March 2020 | VOL. 19, NO. 3| www.McGowan.pitt.edu
DARPA Awards $22M for ‘Smart’ Device that Regenerates Muscle
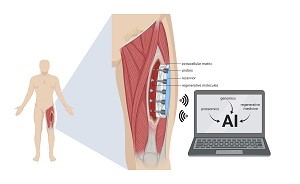
A multi-institution research team led by the University of Pittsburgh secured a $22 million grant from the Defense Advanced Research Projects Agency (DARPA) to develop a device combining artificial intelligence, bioelectronics and regenerative medicine to regrow muscle tissue, especially after combat injuries.
McGowan Institute for Regenerative Medicine affiliated faculty members involved in this effort include:
- Stephen Badylak, DVM, PhD, MD, professor of surgery at Pitt and deputy director of the McGowan Institute for Regenerative Medicine (principal investigator)
- Yoram Vodovotz, PhD, professor in the Department of Surgery, University of Pittsburgh, and director of the Center for Inflammation and Regeneration Modeling at the McGowan Institute
- Ruben Zamora, PhD, research professor in the Department of Surgery, University of Pittsburgh
- Douglas Weber, PhD, associate professor in the Department of Bioengineering, University of Pittsburgh
- Bryan Brown, PhD, associate professor in the Department of Bioengineering, University of Pittsburgh
- Tzahi Cohen-Karni, PhD, assistant professor in the Department of Biomedical Engineering at Carnegie Mellon University
- Adam Feinberg, PhD, professor in the Departments of Biomedical Engineering and Materials Science and Engineering at Carnegie Mellon University
Researchers at Carnegie Mellon University, Northwestern University, Rice University, University of Vermont, University of Wisconsin and Walter Reed National Military Medical Center also are part of this four-year initiative.
When more than 20% of a muscle is damaged, as is common for soldiers wounded in recent overseas conflicts, the tissue can’t regenerate and a stiff scar forms in place of the missing muscle, which often leads to significant disability.
“With these severe injuries it’s been drilled into us through all of our training that functional muscle replacement is not possible,” said principal investigator Dr. Badylak. “The sort of technology we’re developing offers hope where there otherwise would have been no hope.”
Dr. Badylak envisions creating a device that would change the environment inside larger wounds to help them heal the way smaller wounds do naturally.
Smaller, self-healing wounds typically switch from inflammatory to anti-inflammatory conditions a couple weeks after the initial injury. Dr. Badylak imagines kicking larger wounds into anti-inflammatory mode as early as day three or four, and then again a few days later, repeating the cycle until the muscle rebuilds itself, similar to the way fetal wounds heal without forming a scar.
All of that would be accomplished by a smart device implanted inside the wound.
The device will monitor key molecular signals at each stage of healing — from the first hours after injury to the days and weeks that follow — and deliver specific molecules at specific times under the direction of artificial intelligence.
The first two years of the project will involve developing the device, then the next two years will involve close collaboration with surgeons at Walter Reed, who treat patients with major muscle loss, to refine the design so that it’s suitable for the clinic.
Meanwhile, the researchers will be working with industry partners and the U.S. Food & Drug Administration to identify and clear regulatory hurdles that might slow down clinical translation. For instance, it’s possible to test whether the components of the device are safe to use in the human body while the overall design is evolving.
“We’re developing the science and the device in mostly an academic setting,” Dr. Badylak said. “If that’s done without consideration of regulatory and industry requirements, patients would never see it because it would remain buried in institutions with no clear path for clinical translation.”
One of the companies engaging in this process is ECM Therapeutics, which Dr. Badylak spun out of Pitt in 2018 to speed up the clinical translation of several extracellular matrix technologies developed by his lab. Dr. Badylak and Pitt both have a financial stake in the company.
Additional investigators on the grant include Paul Cohen, PhD, and Milos Hauskrecht, PhD, of Pitt; Jonathan Rivnay, PhD, of Northwestern University; Jacob Robinson, PhD, Ashok Veeraraghavan, PhD, and Omid Veiseh, PhD, of Rice University; Gary An, MD, and Robert Chase Cockrell, PhD, of the University of Vermont; Peng Jiang, PhD, of the University of Wisconsin; and Eric Elster, MD, and Seth Schobel, PhD, of Walter Reed.
Illustration: “Smart” Wound-Healing Device: The device will go onto injured muscle to deliver and measure molecules in real time, under the guidance of artificial intelligence. UPMC.
McGowan Institute for Regenerative Medicine Holds Its Annual Scientific Retreat
 The McGowan Institute for Regenerative Medicine held its 2020 Scientific Retreat March 9-10, 2020. The focus was on peer-to-peer networking, and the retreat provided many opportunities to explore collaborative endeavors with other researchers, participating guests, and external partners who are working to bring regenerative medicine technologies to clinical use.
The McGowan Institute for Regenerative Medicine held its 2020 Scientific Retreat March 9-10, 2020. The focus was on peer-to-peer networking, and the retreat provided many opportunities to explore collaborative endeavors with other researchers, participating guests, and external partners who are working to bring regenerative medicine technologies to clinical use.
The participation and contributions of the guests and external collaborators – along with McGowan Institute for Regenerative Medicine affiliated faculty and trainees – provided for insightful discussions and identification of opportunities for partnerships. This year’s program committee (listed below) designed an exciting program:
- Julie Phillippi, PhD (Chair) – University of Pittsburgh
- Bryan Brown, PhD – University of Pittsburgh
- Patrick Cantini – University of Pittsburgh/McGowan Institute
- Andy Duncan, PhD – University of Pittsburgh
- Don Taylor, PhD – University of Pittsburgh/PittSciVelo
- Rosalyn Abbott, PhD– Carnegie Mellon University
- Abigail Allen (trainee member) – University of Pittsburgh
- Piyumi Wijesekara Kankanange (trainee member) – Carnegie Mellon University
Program Highlights
The Director’s Welcome and State of the Institute Address was presented by McGowan Institute Director William Wagner, PhD, Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh. Dr. Wagner also introduced Paula K. Davis, MA, Assistant Vice Chancellor for Health Sciences Diversity, Schools of Health Sciences, University of Pittsburgh. Ms. Davis discussed the importance of diversity in interdisciplinary biomedical research.
The Retreat included numerous general sessions throughout the 2-day event with a focus on these research and/or strategic planning and educational areas:
- Grand Challenges in Regenerative Medicine
- Synthetic Biology and Regenerative Medicine
- Translating Early Stage University Research into the Healthcare Marketplace
- Regenerative Tissue Therapies and Cancer Risk/Progression
- Pulmonary Injury Repair, Regeneration and Transplantation
- Regenerative Medicine Technology Pitch Competition (co-sponsored by sciVelo and McGowan Institute)
- Fireside Chat with Local Entrepreneurs
- Commercialization Office Scheduled Consultations
- Invention Disclosure Sprints
- Student Poster Teasers and Poster Presentations
- Trainee Workshop
 All attendees were invited to view the screening of Burden of Genius, a film on the life and career of Dr. Thomas Starzl, the father of transplantation. Carl Kurlander, Documentarian and Filmmaker, Visiting Distinguished Senior Lecturer, Faculty Adviser, Pitt In Hollywood, University of Pittsburgh, provided opening remarks on the making of the film.
All attendees were invited to view the screening of Burden of Genius, a film on the life and career of Dr. Thomas Starzl, the father of transplantation. Carl Kurlander, Documentarian and Filmmaker, Visiting Distinguished Senior Lecturer, Faculty Adviser, Pitt In Hollywood, University of Pittsburgh, provided opening remarks on the making of the film.
The Retreat program also included 21 presentations addressing a great cross-section of scientific topics given by McGowan Institute affiliated faculty and 17 invited researchers from other institutions and agencies who are not formally affiliated with the McGowan Institute (in alphabetical order):
- Seth Boudreaux, PhD, Senior Manager, Business Development and Licensing Center for Technology Transfer and Enterprise Creation, Carnegie Mellon University: Technology Pitch Competition judge and Commercialization Office Advisor
- Andrew Brown, PhD, Assistant Director, Commercial Translation Programs, sciVelo; Clinical Assistant Professor, Department of Periodontics and Preventative Medicine, University of Pittsburgh School of Dental Medicine: Commercialization Office session organizer and chair and Fireside Chat with Local Entrepreneurs panelist
- Marta Bueno, PhD, Research Assistant Professor of Medicine, Aging Institute & Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh: “Mitochondrial dysfunction in aging and pulmonary fibrosis”
- David Chi, MD, Associate Professor, Department of Otolaryngology, Clinical Director, Division of Pediatric Otolaryngology, Children’s Hospital of Pittsburgh of UPMC: A Patient Story of McGowan/CHP Partnership and New Pediatric Initiatives with the Sutton Family
- Erica Comber, Doctoral Student, Laboratory of Keith Cook, Department of Biomedical Engineering, Carnegie Mellon University: “De novo lung biofabrication”
- Evan Delgado, PhD, Postdoctoral Associate, Department of Pathology, University of Pittsburgh: Poster Teaser Presentations co-organizer and co-chair
- Alex Ducruet, PhD, CLP Director, Licensing and Intellectual Property, Innovation Institute, University of Pittsburgh: Commercialization Office Advisor and Fireside Chat with Local Entrepreneurs panelist
- Asim Ejaz, PhD, Research Assistant Professor, Department of Plastic Surgery, University of Pittsburgh School of Medicine: “Adipose derived stem cells: From bench to bedside”
- Ronald Fortunato, BS, PhD Student, Department of Mechanical Engineering and Materials Science, University of Pittsburgh, Poster #22: “Effect of macro-calcification on the failure mechanics of intracranial aneurysmal wall tissue”
- Ron Jankowski, PhD, Vice President of Scientific Affairs, Cook MyoSite: Academic vs. Industry Career Paths panel member
- Anna Kalmykov, MS, PhD Student, Department of Biomedical Engineering, Carnegie Mellon University, Poster #59: “Organ-on-e-chip: Three-dimensional self-rolled biosensor array for electrical interrogations of electrogenic spheroids”
- Samira Kiani, MD, Associate Professor, Department of Pathology, Division of Experimental Pathology, School of Medicine, Pittsburgh Liver Research Center, University of Pittsburgh: “Synthetic control of gene regulatory networks by CRISPR: The roadmap to human translation”
- Farzaneh Moghadam, BS, PhD Student, Department of Pathology, University of Pittsburgh, Poster #11: “CRISPR-based transcriptional repression to reform immunomodulation in vivo”
- Scott Morley, MBA, Coulter Program Director and EIR Innovation Institute, University of Pittsburgh: Translating Early Stage University Research into the Healthcare Marketplace session co-organizer and Commercialization Office session organizer
- Kentaro Noda, PhD, Research Assistant Professor, Department of Cardiothoracic Surgery, University of Pittsburgh Medical Center: “Donor lung preservation and transplantation”
- Alexis Nolfi, BS, PhD Student, Department of Bioengineering, University of Pittsburgh, Poster #37: “Contact lens delivery of interleukin-4 for treatment of dry eye disease promotes antiinflammatory macrophage populations”
- Ngoc Pham, BS, PhD Student, Graduate School of Pharmaceutical Sciences, Duquesne University, Poster #39: “Hydrogel-enabled intratumoral co-delivery of anti-PD-1 antibody and adenosine deaminase in a mouse model of renal cell carcinoma”
- Sunder Sims-Lucas, PhD, Assistant Professor, Department of Pediatrics, Children’s Hospital of Pittsburgh of UPMC: “Uncovering kidney injury modulators to design therapeutics”
- Elaine Soohoo, PhD, Biomedical Engineer, Division of Circulatory Support, Structural and Vascular Devices, Office of Cardiovascular Devices, U.S. Food and Drug Administration: Academic vs. Industry Career Paths panel member
- Dennis P. Swanson, MS, Assistant Dean for Special Projects, Professor, Pharmacy and Therapeutics, University of Pittsburgh: Commercialization Office Advisor
- Elizabeth Wayne, PhD, Assistant Professor, Departments of Biomedical Engineering and Chemical Engineering, Carnegie Mellon University: Academic vs. Industry Career Paths panel member
- Huaiying Zhang, PhD, Assistant Professor, Department of Biological Sciences, Carnegie Mellon University: “Optogenetic control of telomere clustering by protein phase separation in ALT cancer”
- Daniel Zuppo, BS, PhD Student, Department of Developmental Biology, University of Pittsburgh, Poster #19: “foxm1 is required for cardiomyocyte proliferation after zebrafish cardiac injury”
Poster Session
The poster session was effective in introducing the focus of the Retreat and interests of the faculty and the guests. McGowan Institute faculty member Andrew Duncan, PhD, Assistant Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. Thanks to sciVelo for sponsoring the poster session. The winners of the poster session were:
Cell and Gene Therapy
First Place
Meghan Mooring
Hepatocyte Stress Increases Expression of YAP and TAZ in Hepatocytes to Promote Parenchymal Inflammation and Fibrosis
Mentor: Dean Yimlamai, MD, PhD
Dept. of Pediatrics
University of Pittsburgh
Second Place
Farzaneh Moghadam
CRISPR-based transcriptional repression to perform immunomodulation in vivo
Mentor: Samira Kiani, MD
Dept. of Pathology
University of Pittsburgh
Third Place
Christopher Reyes
Regulation of Smooth Muscle Cell Proliferation and Function by Mitofusin-1
Mentor: Sruti Shiva, PhD
Dept. of Bioengineering
University of Pittsburgh
Computation and Modeling
First Place
George Gabriel
Brain Abnormalities and Neurobehavioral Deficits in a Mouse Model of Hypoplastic Left Heart Syndrome
Mentor: Cecilia Lo, PhD
Dept. of Developmental Biology
University of Pittsburgh
Second Place
Lu Liu
Sustained Analgesic and Anti-inflammatory Efficacy of a Single Dose COX-2 Inhibiting Nanomedicine in a Mouse CFA-induced Inflammatory Model
Mentor: Jelena Janjic, PhD
Graduate School of Pharmaceutical Sciences
Duquesne University
Third Place
Sommer Anjum
Mechanics of passive cell rearrangement during epithelial convergent extension: understanding experimental observations through theory
Mentor: Lance Davidson, PhD
Dept. of Bioengineering
University of Pittsburgh
Medical Devices
First Place
Tyler Meder
Assessment of a Peripheral Nerve Extracellular Matrix Derived Hydrogel for Improving Functional Recovery Following Nerve Reconstruction
Mentor: Bryan Brown PhD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Second Place
Alexis Nolfi
Contact Lens Delivery of Interleukin-4 for Treatment of Dry Eye Disease Promotes Anti-inflammatory Macrophage Populations
Mentor: Bryan Brown PhD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Third Place
Daniel San Roman
Functionalized Out-of-Plane Graphene Microelectrode Arrays for Sensitive Biomolecule Sensing
Mentor: Tzahi Cohen-Karni, PhD
Dept. of Material Science
Carnegie Mellon University
Tissue Engineering
First Place
Anna Kalmykov
Organ-on-e-chip: three-dimensional self-rolled biosensor array for electrical interrogations of electrogenic spheroids
Mentor: Tzahi Cohen-Karni, PhD
Dept. of Biomedical Engineering
Carnegie Mellon University
Second Place
Yoojin Lee
Matrix-bound Nanovesicles as a Source of Lysyl Oxidase
Mentor: Stephen Badylak, MD, PhD, DVM
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Third Place
Zhong (Alan) Li
A Multi-tissue Chip for the Modeling of Osteoarthritis Pain
Mentor: Rocky Tuan, PhD
Dept. of Orthopaedic Surgery
University of Pittsburgh
People’s Choice
Farzaneh Moghadam
CRISPR-based transcriptional repression to perform immunomodulation in vivo
Mentor: Samira Kiani, MD
Dept. of Pathology
University of Pittsburgh
Poster Awards sponsored by sciVelo.
First place: $200 (each category)
Second place: $125
Third place: $75
CATER Awards
First Place
Meghan Mooring
Hepatocyte Stress Increases Expression of YAP and TAZ in Hepatocytes to Promote Parenchymal Inflammation and Fibrosis
Mentor: Dean Yimlamai, MD, PhD
Dept. of Pediatrics
University of Pittsburgh
Second Place
George Gabriel
Brain Abnormalities and Neurobehavioral Deficits in a Mouse Model of Hypoplastic Left Heart Syndrome
Mentor: Cecilia Lo, PhD
Dept. of Developmental Biology
University of Pittsburgh
Third Place
Laura Molina
Novel Model of Bile Duct Paucity Demonstrates the Critical Role of Yap1 in Biliary Morphogenesis in Development and Regeneration
Mentor: Paul Monga, MD
Dept. of Pathology
University of Pittsburgh
CATER Awards sponsored by McGowan Institute.
First place: $200
Second place: $125
Third place: $75
A special thank you is extended to all who made this year’s Retreat a success!
See the full Retreat Program here.
Photos Credit: McGowan Institute
RESOURCES AT THE MCGOWAN INSTITUTE
April Histology Special
April Showers mean big savings at the McGowan Histology Core Lab.
Herovici stain is used to differentiate young and mature collagen as well as identify reticulum fibers within tissue. Herovici’s solution stains young collagen and reticulum blue and mature collagen red while providing a yellow cytoplasm counterstain. Nuclei are stained blue to black with Weigert’s Hematoxylin.
 You’ll receive 25% off Herovici Staining for the entire month of April when you mention this ad.
You’ll receive 25% off Herovici Staining for the entire month of April when you mention this ad.
Contact Julia at the McGowan Core Histology Lab:
Julia Hart
Hartj5@upmc.edu
412-624-5265
Save the Date!
9th International Symposium on Regenerative Rehabilitation
 With a theme of “Where Applied Biophysics Meets Tissue Engineering & Cellular Therapies”, the Symposium is scheduled for December 3-5, 2020 at the University of Texas at Austin. The program features renowned researchers and clinicians from around the world, focusing on the emerging field of Regenerative Rehabilitation. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
With a theme of “Where Applied Biophysics Meets Tissue Engineering & Cellular Therapies”, the Symposium is scheduled for December 3-5, 2020 at the University of Texas at Austin. The program features renowned researchers and clinicians from around the world, focusing on the emerging field of Regenerative Rehabilitation. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
AWARDS AND RECOGNITION
2020 CSC Awards Winners: Drs. Fabrisia Ambrosio and Bryan Brown and Ms. Alexis Nolfi
The Carnegie Science Center established the Carnegie Science Awards program in 1997 to recognize and promote outstanding science and technology achievements in western Pennsylvania.
The Carnegie Science Awards have honored the accomplishments of more than 600 individuals and organizations whose contributions in the fields of science, technology, and education have impacted our region’s industrial, academic, and environmental vitality.
The 24th Annual Carnegie Science Awards celebration will be held on September 17, 2020, during which time the following McGowan Institute for Regenerative Medicine faculty members and student will be presented with awards for their tremendous work and their impact on the vitality of science in the region.
- Life Sciences Award category: Fabrisia Ambrosio, PhD, MPT
- University/Post-Secondary Educator Award category: Bryan Brown, PhD
- The College/University Student Award category: Alexis Nolfi
The Life Sciences Award recognizes and honors scientific advances in new and innovative biomedical and life sciences endeavors that benefit the economy, health, or societal wellbeing of the region.
 Dr. Ambrosio is an internationally recognized scientist and leader. Her research has changed the fundamental approach to the integration of rehabilitation and regenerative medicine. Once treated as two distinctly separate fields, Dr. Ambrosio was one of the first to identify the importance of merging rehabilitation science and regenerative medicine and is considered the founder of the field of regenerative rehabilitation. This new field has resulted in significant advances in the complexity of cases that are successfully treated, and the reduction in the time to recovery for common causes of disability. She has been at the forefront of demonstrating the positive outcomes of this amalgamation and has organized conferences to facilitate the coalescence of regenerative rehabilitation. As a result of her preeminence as a scientist, she was recently selected to be the Director of Rehabilitation for UPMC International.
Dr. Ambrosio is an internationally recognized scientist and leader. Her research has changed the fundamental approach to the integration of rehabilitation and regenerative medicine. Once treated as two distinctly separate fields, Dr. Ambrosio was one of the first to identify the importance of merging rehabilitation science and regenerative medicine and is considered the founder of the field of regenerative rehabilitation. This new field has resulted in significant advances in the complexity of cases that are successfully treated, and the reduction in the time to recovery for common causes of disability. She has been at the forefront of demonstrating the positive outcomes of this amalgamation and has organized conferences to facilitate the coalescence of regenerative rehabilitation. As a result of her preeminence as a scientist, she was recently selected to be the Director of Rehabilitation for UPMC International.
With the goal of advancing the science underlying the prescription of rehabilitation protocols, Dr. Ambrosio has used cellular and tissue engineering therapeutics to improve skeletal muscle healing after injury and in the setting of disease. She has used pharmacologic, genetic, and mechanical strategies as a means to modulate the muscle microenvironment and enhance the regenerative response of injured or diseased skeletal muscle. Dr. Ambrosio’s translational investigations have revealed the vast potential to refine clinical rehabilitation through an improved understanding of the cellular response to exercise-based loading. Her work has been recognized in the form of “Paper of the Year” awards in 2016 and 2017, and she is the highest NIH-funded investigator within all US departments of physical medicine and rehabilitation.
More broadly, Dr. Ambrosio’s research has served to launch the development of a novel field, Regenerative Rehabilitation, which represents an intersection of rehabilitation science and regenerative biology. As a driver of this field, Dr. Ambrosio is Founding Course Director of the Annual International Symposium on Regenerative Rehabilitation, now in its ninth year. The objective of this Symposium series is to catalyze novel interactions and research directions among scientists and clinicians conducting research in regenerative medicine and/or rehabilitation. Dr. Ambrosio is also the Founding Director of the International Consortium for Regenerative Rehabilitation. This Consortium comprises fifteen institutions from around the world and has the goal of capitalizing on synergies between the fields of rehabilitation and regenerative medicine by developing a coordinated, international plan for advancing Regenerative Rehabilitation research. In 2016, Dr. Ambrosio assumed the role of co-Director of the NIH-funded Alliance for Regenerative Rehabilitation Research & Training (AR3T). AR3T, which is considered a center of excellence by NIH, helps to develop research collaborations, provides educational opportunities, and funds pilot projects and technology development projects that will benefit the research community.
Dr. Ambrosio’s science and development of innovative intervention protocols are creating significant shifts in treatment strategies, which have increased the types of cases that can be addressed, as well as the speed and level of recovery. These outcomes have enhanced the recognition of the region as the preeminent place for regenerative therapies….and has benefited the residents of the region who have received these therapies as they have had greater success in returning to “normal” quality of life a greater percentage of the time.
Along these lines, to effectively design interventions to optimize regenerative processes, a comprehensive knowledge of extrinsic factors driving tissue alterations is also critical. Dr. Ambrosio and colleagues have demonstrated that exposure to inorganic arsenic—at concentrations found in drinking water supplies serving over 140 million individuals worldwide and over 3 million in the US—disrupts muscle composition and impairs stem cell function. It is hypothesized that the effects of this common environmental contaminant may contribute to the muscle weakness and fatigue in exposed individuals around the world. Dr. Ambrosio work in this area is targeted toward the development of prevention and/or intervention strategies to improve health outcomes of individuals living in arsenic-endemic areas. Finally, Dr. Ambrosio is the principal investigator on over $2 million annually in grant funding. This funding brings students and jobs to the area and furthers Pittsburgh’s identity as a top city for meds and eds.
The University/Post-Secondary Educator Award recognizes educators for innovation and impact in science, technology, engineering, and math (STEM) education, including: inspiring students to understand, appreciate, and apply science, technology, engineering, or math; and strengthening the teaching profession through the spread of innovative practices.
 Dr. Brown’s effectiveness and impact substantially exceeds the work product of most academics who have a more senior status. The teaching, research and outreach programs that he has established through his own initiative are both impressive and impactful, and his participation in other departmental and school-wide initiatives adds significant value at all levels. Amongst his peers, Dr. Brown is considered a leader in research and a leader in the education of future researchers at the undergraduate and the graduate levels.
Dr. Brown’s effectiveness and impact substantially exceeds the work product of most academics who have a more senior status. The teaching, research and outreach programs that he has established through his own initiative are both impressive and impactful, and his participation in other departmental and school-wide initiatives adds significant value at all levels. Amongst his peers, Dr. Brown is considered a leader in research and a leader in the education of future researchers at the undergraduate and the graduate levels.
He currently teaches both undergraduate and graduate courses including “Bioengineering Thermodynamics” and “Extracellular Matrix in Tissue Biology and Bioengineering” – which he developed as a partnership between Bioengineering and Pathology. Beyond acting as an instructor for these more typical bioengineering courses, Dr. Brown also teaches “Societal, Political, and Ethical Issues in Bioengineering and Biotechnology.” This is a distinct course, as none of the other engineering departments offer a full semester ethics course. As bioengineering represents a broad major with myriad subfields with a direct impact upon human health, it is important that ethics is taught effectively covering broad content. Dr. Brown covers not only typical engineering ethics, but also topics crossing fields including medicine and biotechnology as well as cutting edge areas such as genetic engineering and stem cells, among many others. Of note, Dr. Brown has been able to include many current issues as well as issues he has faced during the course of his own work, effectively engaging and preparing his students for discussions which might otherwise be difficult or uncomfortable, but will most certainly be faced during the course of their careers.
In terms of “classroom effectiveness”, Dr. Brown has been extremely successful. His average evaluation scores are 4.25/5.0, well above average for the school of engineering.
These more typical teaching accomplishments are just the beginning of the story. Dr. Brown also serves as Track Coordinator for Tissue Engineering and Regenerative Medicine within the Bioengineering program and Director of Educational Outreach for the McGowan Institute for Regenerative Medicine. In these roles, he has a direct impact upon graduate education, public awareness, and training in the field of Regenerative Medicine.
Dr. Brown is widely recognized as an effective mentor of students at all levels. In his laboratory, Dr. Brown has been a consummate and caring mentor for 1 research assistant professor, 1 visiting scholar, 3 post-doctoral fellows (1 current + 2 past), 9 PhD (5 current, 4 graduated), 1 MS, more than 50 undergraduate students (at least 50% of which are underrepresented/minority students), and 5 high school interns. Of particular note, Dr. Brown’s graduate students have received more than 10 major fellowships including NIH T32 (4), NSF-GRFP (2), NIH F31 (1), NIH TL1 Clinical and Translational Science Fellowship (1), Fullbright Scholarship (1), K. Leroy Irvis Fellowship (1) and the Gerald McGinnis Fellowship (1). In addition to their scientific achievements, Dr. Brown and his students have received multiple awards for innovation and entrepreneurship. As a single example, Dr. Brown’s students have been winners of the highly competitive Michael Wells Student Healthcare Entrepreneurship Competition twice in recent years. These student accomplishments and awards speak volumes to the high quality of mentorship that Dr. Brown provides and its impact upon their future careers.
Dr. Brown also has a strong commitment to diversity in science. He is a mentor in the Pitt STRIVE, National Science Foundation, Alliances for Graduate Education Mentor and the Professoriate Program. This program seeks to prepare students from underrepresented backgrounds for careers in academia. He also participates in the Pitt SPAEP and EXCEL summer research programs through the Offices of Diversity of the University of Pittsburgh School of Medicine and School of Engineering to offer students from underrepresented backgrounds internships in his laboratory.
Dr. Brown has also organized and launched the McGowan Institute for Regenerative Medicine Summer School. This program has now provided nearly 100 with a hands-on experiential learning program within the multidisciplinary field of regenerative medicine.
In short, though he is early in his career and despite his many other research commitments, Dr. Brown has had an outsized impact upon education in the field of Regenerative Medicine. This includes mentoring of literally hundreds of students at all academic levels within the University of Pittsburgh, interacting with members of the local community at a lay level, and providing structured hands on training programs with a focus upon diversity. These activities are sure to have an impact, both locally, nationally, and even internationally.
The College/University Student Award recognizes one student working towards a degree in a science, technology, engineering, or math (STEM) field, for impact and innovation: preparing youth to consider career opportunities in STEM fields and pursuing research that contributes to the societal or economic wellbeing of the region.
 Ms. Nolfi is an academic superstar. She graduated from the University of Pittsburgh summa cum laude with a double major in Bioengineering and Psychology, and a minor in Chemistry. As recognition for her achievements as an undergraduate, Ms. Nolfi was awarded the prestigious Emma Locke Award – an honor presented to the top female undergraduate at the University of Pittsburgh each year. She also received numerous scholarships during this time. During her graduate work, Ms. Nolfi has also been the recipient of multiple prestigious awards and fellowships, including the NSF Graduate Research Fellowship, an NIH T-32 Fellowship and a scholarship from the ARCS foundation. These highly competitive and prestigious awards signify that Ms. Nolfi is one of the top students in our department, and perhaps at the University of Pittsburgh.
Ms. Nolfi is an academic superstar. She graduated from the University of Pittsburgh summa cum laude with a double major in Bioengineering and Psychology, and a minor in Chemistry. As recognition for her achievements as an undergraduate, Ms. Nolfi was awarded the prestigious Emma Locke Award – an honor presented to the top female undergraduate at the University of Pittsburgh each year. She also received numerous scholarships during this time. During her graduate work, Ms. Nolfi has also been the recipient of multiple prestigious awards and fellowships, including the NSF Graduate Research Fellowship, an NIH T-32 Fellowship and a scholarship from the ARCS foundation. These highly competitive and prestigious awards signify that Ms. Nolfi is one of the top students in our department, and perhaps at the University of Pittsburgh.
Ms. Nolfi was an honorable mention in the 2019 Carnegie Science Awards University Post-Secondary Student Category. Since that time, she has continued to excel, both academically – maintaining a 4.0 GPA – and scientifically – publishing additional manuscripts, making additional presentations at national conferences, and winning the Pitt Innovation Challenge, a $100,000 prize for promising new technology which addresses unmet clinical needs.
Ms. Nolfi also finds the time to participate in significant service and outreach activities. For example, she continues to serve as an Instructor for the Department of Bioengineering Tissue Engineering Summer Camp. This camp is geared towards engaging students, primarily from underrepresented/disadvantaged backgrounds, in Biomedical Engineering. Ms. Nolfi helped design modules which demonstrate concepts of tissue engineering to students. Ms. Nolfi has also presented these modules to groups of students at North Carolina A&T University. More recently, she helped to establish the University of Pittsburgh Society for Biomaterials Student Chapter, which has organized many excellent events for student career development.
Ms. Nolfi has participated in a range of research projects related to women’s health. Her work with Dr. Pamela Moalli sought to characterize the immune response to surgical mesh materials for pelvic organ prolapse and to relate the response to the type and rates of complications in women. While the exact contributions of Ms. Nolfi’s efforts are far too numerous to list here, I would like to emphasize that she, in a short time, mastered all of the techniques required to complete this work. This is no small task considering that this includes aspects of physiology, immunology, urogynecology, surgery, and molecular biology. Ms. Nolfi was an author on six manuscripts resulting from this work, including one first author publication – clearly demonstrating both her productivity and mastery of the subject. Currently, Ms. Nolfi is working at the McGowan Institute for Regenerative Medicine. Her project seeks to examine the role of macrophage phenotype modulation in the downstream success of biomaterials. Ms. Nolfi is now working to demonstrate that we can control macrophage polarization through a novel drug delivery method, utilizing in vivo imaging techniques to monitor this release, and examining the effects of such strategies on downstream tissue integration. Additionally, Ms. Nolfi is participating in another project to develop a clinically relevant rabbit model of pelvic organ prolapse and repair. To date, Ms. Nolfi’s work on these subjects has led to two additional manuscripts, one as a co-author and a review as first author, each of which have now been submitted for publication. Most recently, Ms. Nolfi designed a method for the coating of contact lenses to aid in the treatment of dry eye. This concept led Ms. Nolfi to enter and win the Pitt Innovation Challenge, a $100,000 prize which will now be used to further develop her idea.
Ms. Nolfi is dedicated to academic excellence, research productivity, and community outreach. She is passionate about the communication of knowledge and encouraging others to participate in science. She has been a member of many volunteer and outreach activities as well as tutoring. In the laboratory, Ms. Nolfi has been a dedicated mentor to 8 high school or undergraduate students. She has always taken the time needed to be sure that they understood their projects as well as the underlying science. As a result, five of these students were able to prepare abstracts for presentation at the Biomedical Engineering Society and for publication in the University of Pittsburgh Undergraduate research journal Ingenium. It is clear that these interactions and her experiences in tutoring outreach have had a profound impact upon her decision to pursue a career in an academic setting. Ms. Nolfi will continue to both find success in her graduate program and also go on to be an excellent educator, researcher, and innovator with a significant impact upon human health.
Congratulations, Drs. Ambrosio and Brown and Ms. Nolfi!
SCIENTIFIC ADVANCES
UPMC Celebrates 500th Adult Living-Donor Liver Transplant

Celebrating another milestone in its pioneering role in liver transplantation, UPMC has performed it’s 500th adult living-donor liver transplant (LDLT).
“Living donation offers an option for those who won’t qualify for a deceased donor transplant or who simply cannot wait” said McGowan Institute for Regenerative Medicine affiliated faculty member Abhinav Humar, MD, Chief, Division of Transplantation, UPMC. “There just aren’t enough livers to go around for all who need them.”
During a LDLT a piece of the donor’s healthy liver is removed and transplanted into another person. The procedure works because of the liver’s unique ability to regenerate or regrow. The piece of donated liver grows into a healthy and functioning liver for the recipient.
“It’s an outstanding example of what people are capable of doing for others,” said Dr. Humar. “You can see right away that there’s a bond— both physical because of the organ, and emotional, because many of our donors and patients stay in touch for their lifetime.”
More than 12,000 Americans are waiting for a liver transplant — yet only about 8,000 deceased-donor livers become available each year. Living-liver donation enables UPMC to provide more options for patients and more-timely care without the need for a deceased donor. UPMC is the national leader in LDLTs, performing more than any other medical center in the country.
The Ever-Changing Landscape of the Eye
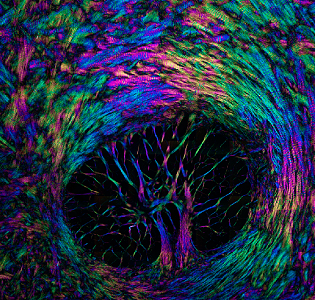
Elaine Vitone, PittMed, recently visited with McGowan Institute for Regenerative Medicine affiliated faculty member Ian Sigal, PhD, Assistant Professor and the founding Director of the Laboratory of Ocular Biomechanics in the University of Pittsburgh Department of Ophthalmology. There she learned about Dr. Sigal’s work and shared her experience in her article (with video), “The Forest, the Trees, and the Leaves.”
Over time in his research, Dr. Sigal learned that changes within the eye don’t happen in a vacuum. Hence, he studies the dynamics of the complex organ in its entirety.
Dr. Sigal notes that eyes are really, really complicated. They are full of fluid, and the amount of that fluid—and the pressure that its volume exerts—varies widely from person to person, as well as over the course of a lifetime, and even over the course of the day. The thinking, historically, was that glaucoma was the result of an excess of pressure pounding away year after year.
But as it turns out, research has revealed, plenty of healthy eyes have high pressure but never develop glaucoma.
So, Dr. Sigal’s central driving question shifted. It’s no longer about asking how nerve fibers die, or why disease happens. It’s: What is going on in healthy eyes, in all their confounding varieties?
“We don’t understand what is happening in glaucoma, because we really don’t understand the normal eye,” he says. “The most amazing thing is not that somebody loses vision. It’s that most people don’t, through our whole life. We go through so many things in our life, and this incredibly complicated, sensitive system still works.”
Illustration: The eye is a biomechanical wonder. A delicate balance of forces is at work every time you focus on a word or follow a line of text across a page. Fluctuating pressure pounds on the back of the eye, where the optic nerve begins; and in some people, those nerve fibers deteriorate, causing vision loss (glaucoma). Yet a certain amount of pressure is necessary for the organ to maintain its shape and function. The eye is like a soccer ball, says Dr. Sigal. “At some point, if it’s too deflated, it just doesn’t work. You can’t play.” Credit: Ian Sigal/Laboratory of Ocular Biomechanics.
Support for Female Pelvic Floor Research

The paper published by Steven Abramowitch, Associate Professor of Bioengineering at the University of Pittsburgh, and McGowan Institute for Regenerative Medicine affiliated faculty member Pamela Moalli, MD, PhD, Associate Professor, Department of Obstetrics, Gynecology & Reproductive Sciences, Division of Urogynecology & Pelvic Reconstructive Surgery, Magee-Womens Hospital of UPMC, University of Pittsburgh, was recently recognized by the editors of the Journal of Biomechanical. The article details complications associated with mechanical loads on synthetic mesh used in pelvic organ prolapse and will be listed as an Editors’ Choice paper in JBME’s Annual Special Issue February 2020.
Pelvic organ prolapse (POP) is a condition where the organs in the pelvis push against the vagina, creating a “bulge” that can extend outside of the body. It results from a weakening of the muscles and tissues that help support the pelvic organs. Despite the fact that 12.6 percent of women in the U.S. will undergo major surgery for POP by the age of 80, most of the studies surrounding these devices were conducted as they are applied to hernias in the abdomen. According to recent research, pelvic floor applications using this technology seem to be more vulnerable to mesh-related complications.
The team’s research in the Swanson School of Engineering and the Center for Interdisciplinary Research in Female Pelvic Health uses experimental and computational methods to examine the mechanical behavior of mesh so that it can be optimized for the female pelvic floor environment.
“The textile and structural properties of mesh have proven to be an important factor in its efficacy – particularly the pore size, which has shown to increase complications when less than 1mm in size,” said Dr. Abramowitch. “Even though these devices are widely used, the in vivo mechanical behavior of synthetic mesh is largely unknown, as is the impact of its mechanics on surrounding biological tissues.”
Though most vaginal mesh is developed with pore sizes large enough to minimize complications, researchers have recently discovered that mechanical loading significantly alters pore dimensions. While previous studies have looked at the effects of uniaxial loading, Dr. Abramowitch and his group are broadening research in this area by quantifying multiaxial loading.
“Transvaginal meshes, which are most commonly associated with complications and have been recently banned from use in the United States, are fixed at multiple locations in the pelvis. This creates multi-directional forces that cause the mesh to change shape in specific regions,” he explained. “Interestingly, our simulations predict the locations where the most shape change occurs, and they happen to be consistent with the most common sites for complications. This gives us a great possible lead to better understanding the mechanisms that cause mesh complications.”
Dr. Abramowitch’s group developed an experimental model to quantify pore dimensions in response to clinically relevant mechanical forces and a computational model to simulate the mechanical behavior of transvaginal mesh in response to these forces. By developing these models, they will be able to examine a wide range of mechanical conditions, predict mesh behavior, and eventually optimize devices for the female pelvic floor.
This research recently led to a $2,500,000 award from the National Institutes of Health to create a novel repair device designed for the vagina that may improve outcomes in POP surgery. Drs. Abramowitch and Moalli will lead this effort.
Alcohol in Her Urine, But Not in Her Blood: Putting the Puzzle Pieces Together

Auto-brewery syndrome is a rare medical condition in which intoxicating quantities of ethanol are produced by specific types of yeast or bacteria through endogenous fermentation in the digestive system. McGowan Institute for Regenerative Medicine affiliated faculty member Kenichi Tamama, MD, PhD, FCAP, Associate Professor of Pathology at the University of Pittsburgh School of Medicine and Director of the Toxicology Laboratory, and colleagues have reported in the Annals of Internal Medicine the first known case of a woman who urinates alcohol after a never-before-seen condition effectively turned her bladder into a microbrewery.
A 61-year-old woman with cirrhosis and poorly controlled diabetes presented to the University of Pittsburgh Medical Center for placement on the liver transplant waitlist. Even though the woman denied drinking any alcohol, she was taken off the waiting list for a donor organ at another hospital, and was instead referred for treatment for alcohol abuse, says Dr. Tamama.
Doctors at UPMC again found alcohol in her urine, but further tests revealed that there was no alcohol in her blood. Stumped, Dr. Tamama, a pathologist, began to investigate. As Dr. Tamama conducted some basic tests, he found that the woman’s urine contained yeast.
According to Dr. Tamama, this is what the team believes occurred: The patient had poorly controlled diabetes, one of the causes of liver disease. It also spurs chronically high blood sugar, with spillover into the urine. The sugar, Dr. Tamama said, likely fueled a yeast overgrowth in the woman’s bladder.
Specifically, she had an abundance of Candida glabrata, which is closely related to brewer yeast. So, the microbes began converting their sugar supply into alcohol.
“The experience we describe here of two liver transplant teams at different institutions demonstrates how easy it is to overlook signals that urinary auto-brewery syndrome may be present,” the study researchers said. The team called for standardized guidelines for alcohol abstinence monitoring. Dr. Tamama said he expects that auto-brewery syndrome will be rare.
The case report said the patient was reconsidered for a transplant.
“This is a game-changer for the patient,” Dr. Tamama said.
Dr. Anna Balazs: Shining a New Light on Biomimetic Materials

Advances in biomimicry – creating biological responses within non-biological substances – will enable synthetic materials to behave in ways that were typically only found in Nature. Light provides an especially effective tool for triggering life-like, dynamic responses within a range of materials. The problem, however, is that the applied light is typically dispersed throughout the sample and thus, it is difficult to localize the bio-inspired behavior to the desired, specific portions of the material.
A convergence of optical, chemical and materials sciences, however, has yielded a novel way to utilize light to control the local dynamic behavior within a material. In a general sense, the illuminated material mimics a vital biological behavior: the ability of the iris and pupil in the eye to dynamically respond to the incoming light. Furthermore, once the light enters the sample, the material itself modifies the behavior of the light, trapping it within regions of the sample.
The latest research from the University of Pittsburgh’s Swanson School of Engineering, Harvard University and McMaster University, reveals a hydrogel that can respond to optical stimuli and modify the stimuli in response. The group’s findings of this opto-chemo-mechanical transduction were published in the Proceedings of the National Academy of Sciences.
The Pitt authors include McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical and Petroleum Engineering and John A. Swanson Chair of Engineering; and Victor V. Yashin, PhD, Visiting Research Assistant Professor. Other members include Joanna Aizenberg, PhD, Amos Meeks (co-first author) and Anna V. Shneidman, Wyss Institute for Biologically Inspired Engineering and Harvard John A. Paulson School of Engineering and Applied Sciences; Ankita Shastri, PhD, Harvard Department of Chemistry and Chemical Biology; and Fariha Mahmood, Derek Morim, PhD (co-first author), Kalaichelvi Saravanamuttu, PhD, and Andy Tran, McMaster University, Ontario, Canada.
“Until only a decade or so ago, the preferred state for materials was static. If you built something, the preference was that a material be predictable and unchanging,” Dr. Balazs explained. “However, as technology evolves, we are thinking about materials in new ways and how we can exploit their dynamic properties to make them responsive to external stimuli.
“For example, rather than programming a computer to make a device perform a function, how can we combine chemistry, optics and materials to mimic biological processes without the need for hard-wired processors and complex algorithms?”
The findings continue Dr. Balazs’ research with spiropyran (SP)-functionalized hydrogels and the material’s photo-sensitive chromophores. Although the SP gel resembles gelatin, it is distinctive in its ability to contain beams of light and not disperse them, similar to the way fiber optics passively control light for communication. However, unlike a simple polymer, the water-filled hydrogel reacts to the light and can “trap” the photons within its molecular structure.
“The chromophore in the hydrogel plays an important role,” she explains. “In the absence of light, the gel is swollen and relaxed. But when exposed to light from a laser beam about the width of a human hair, it changes its structure, shrinks and becomes hydrophobic. This increases the polymer density and changes the hydrogel’s index of refraction and traps the light within regions that are denser than others. When the laser is removed from the source, the gel returns to its normal state. The ability of the light to affect the gel and the gel in turn to affect the propagating light creates a beautiful feedback loop that is unique in synthetic materials.”
Most surprisingly, the group found that the introduction of a second, parallel beam of light creates a type of communication within the hydrogel. One of the self-trapped beams not only controls a second beam, but also the control can happen with a significant distance between the two, thanks to the response of the hydrogel medium. Dr. Yashin notes that this type of control is now possible because of the evolution of materials, not because of advances in laser technology.
“The first observation of self-trapping of light occurred in 1964, but with very large, powerful lasers in controlled conditions,” he said. “We can now more easily achieve these behaviors in ambient environments with far less energy, and thus greatly expand the potential use for non-linear optics in applications.”
The group believes that opto-chemo-mechanical responses present a potential sandbox for exploration into soft robotics, optical computing and adaptive optics.
“There are few materials designed with a built-in feedback loop,” Dr. Balazs said. “The simplicity of the responses provides an exciting way to mimic biological processes such as movement and communication, and open new pathways toward creating devices that aren’t reliant on human control.”
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#207 –– Dr. John Kellum discusses his research in acute kidney injury and sepsis.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Barone WR, Knight KM, Moalli PA, Abramowitch SD
Title: Deformation of Transvaginal Mesh in Response to Multiaxial Loading
Summary: Synthetic mesh for pelvic organ prolapse (POP) repair is associated with high complication rates. While current devices incorporate large pores (>1 mm), recent studies have shown that uniaxial loading of mesh reduces pore size, raising the risk for complications. However, it is difficult to translate uniaxial results to transvaginal meshes, as in vivo loading is multidirectional. Thus, the aim of this study was to (1) experimentally characterize deformation of pore diameters in a transvaginal mesh in response to clinically relevant multidirectional loading and (2) develop a computational model to simulate mesh behavior in response to in vivo loading conditions. Tension (2.5 N) was applied to each of mesh arm to simulate surgical implantation. Two loading conditions were assessed where the angle of the applied tension was altered and image analysis was used to quantify changes in pore dimensions. A computational model was developed and used to simulate pore behavior in response to these same loading conditions and the results were compared to experimental findings. For both conditions, between 26.4% and 56.6% of all pores were found to have diameters <1 mm. Significant reductions in pore diameter were noted in the inferior arms and between the two superior arms. The computational model identified the same regions, though the model generally underestimated pore deformation. This study demonstrates that multiaxial loading applied clinically has the potential to locally reduce porosity in transvaginal mesh, increasing the risk for complications. Computational simulations show potential of predicting this behavior for more complex loading conditions.
Source: J Biomech Eng. 2019 Feb 1;141(2).
GRANT OF THE MONTH
PI: Cecelia Yates
Title: FibroKine™ biomimetic peptides as potential targeted therapeutic treatment of pulmonary fibrosis
Description: Fibrotic diseases constitute a significant health problem in the US with the ability to impact any organ that is scarred from chronic disease, including the heart, lung, liver, arteries, and skin. In some cases, progressive and life-threatening diseases follow, but effective therapies don’t yet exist. In response, Dr. Yates has developed FibroKine™, a chemokine-based class of biomimetic peptides that are potential therapeutic agents for the targeted treatment of tissue fibrosis. Support from CSL Behring will allow the Yates group to test FibroKine™ peptide ability to effectively treat and halt the progression of pulmonary fibrosis.
Source: CSL Behring-Science Center Research Acceleration Initiative
Amount: $250,000
