McGowan Institute for Regenerative Medicine to Co-Direct $75 Million Second Phase of National Effort to Aid Wounded Warriors
 The Armed Forces Institute for Regenerative Medicine (AFIRM) will continue its efforts to apply the latest in tissue engineering and other regenerative medicine techniques to the treatment of battlefield injuries in a $75 million, 5-year second phase.
The Armed Forces Institute for Regenerative Medicine (AFIRM) will continue its efforts to apply the latest in tissue engineering and other regenerative medicine techniques to the treatment of battlefield injuries in a $75 million, 5-year second phase.
The AFIRM-II consortium of more than 30 academic centers and industry partners will be led by Anthony Atala, MD, director of the Wake Forest Institute for Regenerative Medicine, and co-directed by Rocky Tuan, PhD, associate director, McGowan Institute for Regenerative Medicine, and director of the Center for Military Medicine Research at the University of Pittsburgh.
“For the next five years, AFIRM-II will aim to develop novel therapies for severely damaged limbs, reconstruct facial and skull injuries with tissue engineering approaches, regenerate skin for burns, find new ways to prevent rejection of composite tissues, such as hand transplants, and much more,” Dr. Tuan said. “We’ve accomplished a great deal in the first phase of this work, and we are delighted to have the opportunity to bring these innovative techniques to the clinical setting.”
Since its inception in 2008, AFIRM efforts have resulted in clinical studies of face transplantation, minimally invasive surgery for craniofacial injuries, scar reduction treatments, fat grafting for reconstructive surgery, and new treatments for burns.
“When warriors come back from the battlefield with serious life-changing injuries, it is our job to find new and innovative ways to help them. Ultimately, we’d like to create new treatments to repair these severe injuries as if they never happened,” said Maj. Gen. Joseph Caravalho Jr., commanding general of the U.S. Army Medical Research and Materiel Command and Fort Detrick. “The science of regenerative medicine is one of the ways we fulfill our promise to service members who put themselves in harm’s way, that we will work our hardest and do our very best to take care of them.”
The AFIRM program not only funds scientific research, but also requires that discoveries be tested and compared so that the most promising therapies – which could benefit civilians as well as soldiers – can be brought to clinical trials. The consortium will work with health professionals at the U.S. Army Institute of Surgical Research and Walter Reed National Military Medical Center.
Government sponsors of AFIRM are the U.S. Army Medical Research and Materiel Command, the Office of Naval Research, the Air Force Medical Service, the Office of Research and Development – Department of Veterans Affairs, the National Institutes of Health, and the Office of the Assistant Secretary of Defense for Health Affairs.
Illustration: McGowan Institute for Regenerative Medicine.
UPCOMING EVENTS
Save the Date: 2014 McGowan Institute Retreat
 The 13th Annual McGowan Institute for Regenerative Medicine Scientific Retreat is set to take place on March 9-11, 2014, at Nemacolin Woodlands Resort.
The 13th Annual McGowan Institute for Regenerative Medicine Scientific Retreat is set to take place on March 9-11, 2014, at Nemacolin Woodlands Resort.
The poster session will begin on the evening of March 9, 2014, at which time there will be an informal mixer.
Under the leadership of McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD, associate professor, Department of Surgery, University of Pittsburgh, director of the Plastic Surgery Laboratory, and co-director of the Adipose Stem Cell Center, the program committee is planning an exciting group of speakers and topics.
Highlights from the 2013 Retreat are in the Archive in the article entitled “McGowan Institute for Regenerative Medicine Holds Its Annual Scientific Retreat.”
Mark the date on your calendars and watch for additional 2014 Retreat news!
Third Annual Regenerative Rehabilitation Symposium
The annual Regenerative Rehabilitation Symposia series is a unique opportunity for students, researchers, and clinicians working in the interrelated fields of regenerative medicine and rehabilitation to meet, exchange ideas, and generate new collaborations and clinical research questions. Jointly organized by the University of Pittsburgh Rehabilitation Institute, the School of Health and Rehabilitation Sciences at the University of Pittsburgh, the McGowan Institute for Regenerative Medicine and the Rehabilitation Research and Development Center of Excellence at the Veterans Affairs Palo Alto Health Care System, the Third Annual Symposium on Regenerative Rehabilitation will be held on April 10-11, 2014 in San Francisco, CA at the Mission Bay Conference Bay at the University of California, San Francisco (UCSF).
The objectives of this event are:
- To promote the clinical translation of regenerative medicine scientific discoveries by communicating and disseminating research findings that demonstrate the synergistic relationship between regenerative medicine and rehabilitation;
- To provide a forum by which scientists and rehabilitation clinicians may interact, exchange ideas, and identify novel research directions relating to the field of regenerative rehabilitation; and
- To introduce the concept of regenerative rehabilitation to graduate students, medical students and medical residents in the rehabilitation field.
For more information on this event, please contact Katy Wharton at: rehabmtg@pitt.edu or whartonkm@upmc.edu or 412-624-5293.
SCIENTIFIC ADVANCES
The Latest Advances in Technology for People with Spinal Cord Injury
 A special issue of The Journal of Spinal Cord Medicine, published by Maney Publishing, examines various advances made in technology to aid people with spinal cord injury (SCI).
A special issue of The Journal of Spinal Cord Medicine, published by Maney Publishing, examines various advances made in technology to aid people with spinal cord injury (SCI).
Scientists from all over the world contributed to the special issue, focusing on five areas: advances in wheelchair technology; wheelchair sports; personal health and safety; innovations in rehabilitation; and closing the gaps in education and employment.
The studies also incorporate the views of the end-users that have participated throughout the research design and development of the new technologies. These advances are empowering those with SCI and enabling them to lead fuller and more active lives, as everyday activities become easier and more accessible. One year after injury, 12% of people with SCI are now employed.
The issue is guest edited by McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, a member of the journal’s Editorial Board. Dr. Cooper serves as Engineering Director of the University of Pittsburgh SCI Model System. His commentary, “Technology, trends, and the future for people with spinal cord injury,” is available for free download, in which he comments: “Ideally, in the future, we will see more teams of scientists, engineers, and clinicians that include people with and without SCI working together to conduct pioneering research, to create transformational technology, and to establish model clinical programs. Assuredly, technology will play a major role to make this vision of the future become reality.”
Applying technology to rehabilitation is helping to restore the abilities to use arms and legs immobilized by spinal cord injury, as well as to control bladder function. Neuroprostheses are devices that use electrodes to deliver electrical stimulation to areas where function has been lost due to injury. They offer tremendous potential for restoring motor, sensory and bladder function in people with SCI. Virtual reality is being utilized in robotic exoskeletons such as ReWalk to enable those with SCI to stand and walk, and in the evaluation of wheelchair driving performance.
Activities like sports are benefiting from these advances too – a growing area, particularly following the 2012 Olympic Games. Equipment such as the hand cycle enables less stressful and more efficient upper body training to aid rehabilitation and boost overall health. GPS is being used to evaluate the details of wheelchair tennis play, enabling coaches to develop and monitor training regimens.
Other advances in wheelchair technology allow for superior use, such as the power wheelchair which is capable of climbing curbs and maneuvering around obstacles. However, a common problem faced by wheelchair users is increased exposure to vibration, which can damage muscles, nerves, and cause back pain. Devices that collect data from wheelchair users indicate the need for vibration-dampening cushions or suspension systems. Prolonged sitting is also a risk factor for pressure ulcers, a debilitating complication that diminishes quality of life. The research shows that cooling the tissues under pressure can reduce the risk for skin breakdown, and suggests the use of temperature control mechanisms in cushions and mattresses.
Illustration: Maney Publishing.
Pitt Scientists Solve Mystery of Basic Cellular Process
 A mix of serendipity and dogged laboratory work allowed a diverse team of University of Pittsburgh scientists to report in Nature Cell Biology that they had solved the mystery of a basic biological function essential to cellular health. McGowan Institute for Regenerative Medicine affiliated faculty members in this team included:
A mix of serendipity and dogged laboratory work allowed a diverse team of University of Pittsburgh scientists to report in Nature Cell Biology that they had solved the mystery of a basic biological function essential to cellular health. McGowan Institute for Regenerative Medicine affiliated faculty members in this team included:
- Valerian Kagan, PhD, DSc, professor and vice chair of the Pitt Graduate School of Public Health’s Department of Environmental and Occupational Health, as well as a professor in the Department of Pharmacology and the Department of Radiation Oncology at the University of Pittsburgh
- Charleen Chu, MD, PhD, professor and the A. Julio Martinez Chair in Neuropathology in the Pitt School of Medicine’s Department of Pathology
- Catherine Baty, DVM, PhD, research assistant professor at the University of Pittsburgh’s Department of Cell Biology, with a secondary position in the Department of Human Genetics
- Simon Watkins, PhD, founder and director of the Center for Biologic Imaging at the University of Pittsburgh and a member of the Pittsburgh Cancer Institute
- Ivet Bahar, PhD, John K. Vries Chair and professor in the Department of Computational & Systems Biology at the University of Pittsburgh, the associate director of the University of Pittsburgh Drug Discovery Institute, and the founding director of the Carnegie Mellon-University of Pittsburgh PhD Program in Computational Biology, and the Center for Computational Biology & Bioinformatics, School of Medicine, University of Pittsburgh
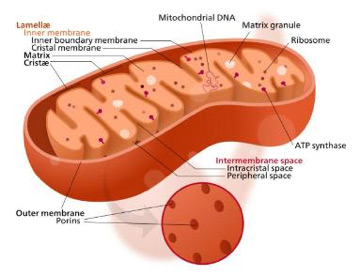
By discovering a mechanism by which mitochondria – tiny structures inside cells often described as “power plants” – signal that they are damaged and need to be eliminated, the Pitt team has opened the door to potential research into cures for disorders such as Parkinson’s disease that are believed to be caused by dysfunctional mitochondria in neurons.
“It’s a survival process. Cells activate to get rid of bad mitochondria and consolidate good mitochondria. If this process succeeds, then the good ones can proliferate and the cells thrive,” said Dr. Kagan, a senior author on the paper. “It’s a beautiful, efficient mechanism that we will seek to target and model in developing new drugs and treatments.”
Dr. Kagan, who, as a recipient of a Fulbright Scholar grant, currently is serving as visiting research chair in science and the environment at McMaster University in Ontario, Canada, likened the process to cooking a Thanksgiving turkey.
“You put the turkey in the oven and the outside becomes golden, but you can’t just look at it to know it’s ready. So you put a thermometer in, and when it pops up, you know you can eat it,” he said. “Mitochondria give out a similar ‘eat me’ signal to cells when they are done functioning properly.”
Cardiolipins, named because they were first found in heart tissue, are a component on the inner membrane of mitochondria. When a mitochondrion is damaged, the cardiolipins move from its inner membrane to its outer membrane, where they encourage the cell to destroy the entire mitochondrion.
However, that is only part of the process, says Dr. Chu, another senior author of the study. “It’s not just the turkey timer going off; it’s a question of who’s holding the hot mitt to bring it to the dining room?” That turns out to be a protein called LC3. One part of LC3 binds to cardiolipin, and LC3 causes a specialized structure to form around the mitochondrion to carry it to the digestive centers of the cell.
The research arose nearly a decade ago when Dr. Kagan had a conversation with Dr. Chu at a research conference. Dr. Chu, who studies autophagy, or “self-eating,” in Parkinson’s disease, was seeking a change on the mitochondrial surface that could signal to LC3 to bring in the damaged organelle for recycling. It turned out they were working on different sides of the same puzzle.
Together with Hülya Bayır, MD, research director of pediatric critical care medicine, Children’s Hospital of Pittsburgh of UPMC and professor, Pitt’s Department of Critical Care Medicine, and a team of nearly two dozen scientists, the three senior authors worked out how the pieces of the mitochondria signaling problem fit together.
Now that they’ve worked out the basic mechanism, Dr. Chu indicates that many more research directions will likely follow.
“There are so many follow-up questions,” she said. “What is the process that triggers the cardiolipin to move outside the mitochondria? How does this pathway fit in with other pathways that affect onset of diseases like Parkinson’s? Interestingly, two familial Parkinson’s disease genes also are linked to mitochondrial removal.”
Dr. Bayir explained that while this process may happen in all cells with mitochondria, it is particularly important that it functions correctly in neuronal cells because these cells do not divide and regenerate as readily as cells in other parts of the body.
“I think these findings have huge implications for brain injury patients,” she said. “The mitochondrial ‘eat me’ signaling process could be a therapeutic target in the sense that you need a certain level of clearance of damaged mitochondria. But, on the other hand, you don’t want the clearing process to go on unchecked. You must have a level of balance, which is something we could seek to achieve with medications or therapy if the body is not able to find that balance itself.”
Illustration: Mitochondria. –Wikipedia.
Unusual Combination Therapy Shows Promise for Preventing Prostate Cancer
 Combining a compound from broccoli with an antimalarial drug prevents prostate cancer in mice, University of Pittsburgh Cancer Institute (UPCI) researchers—including McGowan Institute for Regenerative Medicine affiliated faculty members Simon Watkins, PhD, and Donna Stolz, PhD—discovered.
Combining a compound from broccoli with an antimalarial drug prevents prostate cancer in mice, University of Pittsburgh Cancer Institute (UPCI) researchers—including McGowan Institute for Regenerative Medicine affiliated faculty members Simon Watkins, PhD, and Donna Stolz, PhD—discovered.
The National Cancer Institute-funded research was published in the journal Cancer Research. It is the first such study to show the effectiveness of the combined treatment and provides compelling evidence for human clinical trials.
“Men with prostate cancer suffer significant impairments in quality of life, not only from the disease itself, but also from the treatments,” said senior author Shivendra Singh, PhD, professor in Pitt’s Department of Pharmacology & Chemical Biology. “Because the predominant risk factors for prostate cancer, such as age, race, and genetics, cannot be avoided, there is a great need for preventative treatments for those most at risk.”
Cruciferous vegetables, such as broccoli, watercress, and cabbage, are associated with a lower risk of prostate cancer. The phytochemical sulforaphane in cruciferous vegetables is believed to be responsible.
When scientists tested sulforaphane in the lab, they found it works to prevent early-stage prostate cancer, but not late-stage. Dr. Singh and his colleagues hypothesized that this was due to a cellular mechanism called autophagy, which limits the ability of drugs to destroy cancer.
The antimalarial drug chloroquine inhibits autophagy. When chloroquine and sulforaphane were given to mice predisposed to prostate cancer, only 12 percent of the mice developed late-stage prostate cancer, compared to half in the control group.
“These results are very promising, but I do not recommend that men take chloroquine while eating broccoli in an attempt to prevent prostate cancer,” said Dr. Singh. “Certainly eating broccoli and other cruciferous vegetables is good for you, but chloroquine can have side effects, and it has not been tested in humans for the purpose of preventing prostate cancer.”
Dr. Watkins is founder and director of the Center for Biologic Imaging at the University of Pittsburgh and a member of the Pittsburgh Cancer Institute. He is also the director of the Graduate Program and a professor (with tenure) within the Department of Cell Biology and Physiology. In addition to his responsibilities to the university, Dr. Watkins serves as a member on the research advisory board of Children’s Hospital of Pittsburgh of UPMC.
Dr. Stolz is associate director of the Center for Biologic Imaging and associate professor in the Department of Cell Biology and Physiology at the University of Pittsburgh.
NSF Grant Explores “Materials That Compute”
 A computational “fabric” envisioned by University of Pittsburgh researchers could lead to the development of clothing that could respond to external stimuli, monitor vital signs of patients or athletes, and help the visually impaired “sense” their surrounding environment.
A computational “fabric” envisioned by University of Pittsburgh researchers could lead to the development of clothing that could respond to external stimuli, monitor vital signs of patients or athletes, and help the visually impaired “sense” their surrounding environment.
The research, recently funded by a $700,000 National Science Foundation Integrated NSF Support Promoting Interdisciplinary Research and Education (INSPIRE) grant, builds upon the already-established research of McGowan Institute for Regenerative Medicine affiliated faculty member and principal investigator Anna C. Balazs, PhD, Distinguished Robert v. d. Luft Professor of Chemical Engineering, and Steven P. Levitan, PhD, the John A. Jurenko Professor of Computer Engineering at Pitt’s Swanson School of Engineering. The two are integrating Dr. Balazs’ research into Belousov-Zhabotinsky (BZ) gel, a substance that oscillates in the absence of external stimuli, with Dr. Levitan’s expertise in computational modeling and oscillator-based computing systems.
“Although BZ gels have been investigated since the 1990s, this research moves in a new direction beyond logic operations – in essence creating materials that compute,” Dr. Balazs explains. “The material would be an integrated sensing, computing, and responsive device without an external power source that could act as a “sixth sense” for those who wear it.”
Drs. Balazs and Levitan propose utilizing the chemo-responsive nature of the BZ gels to create a chemical-based computational fabric that would be lightweight and mechanically compliant, and would be human-centric, sensing and responding to human touch and motion. The material would perform autonomously for up to several hours without connections to an external power supply.
The BZ reactions within the gels would perform information processing between sets of stored or learned patterns and stimuli in the form of light, pressure, or chemistry. This ability for the material to interpret a stimulus, send out a signal, and respond in kind will be a key part of the research. “In essence, we let the physics do the computing.”
“The real leverage for this project is capitalizing on the gels’ natural oscillation to communicate at a human scale that can sense the surrounding environment, process information, and react to complex stimuli,” Dr. Levitan adds. “The fabric would most likely require a piezoelectric film to generate an electric field, allowing it to interface with embedded electronics.”
The 5-year grant will allow the researchers to further the computational modeling of how such a BZ gel fabric would function, with the goal that others would be able to fabricate the material.
“Imagine this fabric helping a burn patient who has lost the sense of touch know whether he is in contact with a hot or cold material, or the fabric integrated into a jogging suit that can monitor and display your pulse, pressure, and respiration,” Dr. Balazs says. “By eliminating the need for external wiring or typical computer processors, this sensing fabric could help to change human quality of life.”
Illustration: McGowan Institute for Regenerative Medicine.
Drs. Rubin and Marra Comment on Denmark Stem Cell-Enriched Fat Grafts Clinical Study
 In Denmark, the first clinical study shows the potential of stem cell-enriched fat grafts to transform reconstructive surgery. McGowan Institute for Regenerative Medicine faculty members—J. Peter Rubin, MD, and Kacey Marra, PhD—weigh in on the results.
In Denmark, the first clinical study shows the potential of stem cell-enriched fat grafts to transform reconstructive surgery. McGowan Institute for Regenerative Medicine faculty members—J. Peter Rubin, MD, and Kacey Marra, PhD—weigh in on the results.
“Our promising results suggest that stem-cell enriched fat grafting might prove to be an attractive alternative to major tissue augmentation, such as breast reconstruction after cancer with allogeneic material or major tissue flap surgery, with fewer side effects and more satisfying cosmetic results”, explains Stig-Frederik Trojahn Kølle, MD, a member of the research team from Copenhagen University Hospital in Denmark, in The Lancet.
Increasingly, autologous fat grafting or lipofilling, in which an individual’s own fat is harvested to increase the volume of fat in another area of their body, is being used in reconstructive surgery, with the majority of board certified plastic surgeons in the USA who do breast reconstruction using this technique.
But despite the technique’s potential, resorption rates (the percentage of the transferred fat that does not survive) of up to 80% have been reported. Recent animal studies have shown that fat grafts enriched with culture-expanded adipose [fat]-derived stem cells (ASCs) can substantially improve graft survival.
The cell-treated grafts retained 80.9% of the initial volume compared with 16.3% for the control grafts, with significantly higher amounts of adipose tissue and newly formed connective tissue, and significantly less necrosis 4 months after the transplant.
Writing in a linked Comment in The Lancet, J. Peter Rubin, MD, and Kacey Marra, PhD, University of Pittsburgh and McGowan Institute for Regenerative Medicine faculty members in the USA, say, “These therapies could revolutionize breast reconstruction after cancer and reconstruction of deformities after trauma, for example.”
However, they add, “A crucial open question that will affect use of this therapy is whether there is an optimum cell dose for efficacy, and/or if there is a critical threshold cell dose for therapeutic effect…Another unresolved issue for this therapy is whether high concentrations of stem cells can stimulate the growth of residual breast cancer cells…This issue will be best addressed in large clinical trials.”
Dr. Rubin said the study is “very” important.
“We’ve known that this works in animals. What’s been missing is good data on humans,” said Dr. Rubin.
These early results offer a “proof of principle,” and need to be followed up with clinical trials of actual patients, rather than healthy volunteers, Dr. Rubin said.
Dr. Rubin is chair, Department of Plastic Surgery, director of the Center for Innovation in Restorative Medicine, UPMC endowed professor of plastic surgery, and professor of bioengineering, University of Pittsburgh. Dr. Marra is associate professor, Department of Surgery, University of Pittsburgh, and director of the Plastic Surgery Laboratory as well as co-director of the Adipose Stem Cell Center.
 Regenerative Medicine Podcast Update
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#128 –– Dr. Glenn Prestwich is Presidential Professor of Medicinal Chemistry at The University of Utah. Dr. Prestwich discusses his experience in translating research from the bench to the bedside.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
