November 2017 | VOL. 16, NO. 11| www.McGowan.pitt.edu
As We Celebrate Thanksgiving, Let Us Extend Our Thanks…
![]()
- To the 240+ scientists, engineers and clinicians that are the teams of professionals who are committed to the development and translation of regenerative medicine and medical device-based therapies;
- To the dedicated support staffs who make it possible for the scientists, engineers and clinicians to pursue the goals and objectives of the Institute;
- To the hundreds of student trainees who are learning the fundamentals and developing the applications that continue to make regenerative medicine the promise of the future in improved health care and quality of life;
- To the many agencies, foundations, companies, and individual donors without whose fiscal support and encouragement the outcomes that we give thanks for would not have been possible, and;
- To the many patients who have trusted our teams. These individuals are the true pioneers and the heart of the achievements we recognize.
Since the formation of the McGowan Center for Artificial Organ Development (1992) and the McGowan Institute for Regenerative Medicine (2001) there have been many accomplishments advancing the science and clinical achievements in regenerative medicine-based therapies. We see progress in the areas of medical devices, tissue engineering and cell-based therapies, and the prospects for the future are many. Thanks for the opportunities and outcomes created by all who participate in or support the initiatives of the McGowan Institute. Best wishes for another successful year!
RESOURCES AT THE MCGOWAN INSTITUTE
December Histology Special
Frozen Section Special
Santa isn’t the only one hard at work this winter. At the McGowan Histology Core we are cutting frozen sections and offering 25% off your entire frozen project bill.
We offer a high degree of technical expertise and can accommodate even the most specialized service needs.
You will receive 25% off all of your frozen section needs in December when you mention this ad.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Flow Cytometry At Work – Determining Apoptosis in Normal, Metaplastic, and Neoplastic Esophageal Epithelial Cells Treated with Solubilized Extracellular Matrix
Lindsey Saldin, bioengineering PhD student, in the laboratory of McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor in the Department of Surgery and director of the Center for Pre-Clinical Tissue Engineering within the Institute, recently used flow cytometry to aid in her research towards tissue engineering treatments for esophageal adenocarcinoma. Her experience follows:
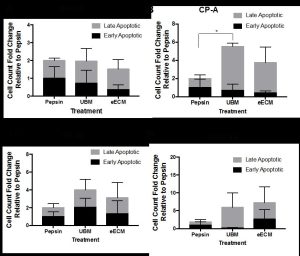
Figure 1. The fold change of early and late apoptotic cells with pepsin-solubilized urinary bladder matrix (UBM) or esophageal mucosa (eECM) treatment compared to pepsin negative control is shown for normal Het-1A (A), cancer pre-cursor Barrett’s CP-A (B), and two types of esophageal adenocarcinoma OE33 (C) and SK-GT-4 (D) esophageal epithelial cell lines. Early/late apoptosis was determined using Propidium Iodide/Annexin V staining.
The incidence of esophageal adenocarcinoma (EAC) is increasing at a faster rate than any other cancer type in the United States [1]. The standard of care is esophagectomy, a procedure with a mortality rate of 1-18% [2-5] and complication rate as high as 64% [3]. In 2011, the Badylak laboratory showed that long segment mucosal resection of EAC confined to the mucosa (i.e., stage T1a) followed by placement with an extracellular matrix (ECM) bioscaffold was shown to minimize or prevent subsequent stricture, promote the replacement of a functional esophageal mucosa, and perhaps most importantly, was not associated with recurrence of EAC in 5 patients [6]. Since the 2011 report, 9 additional patients have been treated with similar results (unreported data).The ECM scaffolds had completely degraded within two weeks of placement, showing the bioinductive cues of the extracellular matrix changed the default esophageal healing response in an area of resected cancer. The inductive cues ECM provides to direct cell behavior may be explained by the concept of “dynamic reciprocity,” the bidirectional crosstalk between a cell and its surrounding matrix to dictate cell behavior. Hence, the rationale for ECM as a material post cancer-resection is that restoring normal microenvironmental cues by supplying “normal” ECM to the site of resected cancer may provide an early inductive cue and reprogram the cells toward normalcy.
The objective of the present study was to expose pepsin-solubilized degradation products of the extracellular matrix to normal, Barrett’s metaplastic cells (the esophageal precursor cancer state), and EAC cells, and then determine changes in cell phenotype, functions associated with tumorigenesis, and cancer signaling pathways. Two types of ECM were used, homologous urinary bladder matrix (UBM) and heterologous esophageal mucosa ECM (eECM). It was hypothesized the ECM would downregulate key neoplastic functions and cancer cell signaling pathways, and that eECM would provide a more pronounced effect to modulate EAC behavior as the homologous ECM source.
One of the cell functions investigated was the degree of early and late apoptosis. This study was conducted in collaboration with Lynda Guzik at the McGowan Flow Cytometry Core. Resistance to apoptosis is a hallmark of neoplastic cells [7]. Normal (Het-1A), Barrett’s (CP-A) and EAC (OE33 and SK-GT-4) cell lines were exposed to pepsin-solubilized UBM, eECM, and pepsin negative control for 24h and then evaluated using flow cytometry with Annexin V/Propidium Iodide (PI) staining. Results showed the metaplastic (CP-A) cells increased late apoptosis compared to pepsin control. No other significant differences were found, however, trends toward increased early/late apoptosis for OE33 and SK-GT-4 cancer cells with UBM and eECM treatment were noted. We are grateful for Ms. Guzik’s expertise in 1) developing the experimental design and optimization of experimental conditions; 2) the performance of the experiment while accommodating our schedule; and 3) her help with the analyses of results. We look forward to collaborating with the flow cytometry core in future studies.
References
- Pohl, H. and H.G. Welch, The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst, 2005. 97(2): p. 142-6.
- Luketich, J.D., et al., Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg, 2003. 238(4): p. 486-94; discussion 494-5.
- Atkins, B.Z., et al., Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg, 2004. 78(4): p. 1170-6; discussion 1170-6.
- Swisher, S.G., et al., Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg, 2000. 119(6): p. 1126-32.
- Metzger, R., et al., High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus, 2004. 17(4): p. 310-4.
- Badylak, S.F., et al., Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A, 2011. 17(11-12): p. 1643-50.
- Hanahan, D. and R.A. Weinberg, Hallmarks of cancer: the next generation. Cell, 2011. 144(5): p. 646-74.
If you have a research project where flow cytometry may benefit your efforts, please feel free to contact Lynda Guzik for more information.
EVENTS
SAVE THE DATE-2018 McGowan Institute Retreat

The program chair for the 2018 McGowan Institute Scientific Retreat is Julie Phillippi, PhD. Stay tuned for additional details and registration information.
 Regenerative Medicine Summer School 2018
Regenerative Medicine Summer School 2018
Bryan Brown, PhD has announced the 5th Annual Regenerative Medicine Summer School- June 3-9, 2018
Objective: To provide national and regional students with a week-long didactic and experiential learning experience addressing the science and engineering related to the multidisciplinary field of regenerative medicine.
Target Audience: Undergraduates, enrolled in a science or engineering program that will have completed their 3rd year of study; exceptional candidates who will have completed their 2nd year of undergraduate study will be considered.
Venue: McGowan Institute-Pittsburgh, PA: June 3-9, 2018
Students who are interested in summer internships in addition to the summer school should apply by January 31, 2018.
Recognizing 25 Years of Progress
 In 1992, the McGowan Center for Artificial Organ Development was formed in a small cluster of research laboratories on the 8th floor of Presbyterian University Hospital. The Center was made possible by the donation of $1 million by Mr. and Mrs. William McGowan. Mr. McGowan was the founding CEO of MCI Communications, and had come to Pittsburgh for a heart transplant. In 2001, the University in collaboration with UPMC expanded the scope of the center to that of an institute, where in addition to medical device development, tissue engineering and cell-based therapies were integrated. The expanded enterprise is today’s McGowan Institute for Regenerative Medicine. This multidisciplinary network, now with over 240 affiliated faculty members from 31 academic departments, has pursued an ambitious program focused on its mission to develop technologies that address tissue and organ insufficiency. Central to this mission is the commitment to expedite the translation of emerging technologies into clinically available products and procedures. In addition to the pioneering science, McGowan affiliated faculty-developed technologies have resulted in 26 spin-out companies. The research areas pursued today by Institute members are dynamic and growing, as the Institute seamlessly integrates engineers, clinicians and basic scientists to fulfill its mission.
In 1992, the McGowan Center for Artificial Organ Development was formed in a small cluster of research laboratories on the 8th floor of Presbyterian University Hospital. The Center was made possible by the donation of $1 million by Mr. and Mrs. William McGowan. Mr. McGowan was the founding CEO of MCI Communications, and had come to Pittsburgh for a heart transplant. In 2001, the University in collaboration with UPMC expanded the scope of the center to that of an institute, where in addition to medical device development, tissue engineering and cell-based therapies were integrated. The expanded enterprise is today’s McGowan Institute for Regenerative Medicine. This multidisciplinary network, now with over 240 affiliated faculty members from 31 academic departments, has pursued an ambitious program focused on its mission to develop technologies that address tissue and organ insufficiency. Central to this mission is the commitment to expedite the translation of emerging technologies into clinically available products and procedures. In addition to the pioneering science, McGowan affiliated faculty-developed technologies have resulted in 26 spin-out companies. The research areas pursued today by Institute members are dynamic and growing, as the Institute seamlessly integrates engineers, clinicians and basic scientists to fulfill its mission.
The McGowan Institute has been at the forefront of many advances in the science of regenerative medicine, and in the translation of these emerging technologies to commercial and clinical use. The future for regenerative medicine-based technologies and procedures is bright. Many new devices and procedures are maturing. The basic science programs that support the translational aspects of the program are dynamic and growing. Regenerative medicine offers to be one of the emerging tools to help improve the quality of life and minimize the overall costs of health care. Representative John Maher, Pitt Board of Trustees member, recently presented to Dr. Wagner, Institute Director, a citation from the Commonwealth of Pennsylvania recognizing the 25th anniversary of the Institute and the achievements that have been realized by McGowan affiliated faculty, and their staffs and trainees.
SCIENTIFIC ADVANCES
Dr. Kia Washington to Direct National Eye Transplant Research Program
From the desks of McGowan Institute for Regenerative Medicine affiliated faculty members J. Peter Rubin, MD, and José-Alain Sahel, MD:
We are pleased to announce the establishment of an interdisciplinary research program in the science of eye transplantation. This unique program in the science of eye transplantation is a joint effort between the Department of Ophthalmology and the Department of Plastic Surgery, and is led by McGowan Institute affiliated faculty member Kia Washington, MD, the director, in coordination with Dr. Sahel. In addition to leveraging the skills and expertise of members of the Departments of Ophthalmology and Plastic Surgery, this innovative program will also include experts in immunology, transplant surgery, the neurosciences, and other related disciplines.
The overarching goal of the eye transplant research program is to restore vision following ocular trauma and ischemic or degenerative damage with eye transplantation. This program builds on Dr. Washington’s pioneering work, which has included development of the first orthotopic vascularized eye transplant small animal model. Current program funding is from a multi-million-dollar grant from the Department of Defense Joint Warfighter Medical Research Program awarded to Dr. Washington.
This novel research program embodies a comprehensive approach to addressing the challenges of eye transplantation. Dr. Washington has assembled an experienced multi-disciplinary team of scientific leaders, from both within the University of Pittsburgh and around the nation, to drive this exciting research forward. Her partnership with the Department of Ophthalmology and Dr. Sahel will propel this research and accelerate the pace of discovery in this exciting field.
After earning her MD at Duke University School of Medicine, Dr. Washington completed her residency in Plastic and Reconstructive Surgery at the University of Pittsburgh Medical Center. She then completed a fellowship in hand and upper extremity surgery at the University of Pittsburgh as well. Board-certified in Plastic Surgery and Hand Surgery, Dr. Washington started working as an Assistant Professor of Plastic Surgery in 2012. She is currently the Associate Director of Hand Surgery at UPMC, Section Chief of Plastic Surgery at the VA Pittsburgh Healthcare System, and Co-Director of the Vascularized Composite Allotransplantation and Microsurgery Laboratory in the Department of Plastic Surgery.
Jose-Alain Sahel, MD
Professor and Chairman
The Eye and Ear Endowed Chair
Department of Ophthalmology
Director, UPMC Eye Center
University of Pittsburgh School of MedicinePeter Rubin, MD, FACS
Chair, Department of Plastic Surgery
Director, UPMC Wound Healing Services
UPMC Endowed Professor of Plastic Surgery
Professor of Bioengineering
University of Pittsburgh
Computer Program Helps Doctors Detect Acute Kidney Injury Earlier to Save Lives

Embedding a decision support tool in the hospital electronic health record increases detection of acute kidney injury, reducing its severity, and improving survival, according to new research from the University of Pittsburgh and UPMC.
The results, published in the Journal of the American Society of Nephrology, address one of the most costly and deadly conditions affecting hospitalized patients, providing evidence that computers analyzing changes in renal function can alert doctors of acute kidney injury before the condition is obvious clinically.
“Acute kidney injury strikes one in eight hospitalized patients and, if unchecked, it can lead to serious complications, including the need for dialysis and even death,” said senior author John Kellum, MD, professor of critical care medicine, director of the Center for Critical Care Nephrology at Pitt’s School of Medicine, and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “Our analysis shows that implementation of a clinical decision support system was associated with lower mortality, less need for dialysis, and reduced length of hospital stay for patients diagnosed with acute kidney injury, among other benefits.”
Acute kidney injury is common in hospitalized patients, particularly those in intensive care units and older adults, and refers to a sudden episode of kidney failure or damage that happens within a few hours or days. It causes a build-up of waste products in the blood that can affect other organs, including the brain, heart, and lungs.
While kidney function is monitored using simple blood tests, subtle changes can elude or delay detection of a problem. Failure to recognize and manage acute kidney injury in the early stages can lead to devastating outcomes for patients and increased costs to the health care system. Benefits of earlier detection of acute kidney injury include earlier intervention to mitigate loss of kidney function, and reduced hospital and long-term health care costs as a result of avoiding progression to severe and permanent kidney damage.
In 2013, Dr. Kellum’s team released a computer program within the electronic health record system across 14 UPMC hospitals. The program monitored levels of blood creatinine, a standard measure of kidney function, over time and analyzed changes in those levels. If the levels rose too high or fast, the program fired an alert in the patient’s electronic health record informing doctors that acute kidney injury could be present. It also helped determine the stage of injury based on changes from the patient’s baseline kidney function.
To determine what effect, if any, the computer program was having on physician behavior and patient outcomes, Dr. Kellum and his colleagues analyzed records from more than half a million patients admitted to UPMC. They started a year before the alert system was deployed, and continued for 2 years after. Patients with acute kidney injury had a small yet sustained decrease in hospital mortality of 0.8 percent, 0.3-day shorter length of stay, and a decrease of 2.7 percent in dialysis rates. Even after adjusting for age and severity of illness, these changes remained highly significant.
In absolute terms, the changes are small, but given the annual frequency of acute kidney injury in hospitalized U.S. patients of about 12 percent – or 2.2 million people – these results would translate into more than 17,000 lives and $1.2 billion saved per year.
“Ultimately, we see this as confirmation that a fairly simple clinical decision support system can make a difference,” said co-author Richard Ambrosino, MD, PhD, medical director of clinical decision support and reporting at UPMC’s eRecord. “More sophisticated systems are possible and should have an even greater impact.”
Dr. Kellum, who also is associate director for acute illness at Pitt’s Institute for Precision Medicine, plans to make improvements to the clinical decision support application in the future.
“Working with pharmacists to adjust patient medications and machine-learning experts to better predict which patients will be at greatest risk for adverse events, my team and I hope to make an even greater impact on patient outcomes,” said Dr. Kellum. “Incorporating protein biomarkers and even genomics into the system could one day revolutionize patient care, not just for acute kidney injury, but for other illnesses.”
Dr. Andrew Schwartz: For BCIs to Be Useful, They’ll Need to Be Wireless

McGowan Institute for Regenerative Medicine faculty member Andrew Schwartz, PhD—Distinguished Professor of Neurobiology at the University of Pittsburgh with adjunct appointments at the Center for Neural Basis Cognition (a joint venture of the University of Pittsburgh and Carnegie Mellon University), at the Robotics Institute at Carnegie Mellon University, and at Pitt’s Department of Bioengineering and its Department of Physical Medicine and Rehabilitation—recently participated in MIT (Massachusetts Institute of Technology) Technology Review’s 17th Annual EmTech where this year’s most significant news on emerging technologies was examined. Dr. Schwartz’s session featured “For Brain-Computer Interfaces to Be Useful, They’ll Need to Be Wireless.”
MIT Technology Review’s associate editor for biomedicine, Emily Mullin, MA, reported on Dr. Schwartz’s presentation. She reports that for decades, brain-computer interfaces have been imagined as a way for people who are paralyzed or those who have lost arms to be able to do everyday tasks like brushing their hair or clicking a TV remote—just by thinking about it. Such robotic devices exist today—so far, a handful of patients in research labs around the world have tried them, giving them a limited range of motions. Some of the patients treated by Dr. Schwartz and his collaborators include Tim Hemes, Jan Scheuermann, Nathan Copeland (photos of all here). But researchers are still years away from making these devices practical for use in people’s homes, says Dr. Schwartz.
Today’s brain-computer interfaces involve electrodes or chips that are placed in or on the brain and communicate with an external computer. These electrodes collect brain signals and then send them to the computer, where special software analyzes them and translates them into commands. These commands are relayed to a machine, like a robotic arm, that carries out the desired action. The embedded chips, which are about the size of a pea, attach to so-called pedestals that sit on top of the patient’s head and connect to a computer via a cable. The robotic limb also attaches to the computer. This clunky set-up means patients can’t yet use these interfaces in their homes.
To get there, Dr. Schwartz said, researchers need to size down the computer so it’s portable, build a robotic arm that can attach to a wheelchair, and make the entire interface wireless so that the heavy pedestals can be removed from a person’s head. Dr. Schwartz said he hopes paralyzed patients will someday be able to use these interfaces to control all sorts of objects beyond just a robotic arm.
“Just imagine someone using telemetry going into a smart home and being able to operate all these devices merely by thinking about them,” he said.
Dr. Schwartz said he’s working on such a model with Draper Laboratory, based in Cambridge, MA, but hasn’t been able to get funding to move the project along. “This is very much on the outskirts of science,” said Dr. Schwartz.
EmTech is an annual opportunity to discover future trends and to understand the technologies that will drive the new global economy. It’s where tech, business, and culture converge, and where you gain access to the most innovative people and companies in the world. The 17th annual EmTech examined this year’s most significant news on emerging technologies. MIT Technology Review events consistently attract senior-level business and technology decision makers who drive the global innovation economy.
Illustration: Justin Saglio, MIT Technology Review.
Understanding Addiction in the Adolescent Mind

Several studies have provided strong evidence that adolescents—people in their teens to early twenties—have a higher vulnerability than adults to addictive substances like cocaine. To understand the origin of the age effect requires a sensor to effectively measure how cocaine interacts with different parts of the brain over time.
Bioengineers at the University of Pittsburgh have developed a new method of using synthetic DNA called “aptamers” to view the effect of cocaine on the brain in real-time with a much higher resolution than other techniques. The Royal Society of Chemistry published their results in its Journal of Materials Chemistry B earlier this year (DOI: 10.1039/C7TB00095B).
“Aptamer-based cocaine sensors have been previously developed for detecting cocaine in whole blood samples, outside of the body. We are the first to develop a sensor for detecting cocaine in the brain tissue of live animals,” says Xinyan Tracy Cui, PhD, William Kepler Whiteford Professor of Bioengineering at Pitt’s Swanson School of Engineering and a McGowan Institute for Regenerative Medicine faculty member.
Dr. Cui is principal investigator for a 2-year, $421,185 new study titled “Dual Polymer Coatings for High Fidelity and Stable In Vivo Cocaine Sensing from MEAs.” She and her team will be using the aptamer-based sensors to observe the effects of cocaine use on both adult and adolescent rats and record the differences.
“It remains unknown whether the ‘age effect’ is the result of differences in neuron sensitivity to cocaine or a consequence of how cocaine concentrates in different areas of the brain,” explains I. Mitch Taylor, postdoctoral researcher in the Cui Lab. “By measuring cocaine concentration and following the pharmacokinetics of the drug over repeated use in both adult and adolescent rats, we hope to answer this question.”
In addition to observing the effect of cocaine on the brain, the researchers will be testing a polymer coating that protects the sensors from harmful exposure once they are implanted inside the rats. Past studies have shown that the brain’s biological environment can interfere with the sensors’ ability to relay information to the researchers.
“The sensors worked great for a couple of hours in the brain tissue, and then performance degraded rapidly due to sensor degradation and biofouling,” says Dr. Cui. “The newly funded project will use advanced polymers to stabilize the sensor and minimize the host tissue reactions, with the goal of achieving long term in vivo sensing over the period of days.”
Dr. Cui aims to directly measure real-time cocaine concentration in different locations of the brain with micrometer resolution for at least 72 hours. Conventional methods of measuring cocaine in the brain tissue are only able to measure concentration levels every 5-10 minutes and over a large volume.
The funding comes from a R21 Exploratory/Development grant from the National Institutes of Health (NIH) National Institute on Drug Abuse. The researchers will focus on studying cocaine usage during this grant period with potential to broaden their research efforts down the road.
“Our sensor technology platform is well suited to be developed for detecting other substances in the future,” assures Dr. Cui.
Tracing Cell Death Pathway Points to Drug Targets for Brain Damage, Kidney Injury, and Asthma
University of Pittsburgh scientists—including McGowan Institute for Regenerative Medicine affiliated faculty members Valarian Kagan, PhD, DSc, Simon Watkins, PhD, John Kellum, MD, and Ivet Bahar, PhD—are unlocking the complexities of a recently discovered cell death process that plays a key role in health and disease, and new findings link their discovery to asthma, kidney injury, and brain trauma. The results are the early steps toward drug development that could transform emergency and critical care treatment.
Damaged or malfunctioning cells can wreak havoc on the body, so it’s essential to destroy and recycle them safely and efficiently. One way this is accomplished is through ferroptosis, a highly regulated cell death program that uses iron (“ferro” means iron), and was first discovered in 2012, explained senior author Dr. Kagan, professor in the Pitt Graduate School of Public Health’s Department of Environmental and Occupational Health. The work also was led by Sally Wenzel, MD, director of the University of Pittsburgh Asthma Institute at UPMC, and Hülya Bayɪr, MD, research director of Pediatric Critical Care Medicine at Children’s Hospital of Pittsburgh of UPMC.
To function harmoniously, the billions of cells in the body use a sophisticated and coordinated language to communicate. Last year, the team published two papers that uncovered the signaling language that cells use to initiate ferroptosis. The communication process they found requires a group of naturally occurring oxidized phospholipids called OOH-phosphatidylethanolamines (OOH-PEs). Phospholipids are the basic building blocks that make up cell membranes, which separate what’s inside a cell from everything else outside.
However, if too many of these phospholipid signals are generated and too many cells die, the organs and tissues of the body cannot function normally.
“Discovering ferroptosis was just the tip of the iceberg – it’s essential that we learn how to keep it under control. If we want to do that, we must understand how it works,” said Dr. Kagan. “And, by discovering how several key proteins interact with the phospholipid molecules to cause ferroptosis, that is what we did. Now we can move forward with the translational work of finding ways to limit ferroptosis and prevent the massive cell death that leads to catastrophic organ and tissue failure.”
In the new work, the team used a variety of methods, including a new technique called lipodomics, to discover that the production of OOH-PE is a major crossroads at which a cell decides whether to initiate ferroptosis.
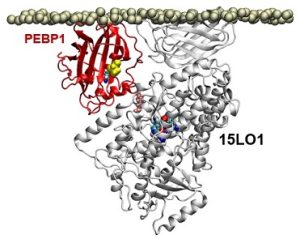
Previous research had shown that OOH-PEs were made by a group of enzymes known as 15-lipoxygenases (15LOs). In the course of its study, the team discovered that a protein called PEBP1 was acting as a “warden” controlling whether the 15LO enzymes made either OOH-PEs or another type of membrane building block. When the cell made increasing amounts of OOH-PEs, it initiated ferroptosis.
The researchers also used several cell culture experiments to demonstrate that PEBP1 and other key players in this pathway play a role in driving ferroptosis in several diseases, such as in kidney cells during renal failure, neurons in brain trauma, and airway cells in asthma. Preventing PEBP1 from binding to 15LOs might be a way to prevent ferroptosis, says Dr. Kagan.
“Better treatments for traumatic brain injury and acute kidney injuries are desperately needed,” said Dr. Bayɪr, co-senior author of the new work. “Gaining insight into these conditions at the molecular level is extremely crucial to developing new therapies.”
In traumatic brain and acute kidney injuries, there is typically an ever-widening area of cell death, indicating that ferroptosis is continuing unabated, even after cells involved in the initial injury have been cleared, said Dr. Bayɪr, also a professor in Pitt’s departments of Critical Care Medicine and Environmental and Occupational Health. This points to a longer therapeutic window of opportunity during which the ferroptotic pathway could be targeted with drugs to halt these devastating injuries.
A future therapy targeting ferroptosis could work differently for asthma, noted Dr. Wenzel, the lead author.
“Asthma is not just about stopping an attack, it’s also about prevention – and both are a major unmet need among patients,” said Dr. Wenzel, also a professor of medicine in Pitt’s School of Medicine. “Our study identified how ferroptotic death signals can damage the cells lining the airway. Targeting these pathways could lead to both preventative and treatment options for asthma exacerbations.”
Illustration: In a computer model, the protein PEBP1 on a cell membrane interacts with the enzyme 15LO1. 2017 Wenzel, et al. Cell.
Pitt and UPMC Researchers Collaborate to Save More Organs for Transplants

Each year, the United States suffers an extreme shortage of organ donations, with only a quarter of patients in need receiving a transplant. Many transplantable organs are lost when a donor’s heart fails, and the organs stop receiving vital blood flow. Researchers at the University of Pittsburgh can potentially double the amount of successful organ donations by developing a novel stent to maintain blood flow to organs, even during the donor’s final heart beats.
The National Institutes of Health awarded a 4-year, $1.3 million R01 grant to a University of Pittsburgh research collaboration between the Department of Surgery and the Department of Industrial Engineering. The study titled “An Organ Perfusion Stent as an Alternative to Surgery in Donor Organ Recovery” will develop a dual chamber organ perfusion stent made of smart material to direct selective blood flow during transplant surgeries.
Leading the study are Principal Investigators Bryan Tillman, MD, PhD, assistant professor in the Division of Vascular Surgery at University of Pittsburgh Medical Center (UPMC); Youngjae Chun, PhD, associate professor of industrial engineering and bioengineering; and Sung Kwon Cho, PhD, associate professor of mechanical engineering and materials science at Pitt’s Swanson School of Engineering. Drs. Tillman and Chun are McGowan Institute for Regenerative Medicine affiliated faculty members.
The stent will isolate visceral arteries—which supply blood to many major organs—without disturbing the heart. To make the stent, the research team will use a superelastic material with a flexible shape memory effect called nitinol, or nickel titanium.
“The shape memory behavior of nitinol is critical for endovascular devices such as stents, filters, and occluders, because at low temperatures, nitinol can easily be collapsed, inserted into a catheter, and delivered into the body,” said Dr. Chun. “Once inside, the body heat will change nitinol’s properties to be superplastic without any actuation force, which is really beneficial for a wide-range of catheter-based procedures.”
The organ perfusion stent can be inserted by a clinician into the femoral artery after the delivery of a guide wire and a catheter. This small puncture or “needlestick” method allows clinicians to maintain selective blood flow to certain organs without disrupting others’ natural functioning. The much larger organ stent in its compressed state can be delivered to the desired organ and deployed.
“We can target the kidneys, pancreas, and liver,” said Dr. Chun. “Transplants involving any major organs connected to the main aorta will be able to benefit from this new technology.”
Other collaborators include William Clark, PhD, professor of mechanical engineering and materials science at Pitt; Ryan Dzadony, MEd, CCP, LP, associate director of the UPMC School of Perfusion; Anthony Demetris, MD, Starzl Professor of Liver and Transplant Pathology at UPMC and McGowan Institute for Regenerative Medicine affiliated faculty member; and Amit Tevar, MD, associate professor of surgery at the Thomas E. Starzl Transplant Institute.
Illustration: Venous dual chamber organ perfusion stent prototype. Note: some materials in the design were purchased from Cook Medical.
Creating Self-Recognizing and Self-Regulating Synthetic Materials
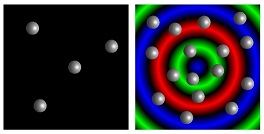
From the smallest cell to humans, most organisms can sense their local population density and change behavior in crowded environments. For bacteria and social insects, this behavior is referred to as “quorum sensing.” Researchers at the University of Pittsburgh’s Swanson School of Engineering have utilized computational modeling to mimic such quorum sensing behavior in synthetic materials, which could lead to devices with the ability for self-recognition and self-regulation.
The findings are based on research into biomimetic synthetic materials by McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, John A. Swanson Chair in Engineering and the Distinguished Professor of Chemical and Petroleum Engineering, and post-doctoral associate Henry Shum, PhD, who is now an assistant professor of applied mathematics at the University of Waterloo. The article, “Synthetic quorum sensing in model microcapsule colonies,” is published in the journal PNAS.
“Quorum sensing (QS) is a distinctive behavior of living organisms that allows them to initiate a specific behavior only when a critical threshold in population size and density are exceeded,” Dr. Balazs explained. “This tunable self-awareness is apparent in macro systems such as bees selecting a site for a new hive, but is vital to cellular systems like bacteria, which produce and secrete signaling molecules that act as “autoinducers” once a specific population is reached. Creating a biomimetic response can allow synthetic materials to effectively “count”; this is, to sense and adapt to their environment once a preprogrammed threshold is reached.”
In a biological system, autoinducers in low concentrations diffuse away and therefore do not trigger response. Hence, the system is in a type of “off” state. However, when the cells reach a specific number or quorum, the production of autoinducers leads to a detection and response. This “on” state increases the production of the signaling molecule and activates further metabolic pathways that are triggered by QS, coordinating the colony behavior.
“However, autoinducers tend to maintain the “on” state once activated so the system is less sensitive to subsequent decreases in the population,” Dr. Shum said. “For self-regulating materials to unambiguously determine their present density, we modeled a colony of immobile microcapsules that release signaling chemicals in a “repressilator” network, which does not exhibit the same “memory” effect. Instead, we found that chemical oscillations emerge in the microcapsule colony under conditions that are analogous to achieving a quorum in biological systems.”
The researchers note that their findings could inspire new mechano-responsive materials, such as polymer gels with embedded QS elements that would activate a certain chemical behavior when compressed, and then switch off when stretched, or when a specific temperature is reached.
“For example, you could have a robotic skin that solidifies to protect itself at a certain temperature, and then becomes “squishy” again when the temperature drops to a nominal level,” Dr. Balazs adds. “Although our work is computational, the results show that the creation of self-recognizing and self-regulating synthetic materials is possible.”
Illustration: Modeled microcapsules (image: grey spheres/gif: small circles) demonstrate “quorum sensing” behavior. University of Pittsburgh Swanson School of Engineering.
AWARDS AND RECOGNITION
BreathoMag Innovation Receives Philips Grand Challenge 2nd Award

Philips Grand Challenge, hosted by Philips and the University of Pittsburgh Innovation Institute, awarded four University of Pittsburgh faculty members a total of $100,000. Each is innovating in the area of sleep and respiratory care.
McGowan Institute for Regenerative Medicine affiliated faculty member Prashant Kumta, PhD, along with Puneeth Shridhar, MD, both teaching engineering, received the competition’s 2nd Award, a total of $30,000. They developed BreathoMag, a dissolvable nasal stent made of magnesium alloy to open the nasal passage. Sinus inflammation, a blocked nose, or a deviated septum can lead to snoring and sleep loss. Silicone and nitinol nasal stents address this problem by opening the nasal airway, but these devices can be uncomfortable and require periodic reinsertion. BreathoMag is a nasal stent composed of a novel magnesium alloy that comfortably opens the nasal passage and then slowly and safely absorbs into the body. Patients can place the stent themselves, making BreathoMag a user-friendly way for patients to sleep, breathe, and feel better.
Also noted, the project of McGowan Institute affiliated faculty member Rory Cooper, PhD, and Mark Greenhalgh, entitled PTS Aware, was a finalist in the competition. PTS Aware is a predictive smartphone application for PTSD mindfulness.
The Philips Grand Challenge is designed to enhance opportunities for innovations developed at the University of Pittsburgh in areas of interest to Philips to advance towards commercialization. The Challenge, is intended to provide financial support that cannot typically be obtained from other traditional sources of funding (including the faculty member’s department/school or external government funding agencies). Philips is a global leader in health technology with more than 40 years of leadership in sleep apnea management, oxygen therapy, noninvasive ventilation and respiratory drug delivery.
Dr. Kumta holds the Edward R. Weidlein Chair Professor with Tenure at the University of Pittsburgh Swanson School of Engineering and School of Dental Medicine and is a professor in the Departments of Bioengineering, Chemical and Petroleum Engineering, Mechanical Engineering and Materials Science, and Department of Oral Biology. He is also Engineering Director of the Center for Craniofacial Regeneration (CCR) and the Founding Director of the Center for Complex Engineered Multifunctional Materials (CCEMM), both at the University of Pittsburgh.
Dr. Cooper is the FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh. Dr. Cooper is Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories, a VA Rehabilitation R&D Center of Excellence in partnership with Pitt.
Illustration: Innovation Institute.
PInCh Awards Focus on Wearable Devices that Address Problems in Health Care

The final event in the Pitt Innovation Challenge (PInCh®) concluded with the award of funding to innovative projects featuring wearable devices that address problems in health care. The challenge, which is in its fourth year, was sponsored by the University of Pittsburgh’s Clinical and Translational Science Institute (CTSI). The central question addressed by this year’s contestants was, “How can we use wearable technology to address an important health problem?”
“Wearable technology capabilities are advancing rapidly, and this year’s PInCh final showcased how this technology can make a difference in health care,” said CTSI director Steven Reis, MD, who also is associate vice chancellor for clinical research, health sciences, and a distinguished professor of medicine at Pitt’s School of Medicine.
Of the 13 winners, two project teams included McGowan Institute for Regenerative Medicine affiliated faculty members. Both winning solutions will also positively impact US military veterans. The projects and teams are:
$125,000 Award Recipient: MOVISU-Fit
Team Members: Krista Kutina, Michael Boninger, Dave Brienza, Katee Coleman, Goeran Fiedler, Alicia Koontz, Mary Miknevich, Kristana Mitchell
Description: Mobile gait training system for lower limb amputees that provides real time visual feedback from data derived from an integrated kinetic sensor in the prosthetic limb.
$30,000 Award Recipient: Manual Wheelchair Virtual Seating Coach
Team Members: S. Andrea Sundaram, Josh Brown, Joshua Chung, Rory Cooper, James Landis
Description: A system to help manual wheelchair users to learn and remember to do adequate pressure reliefs to prevent the development of pressure ulcers.
After two rounds of pre-selection, 13 teams were invited to compete at the final pitch event. Three projects took home $100,000 to $125,000 each in funding, seven received $10,000 to $30,000, and three others received $10,000 on Oct. 25 during an evening filled with live presentations and posters at the University Club in Oakland. Funds to support 2017 PInCh awards were provided by DSF Charitable Foundation, the charitable-giving organization of the David Scaife family.
Congratulations, all!
Top Awards Highlight Work in Badylak and Cooper Laboratories

When Michael Wells had an idea for a healthcare startup when he was a University of Pittsburgh student, he didn’t know where to go for help within the University to move it forward. For the past seven years, the successful healthcare investor and entrepreneur has helped accelerate the culture of innovation and entrepreneurship at Pitt through the Michael G. Wells Competition to ensure that any student with such an idea has access to resources and support to take it all the way. Also, Laurie Kuzneski, together with her husband Andy, created the Kuzneski Innovation Cup. In its second year, the competition assists Pitt innovators with non-healthcare ideas. Both competitions’ judges agreed that choosing this year’s winners was very difficult due to the quality of all the nominees’ presentations.
The top prize of $20,000 for the Wells Competition went to Esophagel, which is developing an extracellular matrix hydrogel that can be delivered to the esophagus to control inflammation and promote tissue remodeling in patient’s suffering from Barrett’s esophagus. The team was previously a winner of the Pitt Innovation Challenge (PInCh) competition and is led by postdoctoral fellow Juan Diego Naranjo, PhD, and PhD candidate Lindsey Saldin. The technology is from the lab of Stephen Badylak, DVM, PhD, MD, deputy director of the McGowan Institute for Regenerative Medicine, who holds over 60 U.S. patents.
In the Kuzneski Innovation Cup competition, the top prize of $15,000 went to PneuMobility, out of Pitt’s Human Engineering Research Lab, led by McGowan Institute affiliated faculty member Rory Cooper, PhD. They have developed PneuChair, a wheelchair powered with compressed air that is lighter than electric-powered chairs and is waterproof. Four of the chairs were built for Morgan’s Wonderland, a theme park for the disabled in Texas that includes a water park. The student innovator, PhD candidate Brandon Daveler, said there is significant investor interest in the PneuChair. “Seeing the impact that this innovation has already made, seeing the smiles on the kids’ faces who couldn’t previously enjoy a water park on their own, makes us even more determined to reach more people,” Mr. Daveler said.
Congratulations, all!
Dr. Robert Parker Receives 2017 Swanson School of Engineering Board of Visitors Award

Recognizing the impact of his tenure on students, faculty, and peers, the Board of Visitors of the University of Pittsburgh’s Swanson School of Engineering recognized McGowan Institute for Regenerative Medicine affiliated faculty member Robert Parker, PhD, with the 2017 Board of Visitors Award. Dr. Parker, Professor and Vice Chair for Graduate Education in the Department of Chemical and Petroleum Engineering, was recognized for faculty excellence in teaching, research, and service, and for contributions to the University, the Swanson School, and the engineering discipline.
“Dr. Parker’s outstanding bona fides not only met our qualifications, but truly exceeded them,” noted Roberta A. Luxbacher, BSChE ’78, Chair of the Board of Visitors. “Most importantly, his passion for education has had a tremendous impact on student success as well as the growth of the Department. On behalf of the Board of Visitors, we are proud to recognize him with this year’s award and thank him for his contributions to the University, the Swanson School, and the field of chemical engineering.”
“In a word, Bob’s contributions to student success, department growth, and research excellence is unparalleled,” added McGowan Institute faculty member Steven Little, PhD, Department Chair and William Kepler Whiteford Professor of Chemical and Petroleum Engineering, who nominated Dr. Parker. “He is a game-changer and inspiration to student and faculty alike, and I can’t thank him enough for his selfless dedication to our Department.”
In Dr. Little’s nomination letter, he noted that in 2017 alone, Dr. Parker’s significant achievements included:
- promotion as the Department’s Vice Chair for Graduate Education
- graduating four PhD students
- publishing eight papers
- directing one of the Department’s National Science Foundation Research Experience for Undergraduates programs
- receiving a 4.75 OTE score in 0500 Process Systems with 63 students
- establishing ENGR 1933: Engineering a Craft Brewery, the only course of its kind taught at an engineering school
- receiving the Swanson School of Engineering’s Outstanding Educator Award
“I am honored to be named the 2017 Board of Visitors Award winner. This award truly recognizes the outstanding team of graduate students and collaborators, both clinical and within the Swanson School of Engineering, that I have had an opportunity to work closely with,” Dr. Parker said. “I thoroughly enjoy the collaborative research and problem-solving environment at Pitt, as well as my classroom interactions teaching the next generation of impactful Chemical Engineers and, for the first time, aspiring Craft Brewers.”
Dr. Parker joined the University of Pittsburgh faculty as an Assistant Professor in 2000 and promoted to Professor in 2014. His research program focuses on systems medicine and the use of mathematical models in the design of clinical decision support systems. In addition to the Outstanding Educator Award, he has been recognized for excellence in education through the Carnegie Science Center Excellence in Higher Education Award and the David L. Himmelblau Award from the Computing and Systems Technology (CAST) Division of AIChE. His commitment to a collaborative future in graduate education formed the basis of two funded Department of Education Graduate Assistance in Areas of National Need (GAANN) training programs, as well as the Systems Medicine Research Experiences for Undergraduates (REU) program.
As Vice Chair for Graduate Education, Dr. Parker is responsible for developing graduate-level training programs to support PhD students, leading graduate recruitment and admission; managing PhD timelines; collaborating with the Swanson School Office of Diversity to maintain a diverse graduate program; serving as faculty advisor of the Department’s Graduate Student Association; and, managing faculty teaching assignments.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#177 –– Dr. Rachelle Palchesko is the Special Faculty Researcher in the Department of Biomedical Engineering at Carnegie Mellon University. Dr. Palchesko discusses her research on the regeneration of the corneal endothelium.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA
Title: Clinical Decision Support for In-Hospital AKI
Summary: AKI carries a significant mortality and morbidity risk. Use of a clinical decision support system (CDSS) might improve outcomes. We conducted a multicenter, sequential period analysis of 528,108 patients without ESRD before admission, from October of 2012 to September of 2015, to determine whether use of a CDSS reduces hospital length of stay and in-hospital mortality for patients with AKI. We compared patients treated 12 months before (181,696) and 24 months after (346,412) implementation of the CDSS. Coprimary outcomes were hospital mortality and length of stay adjusted by demographics and comorbidities. AKI was diagnosed in 64,512 patients (12.2%). Crude mortality rate fell from 10.2% before to 9.4% after CDSS implementation (odds ratio, 0.91; 95% confidence interval [95% CI], 0.86 to 0.96; P=0.001) for patients with AKI but did not change in patients without AKI (from 1.5% to 1.4%). Mean hospital duration decreased from 9.3 to 9.0 days (P<0.001) for patients with AKI, with no change for patients without AKI. In multivariate mixed-effects models, the adjusted odds ratio (95% CI) was 0.76 (0.70 to 0.83) for mortality and 0.66 (0.61 to 0.72) for dialysis (P<0.001). Change in adjusted hospital length of stay was also significant (incidence rate ratio, 0.91; 95% CI, 0.89 to 0.92), decreasing from 7.2 to 6.0 days for patients with AKI. Results were robust to sensitivity analyses and were sustained for the duration of follow-up. Hence, implementation of a CDSS for AKI resulted in a small but sustained decrease in hospital mortality, dialysis use, and length of stay.
Source: J Am Soc Nephrol. 2017 Nov 2. pii: ASN.2017070765. doi: 10.1681/ASN.2017070765. [Epub ahead of print]
GRANT OF THE MONTH
PI: Bryan Tillman
Co-I: Young Jae Chun
Title: An Organ Perfusion Stent as an Alternative to Surgery in Donor Organ Recovery
Description: In the United States, there is a critical shortage of organs for transplant, with only a quarter of the 120,000 patients in need of an organ actually receiving one. The cost of patients awaiting transplant to the healthcare system totals tens of billions of dollars each year. Meanwhile, thousands of organs are discarded each year as a result of injury from inadequate blood flow (ischemic injury) as the donor is dying. The traditional paradigm for consented Donation after Cardiac Death donors has been to discontinue cardiopulmonary life support, leaving organs without adequate blood perfusion until the heart stops. This contrasts with the usual end of life care options for patients who are not donors, in which patients and their families can elect to withhold life support for some body functions (CPR, agents to maintain blood pressure, dialysis, or intubation) while at the same time maintaining life support for other organs. Our proposal details an innovative needlestick approach to maintain selective blood flow to the organs while allowing the donor heart to fail on its own terms. This contrasts to previously reported approaches which would accelerate cardiac death, against ethical principles. We have examined a commercial hybrid prototype Organ Perfusion Stent (OPS) in a pilot study and shown improved blood delivery to organs without a negative impact on cardiac function. Recognizing the logistical challenges of current imaging for device placement in a critically ill patient, such as a donor, we have also developed a portable radiofrequency (RF) approach for stent positioning at bedside. The primary objective of the current approach is to demonstrate that a novel custom made OPS provides objective protection against ischemic organ injury. Our investigation will begin with optimizing the mechanical properties of this novel dual-chambered stent to ensure rapid deployment, successful isolation of the visceral arteries, and simplified retrieval. Next, we will optimize the hemodynamics of the stent to ensure uniform blood flow to the organs. A miniaturized RF antenna will then be directly printed onto the stent structure. In a porcine model of the organ donor, an OPS will be deployed by a needlestick access while under close cardiac monitoring. Positioning approaches using both portable X-ray and RF tag approaches will be compared to gold standard angiography for device positioning. Following a 60 minute period of simulated malperfusion with interval biopsies, both stented and control animals will be recovered with daily assessment to monitor organ function. At two days postoperatively, liver, pancreas and kidney biopsies will be evaluated for ischemic changes histologically. To investigate this device in a human context, an OPS calibrated for human anatomy will then be evaluated for successful deployment and organ perfusion in a heart beating human cadaver model. In summary, we expect that ultimately, an Organ Perfusion Stent may allow every consented donor to successfully donate organs for transplant while respecting the ethics of organ recovery.
Source: National Institute of Biomedical Imaging and Bioengineering
Term: September 15, 2017 – August 31, 2021
Amount: $317,622/year