Gum Disease Treated by Using Homing Beacon to Bring Needed Immune Cells to Inflamed Area
 The red, swollen, and painful gums and bone destruction of periodontal disease could be effectively treated by beckoning the right kind of immune system cells to the inflamed tissues, according to a new pre-clinical study conducted by researchers at the University of Pittsburgh, including McGowan Institute for Regenerative Medicine faculty members Steven Little, PhD, associate professor and chair of the Department of Chemical and Petroleum Engineering, Pitt’s Swanson School of Engineering, and Charles Sfeir, DDS, PhD, director, Center for Craniofacial Regeneration, and associate professor, Departments of Periodontics and Oral Biology, Pitt’s School of Dental Medicine. Their findings, published in the early online version of the Proceedings of the National Academy of Sciences, offer a new therapeutic paradigm for a condition that afflicts 78 million people in the U.S. alone.
The red, swollen, and painful gums and bone destruction of periodontal disease could be effectively treated by beckoning the right kind of immune system cells to the inflamed tissues, according to a new pre-clinical study conducted by researchers at the University of Pittsburgh, including McGowan Institute for Regenerative Medicine faculty members Steven Little, PhD, associate professor and chair of the Department of Chemical and Petroleum Engineering, Pitt’s Swanson School of Engineering, and Charles Sfeir, DDS, PhD, director, Center for Craniofacial Regeneration, and associate professor, Departments of Periodontics and Oral Biology, Pitt’s School of Dental Medicine. Their findings, published in the early online version of the Proceedings of the National Academy of Sciences, offer a new therapeutic paradigm for a condition that afflicts 78 million people in the U.S. alone.
Periodontal disease currently is treated by keeping oral bacteria in check with daily brushing and flossing as well as regular professional deep cleaning with scaling and root planing, which remove tartar above and below the gum line. In some hard-to-treat cases, antibiotics are given. These strategies of mechanical tartar removal and antimicrobial delivery aim to reduce the amount of oral bacteria on the tooth surface, explained co-author and co-investigator Dr. Sfeir.
“Currently, we try to control the build-up of bacteria so it doesn’t trigger severe inflammation, which could eventually damage the bone and tissue that hold the teeth in place,” Dr. Sfeir said. “But that strategy doesn’t address the real cause of the problem, which is an overreaction of the immune system that causes a needlessly aggressive response to the presence of oral bacteria. There is a real need to design new approaches to treat periodontal disease.”
In the healthy mouth, a balance exists between bacteria and the immune system response to forestall infection without generating inflammation, said senior author Dr. Little. But in many people, a chronic overload of bacteria sets up the immune system to stay on red alert, causing harm to the oral tissues while it attempts to eradicate germs.
“There is a lot of evidence now that shows these diseased tissues are deficient in a subset of immune cells called regulatory T-cells, which tells attacking immune cells to stand down, stopping the inflammatory response,” Dr. Little said. “We wanted to see what would happen if we brought these regulatory T-cells back to the gums.”
To do so, the researchers developed a system of polymer microspheres to slowly release a chemokine, or signaling protein, called CCL22 that attracts regulatory T-cells, and placed tiny amounts of the paste-like agent between the gums and teeth of animals with periodontal disease. The team found that even though the amount of bacteria was unchanged, the treatment led to improvements of standard measures of periodontal disease, including decreased pocket depth and gum bleeding, reflecting a reduction in inflammation as a result of increased numbers of regulatory T-cells. MicroCT-scanning showed lower rates of bone loss.
“Mummified remains from ancient Egypt show evidence of teeth scraping to remove plaque,” Dr. Little noted. “The tools are better and people are better trained now, but we’ve been doing much the same thing for hundreds of years. Now, this homing beacon for Treg cells, combined with professional cleaning, could give us a new way of preventing the serious consequences of periodontal disease by correcting the immune imbalance that underlies the condition.”
RESOURCES AT THE MCGOWAN INSTITUTE
Introducing the McGowan Institute Histology Lab
W elcome to The McGowan Institute for Regenerative Medicine Histology Lab!
elcome to The McGowan Institute for Regenerative Medicine Histology Lab!
At the McGowan Histology Lab, you will find friendly and capable help for all of your histology needs. Lori A. Walton (formerly Lori A. Perez) has extensive experience in immunohistochemistry, and has either first authored or contributed to several manuscripts on the subject. Lori has over 20 years of histology experience, and has worked in both diagnostic and research laboratories. She now brings her experience and skill set to the McGowan Institute for Regenerative Medicine.
The Histology Lab offers an extensive selection of special stains, along with routine processing, embedding, cutting and H&E staining. The possibilities with immuno-labeling are virtually endless, and include Peroxidase based reactions and various fluorescent dyes. The laboratory can assist with single, double or Multiplex staining, using quantum dot protocols. Lori specializes in formalin fixed paraffin embedded, immunohistochemistry and frozen IHC.
The McGowan Histology Lab strives to provide the highest quality of histology services for your research needs. We take pride in your success!
Please contact Lori directly at 412-624-5265 or email to perezl@upmc.edu with any questions regarding your project needs.
December Special at the Histology Lab
Ho Ho HEROVICI!
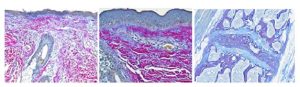
The Herovici combination of picro methyl blue or aniline blue and picro acid fuchsin in proper proportion results in a red stain for Type I mature collagen, while reticulum and newly formed Type III collagen stains blue.
The holidays are a magical time for collagen of all ages! Bring your samples to the McGowan Histology Lab and receive a 30% discount on all Herovici Stains for the entire month of December. Happy Holidays Collagen! Let’s paint the town Red… or Blue.
You’ll receive 10% off Herovici Staining for the entire month of December when you mention this ad.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Introducing the Center for Biologic Imaging
In the last two decades, the evolution of the scientific method has moved traditional disciplines forward and has led to the development of many new fields. The unifying change has been the continued expansive integration of technologies on all fronts, including molecular, biochemical, and computer based. Few fields of endeavor have embraced these changes as much as microscopy. The current research microscope represents the integration of modern optics, robotics, computing, probes, and cameras. This has moved the device from a principally descriptive tool to a primary research tool capable of addressing questions at all levels of resolution to the whole animal.
In many ways the evolution of modern microscopy and its dependence on technology has mirrored the concepts and potential of regenerative medicine. The field is driven by constantly evolving technology and a true understanding of the ultimate utility of a marriage of artificial and natural cellular and tissue resources to build replacement or therapeutically useful tissue homologs.
 Under the direction of Simon Watkins, PhD, the Center for Biologic Imaging (CBI) is housed within the main campus of the University of Pittsburgh. It is the embodiment of a large intellectual and technologic resource directed towards the development and application of microscopic methods for use in biomedical research. Currently, it is probably the largest and most well equipped optical imaging center in the United States. Of course, regenerative medicine is one facet of this drive. The Center is contained within the entire second floor of the BST south and integrates almost all cutting edge imaging technologies. For example, we currently have a total of 19 confocal microscopes; two multiphoton microscopes; three Total Internal Reflection systems; six live cell systems; seven high end upright microscopes; super-resolution microscopes (STORM, PALM, SIM); two transmission electron microscopes; and one high end scanning electron microscope. The Center has four faculty and a total staff of 26 highly-trained microscopists conversant in all aspects of computer aided microscopy and computer aided image processing and analysis.
Under the direction of Simon Watkins, PhD, the Center for Biologic Imaging (CBI) is housed within the main campus of the University of Pittsburgh. It is the embodiment of a large intellectual and technologic resource directed towards the development and application of microscopic methods for use in biomedical research. Currently, it is probably the largest and most well equipped optical imaging center in the United States. Of course, regenerative medicine is one facet of this drive. The Center is contained within the entire second floor of the BST south and integrates almost all cutting edge imaging technologies. For example, we currently have a total of 19 confocal microscopes; two multiphoton microscopes; three Total Internal Reflection systems; six live cell systems; seven high end upright microscopes; super-resolution microscopes (STORM, PALM, SIM); two transmission electron microscopes; and one high end scanning electron microscope. The Center has four faculty and a total staff of 26 highly-trained microscopists conversant in all aspects of computer aided microscopy and computer aided image processing and analysis.
The mission of the CBI is to provide integrated, elite level access and training in the research use, technology development, and industry interactions of computer-aided Microscopy, Biophotonics, and Imaging. The Center is deeply committed to providing open and transparent access to the most cutting edge optical imaging equipment to all biomedical research faculty, with access intended to lead to funded collaborations or with funded collaborations pre-exist and joint outputs such as co-authored publications, IP filings, and grant applications will result. In addition, when practical, research infrastructure will be available for use by external researchers and industry users, subject to the availability of instrument time and support staff.
 The Center has been an integral part of the McGowan Institute since its formation over ten years ago, and Donna Stolz, PhD, the associate director of the CBI, is a core faculty member in the McGowan Institute. We work with almost all of the research teams within the Institute and Bio-Engineering to develop data for papers and grants using the full armamentarium of technologies at our disposal. However, for us to continue to support McGowan-based research, we need to make sure that investigators understand how the Center is supported. Apart from faculty salaries not covered by grants, we are supported entirely by soft money: in other words, our grants and the cost-shared components on grants of our collaborating investigators. We do not receive any support from the institution and prefer it that way as it ensures intellectual autonomy.
The Center has been an integral part of the McGowan Institute since its formation over ten years ago, and Donna Stolz, PhD, the associate director of the CBI, is a core faculty member in the McGowan Institute. We work with almost all of the research teams within the Institute and Bio-Engineering to develop data for papers and grants using the full armamentarium of technologies at our disposal. However, for us to continue to support McGowan-based research, we need to make sure that investigators understand how the Center is supported. Apart from faculty salaries not covered by grants, we are supported entirely by soft money: in other words, our grants and the cost-shared components on grants of our collaborating investigators. We do not receive any support from the institution and prefer it that way as it ensures intellectual autonomy.
Essentially, the work flow is open and easy. We discuss project goals with PIs and then implement solutions, commonly through training or algorithm development. This work does not need to be funded before we start; howeve,r to continue to build partnerships investigators need to have a realistic and pragmatic appreciation of the resources needed to maintain the CBI at the cutting edge. Accordingly, we have recently implemented new reservation strategies, which are optimized and give priority to groups that are actively funding the Center.
We are dedicated to building exciting, cutting edge tools to investigate the molecular, cellular, and tissue phenomena that occur in health and disease. We believe that seeing is believing and quantifying is even better. If you have questions about how the center works, what we can do, and how we can build your program, please contact one of the faculty. See www.cbi.pitt.edu for contact details.
SCIENTIFIC ADVANCES
Traumatic Brain Injury Research Advances with $18.8M NIH Award
 The National Institutes of Health is awarding $18.8 million over 5 years to support worldwide research on concussion and traumatic brain injury. The NIH award, part of one of the largest international research collaborations ever coordinated by funding agencies, will be administered through University of California San Francisco (UCSF) and includes McGowan Institute for Regenerative Medicine affiliated faculty member David Okonkwo, MD, PhD, assistant professor with the Department of Neurological Surgery, University of Pittsburgh Medical Center, director of Neurotrauma and of the Spinal Deformity Program, clinical director of the Brain Trauma Research Center, and associate director of the Center for Injury Research and Control. Dr. Okonkwo will serve as the principal investigator for the University of Pittsburgh in the award.
The National Institutes of Health is awarding $18.8 million over 5 years to support worldwide research on concussion and traumatic brain injury. The NIH award, part of one of the largest international research collaborations ever coordinated by funding agencies, will be administered through University of California San Francisco (UCSF) and includes McGowan Institute for Regenerative Medicine affiliated faculty member David Okonkwo, MD, PhD, assistant professor with the Department of Neurological Surgery, University of Pittsburgh Medical Center, director of Neurotrauma and of the Spinal Deformity Program, clinical director of the Brain Trauma Research Center, and associate director of the Center for Injury Research and Control. Dr. Okonkwo will serve as the principal investigator for the University of Pittsburgh in the award.
The award supports a team of U.S. researchers at more than 20 institutions throughout the country who are participating in the International Traumatic Brain Injury (InTBIR) Initiative, a collaborative effort of the European Commission, the Canadian Institutes of Health Research (CIHR), the National Institutes of Health (NIH), and the U.S. Department of Defense (DOD).
Although the potential long-term harms due to concussions and blows to the head have gained more attention recently – due in part to media coverage of the experiences of athletes and of soldiers returning from the Middle East – traumatic brain injuries, or TBI, that results from automobile crashes or other common accidents impacts many more people.
In the work funded by the NIH grant – which also is supported by contributions from the private sector and from the nonprofit One Mind for Research – the researchers aim to refine and improve diagnosis and treatment of TBI, which often has insidious health effects, but which frequently is undiagnosed, misdiagnosed, inadequately understood, and undertreated, according to UCSF neurosurgeon Geoffrey Manley, MD, PhD, a principal investigator for the grant who will serve as the U.S. research team’s primary liaison to the NIH, and the chief of neurosurgery at the UCSF-affiliated San Francisco General Hospital, a Level-1 trauma center.
New Approach to Lead to Patient-Specific Treatments
The new NIH award funds a continuation and expansion of TRACK-TBI. Among the goals is the creation of a widely accessible, comprehensive “TBI information commons” to integrate clinical, imaging, proteomic, genomic, and outcome biomarkers from subjects across the age and injury spectra. Another goal is to establish the value of biomarkers that will improve classification of TBI and better optimize selection and assignment of patients for clinical trials.
The researchers also aim to evaluate measures to assess patient outcomes across all phases of recovery and at all levels of TBI severity, to determine which tests, treatments, and services are effective and appropriate – depending on the nature of TBI in particular patients.
In addition to Drs. Manley and Okonkwo, principal investigators for the newly funded project include Pratik Mukherjee, MD, PhD, UCSF; Claudia Robertson, MD, Baylor College of Medicine; Joseph Giacino, PhD, Harvard University; Ramon Diaz-Arrastia, MD, PhD, Uniformed Services University of the Health Sciences; and Nancy Temkin, PhD, University of Washington. Each of these leading experts has worked in the TBI field for 2 decades or more.
International Funding and Collaboration
TRACK-TBI clinical enrollment sites throughout the United States will enroll 3,000 patients across the spectrum of mild to severe brain injuries. Clinical, imaging, proteomic, genomic, and clinical outcome databases will be linked into a shared platform that will promote a model for collaboration among scientists within InTBIR and elsewhere.
In addition to the U.S. award, the European Commission, the executive body of the European Union, has awarded €35.2 million (estimated $47,563,417) to fund the Collaborative European NeuroTrauma Effectiveness-TBI (CENTER-TBI) consortium, also part of the InTBIR. This project will collect data in over 5,000 patients across Europe, where 38 scientific institutes and more than 60 hospitals will participate.
In Canada, CIHR and its national partners also have made a multimillion dollar investment in TBI research, the details of which will be formally announced in the near future.
The InTBIR Scientific Advisory Committee met in Vancouver, British Columbia, and awardees from all three jurisdictions (EU, USA, Canada) now are aligning efforts to share resources and collaborate on strategies for achieving the InTBIR goals.
Muscle Morbidity and Reduced Regenerative Capacity
 While the widely reported incidence of arsenic use in past centuries for medicinal, industrial, and homicidal purposes has declined dramatically, modern times have seen a resurgence in the attention paid to this organic metalloid. This is due, in large part, to the increasingly recognized presence of arsenic in the food and drinking supplies serving more than 140 million individuals worldwide and nearly 4 million individuals in the United States alone. Unfortunately, the very same characteristic that makes arsenic such an effective tool for acute poisoning also makes it a dangerous environmental contaminant: it is largely undetected because it is odorless, tasteless and colorless. Increasingly, however, arsenic is being recognized for its adverse, yet clandestine, effects on tissue functioning and regenerative capacity – even at low, everyday concentrations.
While the widely reported incidence of arsenic use in past centuries for medicinal, industrial, and homicidal purposes has declined dramatically, modern times have seen a resurgence in the attention paid to this organic metalloid. This is due, in large part, to the increasingly recognized presence of arsenic in the food and drinking supplies serving more than 140 million individuals worldwide and nearly 4 million individuals in the United States alone. Unfortunately, the very same characteristic that makes arsenic such an effective tool for acute poisoning also makes it a dangerous environmental contaminant: it is largely undetected because it is odorless, tasteless and colorless. Increasingly, however, arsenic is being recognized for its adverse, yet clandestine, effects on tissue functioning and regenerative capacity – even at low, everyday concentrations.
In addition to causing a number of cancers and non-cancer diseases, chronic arsenic exposure causes significant muscle weakness and dysfunction. Sensorimotor impairment and muscle atrophy are observed in 10-14 million individuals exposed daily to arsenic in their drinking water. Thus, in a very significant population, environmental exposure may be surreptitiously contributing to skeletal muscle dysfunction. This is important, as muscle weakness is among the greatest factors contributing to declines in functional mobility and is a strong predictor of mortality.
Collaborative investigations from the laboratories of Aaron Barchowsky, PhD, Professor in the Department of Environmental and Occupational Health at the University of Pittsburgh, and McGowan Faculty Member Fabrisia Ambrosio, PhD, MPT, assistant professor and research scientist in the Department of Physical Medicine and Rehabilitation, have recently demonstrated that environmentally-relevant levels of arsenic found in drinking water dramatically disrupts muscle composition and impairs metabolic functioning. It is hypothesized that these effects contribute to the common symptoms of weakness and fatigue in exposed individuals. Moreover, Ambrosio, Barchowsky and Donna Stolz, PhD have found that muscle stem cells are direct targets of arsenic exposure, findings which may have important implications for how well an individual is able to heal after a traumatic injury or a surgical procedure, for example.
The NIH has recognized the widespread importance of this line of investigation, as evidenced by a grant totalling over $2 million recently awarded to Barchowsky and Ambrosio. This project will investigate the underlying mechanisms for skeletal muscle morbidity and lost regenerative capacity caused by arsenic exposures
Improving the Therapeutic Relevance of Muscle Stem Cells
 As reported by the Stem Cell correspondent Stuart P. Atkinson, the research group of McGowan Institute for Regenerative Medicine faculty member Johnny Huard, PhD, professor in the Departments of Orthopaedic Surgery, Molecular Genetics, Biochemistry, Bioengineering, and Pathology, the Henry J. Mankin endowed chair in orthopaedic surgery research, and the director of the Stem Cell Research Center, has previously isolated and characterized muscle-derived stem cells (MDSCs) which have been shown by various groups by being able to undergo osteogenic differentiation given the correct stimuli. They are therefore a potential alternative to bone marrow-derived mesenchymal stem cells for bone tissue engineering. One of these stimuli is continued exposure to bone morphogenetic proteins (BMP), hindered by the short half-lives in vivo and the requirement of maintaining a localized concentration. The team, including McGowan Institute for Regenerative Medicine faculty member Yadong Wang, PhD, the William Kepler Whiteford professor in bioengineering with adjunct positions in chemical engineering and surgery at the University of Pittsburgh, has also devised a delivery strategy; a poly(ethylene argininylaspartate diglyceride)(PEAD)-heparin complex loaded with BMP2 which forms an emulsion-like aggregation of organic molecules separated from the aqueous phase, or a coacervate, previously used to effectively deliver fibroblast growth factor-2 (FGF2) for therapeutic angiogenesis. Now, in a report in Stem Cells Translational Medicine, they report on the use of this system with BMP2 to stimulate osteogenesis in MDSCs in vitro and in vivo.
As reported by the Stem Cell correspondent Stuart P. Atkinson, the research group of McGowan Institute for Regenerative Medicine faculty member Johnny Huard, PhD, professor in the Departments of Orthopaedic Surgery, Molecular Genetics, Biochemistry, Bioengineering, and Pathology, the Henry J. Mankin endowed chair in orthopaedic surgery research, and the director of the Stem Cell Research Center, has previously isolated and characterized muscle-derived stem cells (MDSCs) which have been shown by various groups by being able to undergo osteogenic differentiation given the correct stimuli. They are therefore a potential alternative to bone marrow-derived mesenchymal stem cells for bone tissue engineering. One of these stimuli is continued exposure to bone morphogenetic proteins (BMP), hindered by the short half-lives in vivo and the requirement of maintaining a localized concentration. The team, including McGowan Institute for Regenerative Medicine faculty member Yadong Wang, PhD, the William Kepler Whiteford professor in bioengineering with adjunct positions in chemical engineering and surgery at the University of Pittsburgh, has also devised a delivery strategy; a poly(ethylene argininylaspartate diglyceride)(PEAD)-heparin complex loaded with BMP2 which forms an emulsion-like aggregation of organic molecules separated from the aqueous phase, or a coacervate, previously used to effectively deliver fibroblast growth factor-2 (FGF2) for therapeutic angiogenesis. Now, in a report in Stem Cells Translational Medicine, they report on the use of this system with BMP2 to stimulate osteogenesis in MDSCs in vitro and in vivo.
Loading of BMP2 into the coacervate was 98.2% efficient, and after 28 days incubation 25% of the BMP2 had been released indicating that the coacervate can efficiently control the release of incorporated BMP2. Using the stimulation of alkaline phosphatase (ALP) expression in the myogenic cell line, C2C12, a common method of determining the activity of BMPs, it was demonstrated that coacervate-BMP2 induced more ALP than control (coacervate alone) and a similar concentration of free BMP2. ALP is also a marker for osteogenic differentiation of MDSCs in response to BMP2, and this system was next used to assess the effectiveness of coacervate-BMP2 on a cell monolayer and cells in a 3D fibrin gel, representing a potential scaffold for cell delivery to a bone defect. In the monolayer system, 100ng of coacervate-BMP2, but not 100ng of free BMP2 stimulated ALP expression at 5 days, while multi-dose treatment of free BMP2 (300ng in total) to stimulate sustained release did allow for ALP expression, which was not significantly different to coacervate-BMP2. mRNA levels of Runx2 and collagen type I were also increased in coacervate-BMP2 and multi-dose free BMP2 treatment to a similar level suggesting that the coacervate system can mediate osteogenic differentiation at lower doses of BMP2. Analysis in the 3D fibrin gel culture system found that while free BMP2 (100ng) this time did allow for ALP expression, perhaps due to reduced growth factor degradation, coacervate-BMP2 stimulated expression significantly more. Furthermore, due to the controlled release, BMP2 levels released from the coacervate, and therefore active, were estimated to be 90% less than free BMP2 at day 5.
Finally, in vivo analysis using a mouse ectopic bone formation model found that MDSCs stimulated with coacervate-BMP2 displayed extensive bone formation at 2 and 4 weeks after implantation, while those treated with free BMP2 had minimal bone formation at 2 weeks but which did increase by week 4. Calcified osteoid matrix was obvious throughout the wound for the coacervate-BMP2 treated cells but was only found in the periphery of the wound for free BMP2 treated cells. Furthermore, within the coacervate-treated wound, the interface between muscle and newly formed bone was detectable, with adjacent myofibers morphologically normal.
Overall, this study demonstrates the effectiveness of the polycation-heparin coacervate delivery system for the binding, protection, and sustained release of BMP2, an important factor in many differentiation studies. While this study does underline the excellent therapeutic potential of this system in bone repair and highlights the potential of MDSCs, this strategy can surely be adapted for uses in many different differentiation strategies, and in the maintenance and production of pluripotent cell types. Furthermore, this could lead to an increased cost effectiveness of many protocols utilizing expensive factors, as lower concentrations of coacervate-BMP2 were seen to function as well as concentrations of free BMP2 at 3 times the level.
Entering a New Dimension: 4D Printing
 Imagine an automobile coating that changes its structure to adapt to a humid environment or a salt-covered road, better protecting the car from corrosion. Or consider a soldier’s uniform that could alter its camouflage or more effectively protect against poison gas or shrapnel upon contact.
Imagine an automobile coating that changes its structure to adapt to a humid environment or a salt-covered road, better protecting the car from corrosion. Or consider a soldier’s uniform that could alter its camouflage or more effectively protect against poison gas or shrapnel upon contact.
A trio of university researchers from the University of Pittsburgh’s Swanson School of Engineering, Harvard School of Engineering and Applied Sciences, and the University of Illinois is proposing to advance 3D printing one step—or rather, one dimension—further. Thanks to an $855,000 grant from the United States Army Research Office, the team hopes to develop 4D materials, which can exhibit behavior that changes over time.
The team includes McGowan Institute for Regenerative Medicine affiliated faculty member and principal investigator Anna Balazs, PhD, the Robert v. d. Luft Distinguished Professor of Chemical Engineering in Pitt’s Swanson School of Engineering and a researcher in the computational design of chemo-mechanically responsive gels and composites. Co-investigators are Jennifer A. Lewis, ScD, the Hansjorg Wyss Professor of Biologically Inspired Engineering at the Harvard School of Engineering and Applied Sciences and an expert in 3D printing of functional materials; and Ralph G. Nuzzo, PhD, the G. L. Clark Professor of Chemistry and Professor of Materials Science and Engineering at the University of Illinois, a synthetic chemist who has created novel stimuli-responsive materials.
The three scientists will integrate their expertise to manipulate materials at nano and micro levels in order to produce, via 3D printing, materials that can modify their structures over time at the macro level. Three-dimensional printing, also known as additive manufacturing, is the process of creating a 3D object based upon a digital model by depositing successive layers of material.
“Rather than construct a static material or one that simply changes its shape, we’re proposing the development of adaptive, biomimetic composites that reprogram their shape, properties, or functionality on demand, based upon external stimuli,” Dr. Balazs explained. “By integrating our abilities to print precise, three-dimensional, hierarchically-structured materials; synthesize stimuli-responsive components; and predict the temporal behavior of the system, we expect to build the foundation for the new field of 4D printing.”
Dr. Lewis added that current 3D printing technology allows the researchers to build in complicated functionality at the nano and micro levels—not just throughout an entire structure, but also within specific areas of the structure. “If you use materials that possess the ability to change their properties or shape multiple times, you don’t have to build for a specific, one-time use,” she explained. “Composites that can be reconfigured in the presence of different stimuli could dramatically extend the reach of 3D printing.”
Since the research will use responsive fillers embedded within a stimuli-responsive hydrogel, Dr. Nuzzo says this opens new routes for producing the next generation of smart sensors, coatings, textiles, and structural components. “The ability to create one fabric that responds to light by changing its color, and to temperature by altering its permeability, and even to an external force by hardening its structure, becomes possible through the creation of responsive materials that are simultaneously adaptive, flexible, lightweight, and strong. It’s this ‘complicated functionality’ that makes true 4D printing a game changer.”
AWARDS AND RECOGNITION
Dr. Kacey Marra Named Associate Editor for Cells Tissues Organs
 McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD, associate professor, Departments of Plastic Surgery and Bioengineering, University of Pittsburgh, and co-director, Adipose Stem Cell Center, has been named an associate editor for the journal, Cells Tissues Organs.
McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD, associate professor, Departments of Plastic Surgery and Bioengineering, University of Pittsburgh, and co-director, Adipose Stem Cell Center, has been named an associate editor for the journal, Cells Tissues Organs.
Dr. Marra is recognized for her interdisciplinary research in the design, synthesis, characterization, and assessment of polymeric biomaterials. Dr. Marra joined the Department of Surgery as an assistant professor in November 2002. Prior to that appointment she was with Carnegie Mellon University as a research scientist at the Institute for Complex Engineered Systems (ICES), and an associated faculty member in the Department of Biomedical Engineering (1998-2002), and the Department of Materials Science Engineering (2000-2002). In 1996-7, Dr. Marra was a post-doctoral fellow at the Emory University School of Medicine with advisor Elliot Chaikof, MD, PhD. At Emory, Dr. Marra worked on the synthesis of novel synthetic blood vessels.
Dr. Marra is actively involved in research efforts investigating polymeric biomaterials for tissue engineering applications. She is the director of the Plastic Surgery Laboratory as well as co-director of the Adipose Stem Cell Center. Her research is focused on the utilization of adipose-derived stem cells for tissue engineering applications such as soft tissue reconstruction. Additionally, a major research effort in her laboratory is the long-term delivery of neurotrophic factors for long gap nerve repair. Dr. Marra participates in numerous outreach programs, including a high school student program she developed in her laboratory (ROHSS: Research Opportunities for High School Students).
Congratulations, Dr. Marra!
 Regenerative Medicine Podcast Update
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#129 –– Dr. Linda Noble-Haeusslein is a Professor with the Department of Neurological Surgery and Department of Physical Therapy and Rehabilitation at the University of California, San Francisco. Dr. Noble-Haeusslein discusses her research in the functional recovery after traumatic brain or spinal cord injury.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
