by children could open the door to saving young lives.
Illustration: Mark Nootbaar / 90.5 WESA.
UPMC Team Performs First U.S. LIVE™ Procedure to Stop Heart Failure Progression
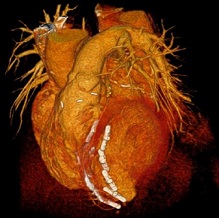
A multidisciplinary team of UPMC physicians, including McGowan Institute for Regenerative Medicine affiliated faculty members, recently completed the first Less Invasive Ventricular Enhancement™ (LIVE™) procedure in the United States to treat patients whose hearts can no longer pump enough blood to the rest of their body due to coronary artery disease.
Ischemic cardiomyopathy, as this condition is known, is one of the leading causes of cardiac death. The condition results when damaged muscle from previous heart attacks prevents the heart from pumping the necessary amount of blood to the body. The scar tissue that develops following the heart attack can lead to an abnormal shape and size of the left ventricle and impaired overall heart function.
“Scar tissue combined with the enlarged size of the left ventricle puts additional stress on the rest of the heart, leading to a gradual heart failure progression,” said McGowan Institute affiliated faculty member Catalin Toma, MD, director of interventional cardiology at UPMC. “Reducing this stress can stop heart failure from advancing, and this procedure provides a less invasive option for slowing the progression to end-stage heart failure.”
Dr. Toma worked side by side with McGowan Institute affiliated faculty member Christopher Sciortino, MD, PhD, surgical director of UPMC’s Advanced Heart Failure Center, along with anesthesiologists and advanced imaging specialists, to use the Revivent TC™ system from BioVentrix Inc. to bring the left ventricle back to its normal shape and size.
The Revivent TC system uses a minimally invasive approach to attach small anchor pairs to the inside and outside of the heart. The anchors pull towards each other, synching any scar tissue away from the heart’s remaining functional tissue, bringing the left ventricle closer to its regular formation.
The LIVE procedure was approved in Europe in 2016, and UPMC’s surgery was the first in the U.S. under a protocol that allows the Revivent TC system to be used as an investigational device in a multicenter clinical study to collect safety and effectiveness data. To date, 130 LIVE procedures have been performed worldwide.
“At UPMC, we see a high volume of patients with advanced heart failure, and we have extensive teams dedicated to their treatment and surgical needs,” said Dr. Sciortino. “We are hopeful that the LIVE procedure will provide a new, less invasive option for preventing heart failure and improving the quality of life for our patients.”
More information on the LIVE procedure is available on the BioVentrix website, and information about heart failure is available on the UPMC Heart and Vascular Institute website.
Illustration: Less Invasive Ventricular Enhancement (LIVE) uses metal anchors to fold over the scarred portion of the heart and fasten it in place. UPMC.
“Cellular” Biology Game
White blood cells are like the “assassins” of systems biology. Some destroy viruses by swallowing them whole, others lie ready to sound the alarm with inflammation, while “natural killer” white blood cells hose down infected cells with a toxin that causes immediate cell death. The human immune system is an intense, fast-paced game of cat and mouse on a cellular level, and thanks to researchers at the University of Pittsburgh, now the game can take place on a cell phone.
McGowan Institute for Regenerative Medicine affiliated faculty member Jason Shoemaker, PhD, assistant professor of chemical engineering at the Swanson School of Engineering, and Robert Gregg, a PhD candidate studying intercellular immunity in Dr. Shoemaker’s group, created the game “Vir-ed”—a virtual reality (VR) education game designed to teach new biology and biochemistry students about the human immune system.
“Systems biology is something you can’t really see, and it’s not a hands-on subject, but it is a holistic tool that can help young minds understand how biological systems function,” says Dr. Shoemaker. “We decided to design the game to create a way for students to be able to visualize what they were studying.”
In Vir-ed (which rhymes with “wired”), players follow a storyline and a series of mini-games while learning how viruses invade host cells, the basic biological mechanisms associated with infection, and how human cells detect viruses. As the game begins, the immersive technology casts the player in the role of the virus, determined to avoid the predatory white blood cells and find a juicy red blood cell to infect.
“The first story shows you how a virus invades a cell, and the second shows you how a cell stops a virus,” explains Mr. Gregg. “Players unlock mini-games by playing through the story mode, and the mini-games require certain achievements to unlock trophies. Each trophy comes with a description and more information about a subject like “DNA” to help educate the player.”
One of the Vir-ed mini-games follows a similar format to the memory game Simon. Players must remember the sequence of a ribonucleic acid (RNA) nucleotide, which consists of the nitrogenous bases adenine, guanine, cytosine, and uracil (AGCU). To complete the mini-game, the player must remember a random assembly of three to six nitrogenous bases with the ultimate goal of getting the order right for a total of 21 cumulative nucleotides.
To develop the software for the game, the Pitt team worked with a nonprofit called Cacti Council—an educational organization that uses computer science to promote critical and creative thinking. A total of 17 people from the Cacti Council team worked on Vir-ed, including graphic designers, programmers, recording artists, and user experience (UX) designers.
“Educational games are tricky,” says Jeremiah Blanchard, a Cacti Council founder. “They’re really an attempt to thread the needle of meeting the requirements for both a game and an educational tool. You have to find the points of overlap. If you do, it can really impact a student’s life in a positive way.”
Vir-ed is already available on the Google Play Store and can be downloaded and viewed on an Android phone and any VR headset. Dr. Shoemaker and Mr. Gregg have almost finished adapting the game for Apple devices, and they are considering adding new features like augmented reality in the future.
“The immediate next step will be to work with the school’s Engineering Education Resource Center to introduce the game to middle and high school students and get feedback on how it performs as an educational tool,” says Dr. Shoemaker.
“Based on what we’ve seen so far, we expect positive response,” adds Mr. Blanchard.
About the Immunosystems Lab
Dr. Shoemaker leads the Shoemaker Immunosystems Lab at Pitt. He and his team of researchers use mathematical models and simulations to better understand immunity and health. By applying the engineering knowledge of the immune system gathered from these models, the team can develop new therapies to promote improved patient outcomes, patient-specific treatment, and immune optimization.
Illustration: Vi-red screen shot.
Modeling Particle Movements on Bees and Bacteria Could Lead to Robotics Advances

Before any business can take place at meetings, whether it be the local borough or a committee at work, a certain number of members need to be in attendance. This is called reaching quorum. It turns out, the same thing also happens on a microscopic level. For instance, bacteria exhibit a behavior called quorum sensing.
“When there are one or two bacteria, they behave in a certain way,” said McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical Engineering and the John A. Swanson Chair of Engineering at the University of Pittsburgh’s Swanson School of Engineering. “But suddenly, when there is a critical threshold of bacteria, the bacteria can sense this increase in population and they dramatically change their behavior.”
In other words, the bacteria have reached a quorum.
Other examples of quorum sensing in living beings include how honey bees select locations for new hives or bioluminescence in deep-sea squid.
Now imagine that type of behavior in machines. That’s what Dr. Balazs and collaborator Henry Shum, PhD, at the University of Waterloo in Canada have been researching. The duo has mimicked quorum sensing behavior in synthetic — nonliving — materials, which could lead to mechanical devices with the ability for self-recognition and self-regulation.
Their research was recently published in the Proceedings of the National Academy of Sciences.
Dr. Balazs studies statistical, mechanical and computer modeling of complex chemical systems.
The findings build off of a previous study the pair published in 2015, where three microscopic synthetic capsules reacted and moved toward each other using chemical signaling.
“They have a bioinspired behavior that enables them to come together and form small groups,” Dr. Shum said.
This most recent study, however, modeled a large number of microcapsules and found that, once quorum was met, synthetic particles reacted autonomously to produce distinct oscillations in chemical signals. The oscillations indicate to the other particles that there is a quorum. “I don’t want to say the particles are self-aware, but they can perform this simple, elementary lifelike function,” Dr. Balazs said.
Dr. Balazs added that these particles’ ability to “count” shows potential blurred lines between what is living and nonliving.
Drs. Balazs and Shum said their findings could lead to advancements in soft robotics, a sub-field dealing with the construction of robots from highly flexible materials, similar to those found in living organisms.
Their findings could also inspire new “mechano-responsive” materials, such as polymer gels with embedded quorum sensing elements that would activate a certain chemical behavior when compressed, and then switch off when stretched or when a specific temperature is reached. For example: “You could have a robotic skin that solidifies to protect itself at a certain temperature, and then becomes ‘squishy’ again when the temperature drops to a nominal level,” Dr. Balazs said.
In the future, Drs. Balazs and Shum will combine aspects of their work by studying how larger microscopic systems react and move toward each other to build different structures.
Illustration: Bees use a process called quorum sensing to decide upon a location for a new hive when their population reaches a specific number. On the microscopic level, particles can also sense a quorum through chemical signals sent to other members of the colony. University of Pittsburgh.
AWARDS AND RECOGNITION
Dr. Marina Kameneva Receives U.S. Patent for DRP Work

McGowan Institute for Regenerative Medicine faculty member Marina Kameneva, PhD, Research Professor of Surgery at the University of Pittsburgh School of Medicine, Professor of Bioengineering, University of Pittsburgh, and Director of the Artificial Blood Program at the McGowan Institute, along with co-inventors Denis Bragin, PhD, and Edwin Nemoto, PhD, from the University of New Mexico, recently received a U.S. patent for their development entitled “Rheological treatment of brain ischemia by drag reducing polymers.”
From the patent summary: The present disclosure provides methods and compositions for the treatment of acute or chronic cerebral ischemia by targeting the very basis of ischemia by improving the rheology of blood flow through arteries and capillaries and as such would be relevant in the treatment, amelioration, or prevention of acute and chronic ischemic conditions of various etiologies. According to a first embodiment, the present disclosure provides linear, blood soluble, non-toxic macromolecules, referred to herein as drag-reducing polymers (DRPs) for the treatment, amelioration, or prevention of brain (and other organs) ischemia and other conditions or injuries causing or potentially leading to ischemic injury. According to a second embodiment, the present disclosure provides a method for treating, ameliorating, or preventing brain (and other organs) ischemia of varying etiologies by the administration of DRP to a subject.
Dr. Jacqueline Kreutzer Named to New Leadership Appointment

Children’s Hospital of Pittsburgh of UPMC announced recently new leadership appointments in its Heart Institute and Division of Pediatric Cardiology. McGowan Institute for Regenerative Medicine affiliated faculty member Jacqueline Kreutzer, MD, professor of pediatrics at the University of Pittsburgh School of Medicine, is now appointed as chief of the Division of Pediatric Cardiology.
An internationally recognized leader in interventional cardiology, Dr. Kreutzer currently is serving as director of the Cardiac Catheterization Laboratory at Children’s Hospital. She completed a pediatric cardiology fellowship and training in interventional cardiology at Boston Children’s Hospital and is board-certified in pediatric cardiology and adult congenital heart disease. Dr. Kreutzer joined Children’s Hospital in 2005.
Dr. Kreutzer has been a clinical champion for the Division of Pediatric Cardiology, distinguished in the field of interventional cardiology innovation, bringing new techniques to Children’s Hospital. She has served as the institutional investigator on numerous clinical trials for investigational devices and currently is the national principal investigator for the Melody transcatheter pulmonary valve post-approval study.
Dr. Kreutzer has authored more than 80 publications and serves as editor and reviewer for several journals, including the Journal of the American College of Cardiology. She has lectured extensively and served in numerous academic positions such as for the American Board of Pediatrics, American College of Cardiology, American Heart Association and the Society of Cardiac Angiography and Intervention.
Congratulations, Dr. Kreutzer!
Partnership for Public Service Honors VA Pittsburgh/Pitt Researcher Dr. Rory Cooper with ‘Oscar’ of Government Service

The Partnership for Public Service honored McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, research scientist at VA Pittsburgh Healthcare System and professor at the University of Pittsburgh, with a Samuel J. Heyman Service to America Medal (Sammie) for his work as director of the Human Engineering Research Laboratory (HERL).
Dr. Cooper received the Sammie in the Science and Environment category for developing adaptive wheelchairs at HERL, a collaboration between Pitt, VA Pittsburgh Healthcare System, and UPMC. Dr. Cooper is HERL’s founding director and its VA senior research career scientist.
An Army veteran, Dr. Cooper was cited in his nomination for designing innovative wheelchairs and other assistive technologies that have markedly improved the mobility and quality of life for hundreds of thousands of veterans and other people with disabilities. He led projects that include development of a wheelchair with robotic arms and hands that can grasp objects, personal vehicles that enable people to access terrain their wheelchairs can’t traverse, and manual wheelchairs with more comfortable and adjustable seats.
Dr. Cooper and six other winners were chosen from 26 finalists and more than 440 nominees by a selection committee that included leaders from government, business, the foundation and nonprofit community, academia, entertainment and the media.
“The federal government is a unique instrument for our country. The 2017 Service to America Medal recipients represent the best in government, the unsung heroes who quietly work behind the scenes to serve their country and the public good,” said Max Stier, Partnership for Public Service president and CEO. “It is important, especially in these uncertain times, to celebrate and recognize the Sammies honorees and their colleagues throughout the government who are making a positive difference in people’s lives.”
Dr. Cooper is the FISA Foundation/Paralyzed Veterans of America chair of the Department of Rehabilitation Science and Technology in Pitt’s School of Health and Rehabilitation Sciences. He also serves as the school’s associate dean for inclusion and a distinguished professor, as well as a professor of bioengineering, physical medicine and rehabilitation, and orthopaedic surgery.
Congratulations, Dr. Cooper!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#176 –– Dr. Mark Slaughter is the Professor and Chair of the Department Cardiovascular and Thoracic Surgery at the University of Louisville School of Medicine. Dr. Slaughter discusses his research on ventricular assist devices.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
We encourage submissions of images that highlight your research. If you have images, or have an idea for an interesting image, please contact Donna Stolz, PhD at donna.stolz@pitt.edu.
PUBLICATION OF THE MONTH
Author: Jeffries RG, Lund L, Frankowski B, Federspiel WJ
Title: An extracorporeal carbon dioxide removal (ECCO2R) device operating at hemodialysis blood flow rates.
Summary: BACKGROUND: Extracorporeal carbon dioxide removal (ECCO2R) systems have gained clinical appeal as supplemental therapy in the treatment of acute and chronic respiratory injuries with low tidal volume or non-invasive ventilation. We have developed an ultra-low-flow ECCO2R device (ULFED) capable of operating at blood flows comparable to renal hemodialysis (250 mL/min). Comparable operating conditions allow use of minimally invasive dialysis cannulation strategies with potential for direct integration to existing dialysis circuitry.
METHODS: A carbon dioxide (CO2) removal device was fabricated with rotating impellers inside an annular hollow fiber membrane bundle to disrupt blood flow patterns and enhance gas exchange. In vitro gas exchange and hemolysis testing was conducted at hemodialysis blood flows (250 mL/min).
RESULTS: In vitro carbon dioxide removal rates up to 75 mL/min were achieved in blood at normocapnia (pCO2 = 45 mmHg). In vitro hemolysis (including cannula and blood pump) was comparable to a Medtronic Minimax oxygenator control loop using a time-of-therapy normalized index of hemolysis (0.19 ± 0.04 g/100 min versus 0.12 ± 0.01 g/100 min, p = 0.169).
CONCLUSIONS: In vitro performance suggests a new ultra-low-flow extracorporeal CO2 removal device could be utilized for safe and effective CO2 removal at hemodialysis flow rates using simplified and minimally invasive connection strategies.
Source: Intensive Care Med Exp. 2017 Sep 6;5(1):41. doi: 10.1186/s40635-017-0154-1.
GRANT OF THE MONTH
PI: Lance Davidson
Title: Biomechanics of Morphogenesis
Description: Physical mechanical processes are central to the morphogenesis of embryos and their organs. The goal of this proposal is to apply a multi-scale analysis of the mechanics of convergent extension, identifying biomechanical mechanisms that establish passive tissue properties such as stiffness as well as active processes that generate forces of extension, regulate cell behaviors and tissue deformation, and how passive mechanics and active force generating processes are coordinated within the frog embryo. Studies outlined in this proposal will answer: (1) How are cell-scale structures and tissue mechanics are integrated during elongation? Early development is marked by dramatic changes in the mechanical properties of embryos. To understand how and why these properties change we test simple models of tissue mechanics based on synthetic closed-cell foams using bioengineering and biophysical methods to disrupt features from large scale architecture to the subcellular actomyosin-dependent cortex. (2) What single-cell mechanical processes contribute to convergent extension? We extend our analysis of cell behaviors to an unbiased approach that combines wide-field confocal microscopy with descriptive biomechanical analyses from the level of the cell, to the local neighborhood, to the strain fields of the entire embryo. Combining analyses of neural plate and paraxial somitic mesoderm we describe the dependence of these movements on planar polarity signaling. Using systems approaches we seek to test the dependencies of specific cell behaviors on both upstream signaling systems and their targeted downstream effectors. (3) How are tissue polarity cues transduced into polarized cell behaviors? We hypothesize that polarized cell behaviors and the oriented forces they generate are the result of cues produced by anisotropic strain. To test the roles of polarized intracellular factors and mechanical strain in organizing cell behaviors we use magnetogenetics and micro-scale tissue stretchers. Results from this project will complement ongoing efforts to identify the molecular regulators of morphogenesis by providing a conceptual framework developing new hypotheses of morphogenesis and bioengineering tools to test them. The significance of our work provides researchers a more complete understanding of the contribution of cell- and tissue-mechanics to development, to understand the role of tissue mechanics in oncogenesis, and to provide fundamental physical principles for future functional tissue engineers.
Source: NIH Department of Health and Human Services
Term: September 1, 2017 – August 31, 2021
Amount: $1,327,207