March 2017 | VOL. 16, NO. 3| www.McGowan.pitt.edu
McGowan Institute for Regenerative Medicine Holds Its Annual Scientific Retreat

The McGowan Institute for Regenerative Medicine held its 2017 Scientific Retreat on March 5-7, 2017. The retreat provided many opportunities to explore collaborative endeavors with other researchers, participating guests, and external partners who are working to bring regenerative medicine technologies to clinical use. The 2017 Retreat program included technical and strategic planning sessions. The program was designed to facilitate insightful discussions and to identify opportunities for partnerships and new initiatives.
Program Highlights
The Keynote Lecture was presented by McGowan affiliated faculty member Dietrich Stephan, PhD, Professor and Chairman of the Department of Human Genetics at the University of Pittsburgh. Dr. Stephan shared his vision on the interface between personalized and regenerative medicine.
The Retreat included numerous general sessions throughout the 3-day event with a focus on these research and/or strategic planning areas: Craniofacial/Craniomaxillofacial, Cardiovascular, Manufacturing, NSF-ERC for Revolutionizing Metallic Biomaterials, DOD Needs, Pediatric, Wound Healing, Burns, Cell Therapy, and Vision & Hearing. Several trainee sessions were organized by Satdarshan (Paul) Singh Monga, MD, Andrew W. Duncan, PhD, and Kacey G. Marra, PhD. A special session on “Clinical Outcomes” was moderated by McGowan Institute faculty member Charles Sfeir, DDS, PhD, Associate Dean of Research in the University of Pittsburgh School of Dental Medicine, and presented by McGowan Institute faculty member Bernard Costello, MD, DMD, FACS, Professor, the Chief/Division of Craniofacial and Cleft Surgery, and the Fellowship Program Director, in the Department of Oral and Maxillofacial Surgery, University of Pittsburgh.
 The poster session was effective in highlighting the focus of the Retreat and interests of the faculty and the guests. McGowan Institute faculty member Andrew Duncan, PhD, Assistant Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. The winners of the poster session were:
The poster session was effective in highlighting the focus of the Retreat and interests of the faculty and the guests. McGowan Institute faculty member Andrew Duncan, PhD, Assistant Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. The winners of the poster session were:
Cell and Gene Therapy
First Place: Bing Han
Omental milky spots as sites for ectopic liver development
Mentor: Eric Lagasse, PharmD, PhD, Dept. of Pathology, University of Pittsburgh
Second Place: David Gau
Small molecule-mediated inhibitions of transcriptional cofactor MKL and its downstream target profilin impedes endothelial cell migration and angiogenesis
Mentor: Partha Roy, PhD, Dept. of Bioengineering, University of Pittsburgh
Third Place: Elizabeth Stahl
Accumulation of bone marrow derived macrophages in the aged murine liver
Mentor: Bryan Brown, PhD, Dept. of Bioengineering, University of Pittsburgh
Computation and Modeling
First Place: Lu Liu and Eric Lambert
High intensity focused ultrasound-sensitive perfluorocarbon nanoemulsions in targeted drug delivery
Mentor: Jelena Janjic, PhD, Graduate School of Pharmaceutical Sciences, Duquesne University
Second Place: Lisa Carey Lohmueller
Predicting all cause mortality for LVAD patients using Bayesian Networks
Mentor: James Antaki, PhD, Dept. of Bioengineering, University of Pittsburgh, and Dept. of Biomedical Engineering, Carnegie Mellon University
Third Place: Emily Ackerman
Controllability analysis of protein-protein interaction networks for antiviral drug development
Mentor: Jason Shoemaker, PhD, Dept. of Chemical and Petroleum Engineering, University of Pittsburgh
Medical Devices
First Place: Firuz Feturi
Ultrasound Triggered-Release Embedded Anti-Rejection Therapy (TREAT) for targeted immunomodulation in vascularized composite allotransplantation
Mentors: Raman Venkataramanan, PhD, Dept. of Pharmaceutical Science, University of Pittsburgh, and Vijay Gorantla, MD, PhD, Clinical Initiatives and Research Innovation, McGowan Institute
Second Place: Akhil Patel
RegenMatrix: collagen-mimetic bioactive hydrogels for growth factor free approach for bone regeneration
Mentor: Shilpa Sant, PhD, Dept. of Pharmaceutical Science, University of Pittsburgh
Third Place: Puneeth Shridhar
Ventriculo amniotic shunt for fetal aqueductal stenosis
Mentor: Youngjae Chun, PhD, Dept. of Bioengineering, University of Pittsburgh
Tissue Engineering
First Place: Chelsea Stowell
Resorbable vascular grafts support early cell infiltration and endothelialization in a porcine vascular access model
Mentor: Yadong Wang, PhD, Dept. of Bioengineering, University of Pittsburgh
Second Place: Andrew Bradshaw
Modeling metastasis from invasion to colonization on a human physiomimetic chip
Mentor: Alan Wells, MD, DMS, Dept. of Pathology, University of Pittsburgh
Third Place: Ehab Tamimi
Biomechanical evaluation of gelatin/fibrinogen electrospun cylindrical scaffolds seeded with 3T3 mouse fibroblasts and porcine smooth muscle cells
Mentor: Jonathan Vande Geest, PhD, Dept. of Bioengineering, University of Pittsburgh
The poster session prizes were sponsored by sciVelo; the prizes were: first place ($200), second place ($125), and third place ($75).
CATER
 First Place: Lindsey Saldin
First Place: Lindsey Saldin
Tissue-specific effects of normal, metaplastic, and neoplastic esophageal extracellular matrix hydrogels
Mentor: Stephen Badylak, DVM, PhD, MD, Dept. of Surgery, University of Pittsburgh
Second Place: Andrew Bradshaw
Modeling metastasis from invasion to colonization on a human physiomimetic chip
Mentor: Alan Wells, MD, DMS, Dept. of Pathology, University of Pittsburgh
Third Place: Colin Beckwitt
Adjuvant statin therapy efficacy is dictated by tumor dormancy and statin lipophilicity in ex vivo and in vivo models of metastatic breast cancer
Mentor: Alan Wells, MD, DMS, Dept. of Pathology, University of Pittsburgh
The CATER prizes were: first place ($200), second place ($125), and third place ($75).
A special thank you is extended to all who made this year’s Retreat a success!
See the full Retreat Program here.
RESOURCES AT THE MCGOWAN INSTITUTE
April Histology Special
April Showers mean big savings at the McGowan Histology Core Lab.
I would like to thank everyone who visited the Histology booth at the McGowan Retreat this year. It was so fun to meet fans of our work, and hopefully we will see a few new faces around the lab this year!
Break out your umbrellas as April is raining down savings on you lucky coupon holders! In addition to the coupons given out at the retreat, I’d like to offer 25% off our Herovici Stain:
Herovici stain is used to differentiate young and mature collagen as well as identify reticulum fibers within tissue. Herovici’s solution stains young collagen and reticulum blue and mature collagen red while providing a yellow cytoplasm counterstain. Nuclei are stained blue to black with Weigert’s Hematoxylin.
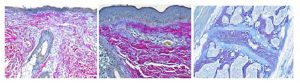
You’ll receive 25% off Herovici Staining for the entire month of April when you mention this ad. Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Flow Cytometry
Evan Delgado, PhD, is a post-doctoral researcher in the laboratory of McGowan Institute for Regenerative Medicine faculty member Andrew Duncan, PhD, Assistant Professor in the Department of Pathology at the University of Pittsburgh. He has been a member of the McGowan Institute for 2 years. His research specifically focuses on the dynamics of polyploidy in hepatocytes and how this relates to tumorigenesis. Dr. Delgado shares his experience with the Flow Cytometry Facility and how it helped his research.
The work in our lab focuses on a phenomenon in liver cells, hepatocytes, called polyploidy. Polyploidy refers to alterations in the number of complete chromosome sets within a cell. Normally, a cell is diploid and contains two sets of chromosomes. Hepatocytes are unique because they are capable of increasing the number of whole chromosome sets while maintaining a homeostatic biology. Sometimes, we can observe hepatocytes with 4, 8, 16, or higher copies of chromosome sets. This occurs when the cell begins to divide, but a failure in cytokinesis forces the cell to not divide even though it has already replicated its DNA.
Even though hepatocytes can be completely normal while containing an extraordinarily high amount of DNA content, we do not know if the DNA copy number is reflective of gene expression within the individual cells. This is important because gene expression relative to polyploidy can explain differential hepatocyte function observed in vivo.
To answer this question, we isolated hepatocytes from mice and utilized flow cytometry to identify hepatocytes based on DNA content. Flow cytometry is a crucial part of our approach because it allows us to identify these differential hepatocyte populations with diploid (2c), tetraploid (4c), octaploid (8c), etc., copies of DNA according to Hoechst intensity (Figure 1A). We are then able to sort these hepatocyte populations into extremely pure sub-fractions with respect to DNA content. Using these sorted hepatocytes, we are able to conduct further downstream gene expression analyses on single cells which are manually isolated under a microscope (Figure 1B, C).
The flow cytometry core facilities provided us with tools critical to our successful isolation of pure hepatocyte populations containing 2 or 4 copies of chromosomes in single cell suspensions. Together, this will enable us to interrogate whether differential gene expression patterns exist between populations of hepatocytes containing varied copies of chromosomes.
Do you have a research project that may benefit from Flow Cytometry analysis or sorting? Contact the facility’s manager, Lynda Guzik, for more information.
SCIENTIFIC ADVANCES
Funding to Establish NIDCR Consortium for Tissue Regeneration Therapies Received from NIH

The University of Pittsburgh School of Dental Medicine received an $11.7 million grant from the National Institute of Dental and Craniofacial Research (NIDCR) to establish a resource center dedicated to advancing therapies for regenerating damaged dental, oral, and craniofacial tissues.
Officially named the Michigan-Pittsburgh-Wyss Resource Center: Supporting Regenerative Medicine in Dental, Oral, and Craniofacial Technologies, Pitt established the center in partnership with the University of Michigan and Harvard University as part of the NIDCR’s Dental, Oral, and Craniofacial Tissue Regeneration Consortium (DOCTRC). The goal of the consortium is to guide new therapies from the research stages through preclinical studies and into human clinical trials.
“There is tremendous value in craniofacial regenerative medicine research, and our goal is to create therapies and technologies that help patients,” said McGowan Institute for Regenerative Medicine faculty member Charles Sfeir, DDS, PhD, principal investigator, associate dean for research and director of Pitt’s School of Dental Medicine Center for Craniofacial Regeneration. “This newly established consortium is dedicated to making the most promising research in this field a clinical reality, and we are proud to be part of this effort at the University of Pittsburgh.”
Pitt, Michigan, and Harvard researchers joined forces during an initial year-long organizational phase funded by an NIDCR planning grant. The current award provides funding for a second 3-year phase, which will consist of researchers evaluating projects based on their clinical and commercial viability. The resource center will then match selected projects with the clinical, scientific, industrial, and regulatory expertise necessary to more efficiently translate the research into clinical trials and eventually clinical practice.
The University of Pittsburgh is uniquely positioned as a leading tissue regeneration hub with a proven record of translating tissue engineering therapies. In addition, the integration between the Center for Craniofacial Regeneration and the McGowan Institute for Regenerative Medicine provides outstanding expertise in regenerating dental and oral cranial tissues.
“This opportunity leverages our substantial base of researchers and support personnel in the regenerative medicine space and provides a focus on the unique challenges faced in the craniofacial area,” said William Wagner, PhD, director of Pitt’s McGowan Institute for Regenerative Medicine and Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh. “I am confident that the investment from the NIH will result in meaningful progress of new therapies toward patients with needs for craniofacial tissue therapy.”
Drs. Sfeir and Wagner are the principal investigators at the resource center in Pittsburgh. David Kohn, PhD, and William Giannobile, DDS, from Michigan; and David Mooney, PhD, from Harvard, will serve as principal investigators at their universities.
In addition to Pitt’s resource center, a second center based at the University of California, San Francisco, also received funding from DOCTRC. Total NIH funding for both resource centers amounts to $24 million over 3 years.
Regenerative Medicine for Whole Organ Replacement

Extracellular matrix (ECM) represents the structural and functional molecules secreted by the resident cells of every tissue in the body. Researchers in the laboratory of Stephen Badylak, DVM, PhD, MD, Professor in the University of Pittsburgh’s Department of Surgery, a deputy director of the McGowan Institute for Regenerative Medicine, and Director of the Center for Pre-Clinical Tissue Engineering within the Institute, have extensive experience isolating and harvesting this ECM and producing usable formats for use in pre-clinical animal and bench top studies. Scientists routinely produce ECM from tissue such as small intestine, urinary bladder, and liver tissue. The cells of these tissues are removed by physical, enzymatic, and/or chemical methods so that only the native ECM remains.
In Dr. Badylak’s lab, research continues on utilizing organ ECM scaffolds to one day use as replacement organs to patients in need. Some organs, like skin and bladders, may be available to patients much sooner than more complicated organs like liver, lungs, and heart.
“These two organs [liver and lungs] have the anatomy where they can hook up the plumbing and … still have the benefit of the remaining failing organ while the new organ is taking hold,” says Dr. Badylak in his interview with Kate Baggaley, Science Writer for NBC. His research team is investigating how best to reseed engineered livers with new cells.
“We’re still trying to figure out what’s the best recipe,” he says.
Watch these videos from the Badylak lab on decellularizing and seeding a liver ECM scaffold.
Another option to acquiring much needed organs is to “build them from scratch” and print them on a 3D printer, later to be individualized for the patient. There’s a lot we still don’t know about how our organs are put together on a microscopic level, and what directions they need from the body.
“I know the general shape of a liver,” Dr. Badylak says. “What I don’t know is how the cells are layered exactly next to each other. Technology has outpaced our understanding of biology, and we need to understand more about what we want to print.”
NSF Award to Develop Ultrasonic Sensors for a Hybrid Exoskeleton Received

The promise of exoskeleton technology that would allow individuals with motor impairment to walk has been a challenge for decades. A major difficulty to overcome is that even though a patient is unable to control leg muscles, a powered exoskeleton could still cause muscle fatigue and potential injury.
However, an award from the National Science Foundation’s Cyber-Physical Systems (CPS) program will enable researchers at the University of Pittsburgh to develop an ultrasound sensor system at the heart of a hybrid exoskeleton that utilizes both electrical nerve stimulation and external motors.
Principal investigator of the 3-year, $400,000 award is Nitin Sharma, PhD, assistant professor of mechanical engineering and materials science at Pitt’s Swanson School of Engineering. Co-PI is McGowan Institute for Regenerative Medicine affiliated faculty member Kang Kim, PhD, associate professor of medicine and bioengineering. The Pitt team is collaborating with researchers led by Siddhartha Sikdar, PhD, associate professor of bioengineering and electrical and computer engineering at George Mason University, who also received a $400,000 award for the CPS proposal, “Synergy: Collaborative Research: Closed-loop Hybrid Exoskeleton Utilizing Wearable Ultrasound Imaging Sensors for Measuring Fatigue.”
“One of the most serious impediments to developing a human exoskeleton is determining how a person who has lost gait function knows whether his or her muscles are fatigued. An exoskeleton has no interface with a human neuromuscular system, and the patient doesn’t necessarily know if the leg muscles are tired, and that can lead to injury,” Dr. Sharma explained. “Electromyography (EMG), the current method to measure muscle fatigue, is not reliable because there is a great deal of electrical “cross-talk” between muscles and so differentiating signals in the forearm or thigh is a challenge.”
To overcome the low signal-to-noise ratio of traditional EMG, Dr. Sharma partnered with Dr. Kim, whose research in ultrasound focuses on analyzing muscle fatigue.
“An exoskeleton biosensor needs to be noninvasive, but systems like EMG aren’t sensitive enough to distinguish signals in complex muscle groups,” Dr. Kim said. “Ultrasound provides image-based, real-time sensing of complex physical phenomena like neuromuscular activity and fatigue. This allows Nitin’s hybrid exoskeleton to switch between joint actuators and FES, depending upon the patient’s muscle fatigue.”
“Right now an exoskeleton combined with ultrasound sensors is just a big machine, and you don’t want to weigh down a patient with a backpack of computer systems and batteries,” Dr. Sharma said. “The translational research with George Mason will enable us to integrate a wearable ultrasound sensor with a hybrid exoskeleton, and develop a fully functional system that will aid in rehabilitation and mobility for individuals who have suffered spinal cord injuries or strokes.”
Catalytic Conveyor Belt
Capitalizing on previous studies in self-powered chemo-mechanical movement, researchers at the University of Pittsburgh’s Swanson School of Engineering and Penn State University’s Department of Chemistry have developed a novel method of transporting particles that utilizes chemical reactions to drive fluid flow within microfluidic devices. Their research, “Harnessing catalytic pumps for directional delivery of microparticles in microchambers,” was published recently in the journal, Nature Communications.
The computational modeling research was led by McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical and Petroleum Engineering at Pitt, with post-doctoral associates Oleg E. Shklyaev, PhD, and Henry Shum, PhD. Experiments at Penn State were conducted by Ayusman Sen, PhD, Distinguished Professor of Chemistry and graduate students S. Das, A. Altemose, I.Ortiz-Rivera, and L. Valdez. Their combined theoretical and experimental findings could enable controllable transport of particles and cells, allowing highly sensitive chemical assays to be performed more rapidly and efficiently.
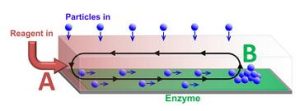
“One of the critical challenges in transporting microparticles within devices is delivering the particle to a specific location,” Dr. Balazs explained. “Much like a conveyor belt in a factory, you want to move the particle within a closed system without any modification to its surface or damage to its structure.”
Dr. Balazs noted that in addition to successfully delivering the particles, the other challenges the researchers faced were maintaining unidirectional flow from point A to point B within a closed chamber, and ensuring that a critical concentration of these particles could be delivered to sensors, which only operate above a critical threshold. The solution was to generate a gradient of a chemical reagent by introducing the reagent at one end of the chamber, point A. Enzymes on the surface of the chamber consumed the reagent so that it was completely depleted at the point B. Since the presence of the reagent increases the fluid density, a density gradient was established between points A and B, leading to convective flow that transported particles like a conveyor belt.
“Previously, to generate spontaneous propulsion of microparticles, one needed to chemically modify the surface of these particles, thus altering their inherent properties,” Dr. Balazs said. “Moreover, modifying the particle’s surface does not necessarily allow you to direct its motion within the chamber. We were able to predicate through our computational models and demonstrate in the experiments performed at Penn State that the flow generated by the catalytic chemical reaction in the chamber could effectively transport particles to a particular sensor, and could permit control over the speed and direction of the particle transport, without having to use an external pump or any modification of the cargo.”
“Utilizing catalytic reactions to drive fluids to controllably transport particulates in solution is a relatively new field, even though it’s what our bodies do at any given moment when converting food to fuel. Replicating it within a synthetic system however is very difficult,” Dr. Sen added. “In our lab, we were able to design a “machine” without the need for a mechanical device that could be used many times over simply by adding fuel to the chamber, while allowing the particle to remain a passive participant along for the ride.”
Illustration: Particles transported along a channel by chemically-driven fluid flow. The flow is generated by reagent entering at one end of the channel (A) and reacting at the enzyme covered surface. The cargo is deposited at position B, which can be controlled by varying the reaction rate. (Oleg E. Shklyaev and Henry Shum)
RECENT EVENTS
Dr. Mark Slaughter is McGowan Institute Distinguished Lecturer

Mark Slaughter, MD was the McGowan Institute’s 2017 Distinguished Lecturer. His lecture on March 23rd was titled “Future Role of MCS in the Treatment of Advanced Heart Failure.” Through the application of mechanical circulatory support, Dr. Slaughter’s aim is to offer individuals with heart failure an alternative to heart transplantation, repairing hearts and helping them recover naturally.
In his lecture Dr. Slaughter addressed the following:
- Improved technology and bioengineering have resulted in devices that are more durable, reliable, smaller/miniaturized, allow less invasive implant techniques and reduced adverse events;
- Survival in appropriately selected patients is now similar to transplant at 3-5 years’
- Improving patient management and identifying best practices will limit outcome variation between programs;
- Smart pumps (pressure sensors) and a physiologic pulse will bring the technology one step closer to mimicking transplant;
- Myocardial recovery is complex, will benefit from LVAD unloading but require adjuvant therapy, and;
- Collaborative efforts between clinicians, engineers and basic scientists will yield solutions to diminish the fatal effects of advanced heart failure.
Dr. Slaughter is the Professor and Chair in the Department Cardiovascular and Thoracic Surgery at the University of Louisville School of Medicine. He is also the director of the Heart Transplant and Mechanical Assist Device program at Jewish Hospital & St. Mary’s HealthCare.
Dr. Kormos Delivers Provost’s Inaugural Lecture

Robert L. Kormos, MD – Brack G. Hattler Professor of Cardiothoracic Transplantation, Professor Cardiothoracic Surgery, Bioengineering and the McGowan Institute delivered the Provost’s Inaugural Lecture on March 21, 2017 entitled “Will Engineering Conquer Biology? Lessons from 30 Years of Mechanical Support for the Failing Heart.”
Dr. Kormos reviewed the progress that has been made over the last 30 years in mechanical circulatory support devices and systems. While significant progress is apparent-especially in the reduced size of pumps and controllers, as well as the reliability of devices and systems, there still are issues such as infections (especially in the drive line), biocompatibility and bearing failure that need to be addressed.
Dr. Kormos noted the key role that Family House has played in helping patients who are on circulatory support being able to leave the hospital. In the early days of the program, Family House was the only allowable destination outside of the hospital while a patient was on circulatory support; today many patients can return to their homes with their implanted device, after sufficient recuperation.
Dr. Kormos highlighted the key role that the UPMC clinical team and the McGowan scientists and engineers have played in the advancement of circulatory support technology. As an example, the most widely used ventricular assist device used today, which employs a rotary pump, was developed here in partnership with a commercial partner. Dr. Kormos did the first implantation of the device in 2000 in Tel Aviv Israel.
He discussed the migration over the years from pulsatile devices, to rotary (non-pulsatile) pumps, and the recent introduction of prototype rotary pump designs that also have a pulsatile component to the flow. He shared the opinion that a pulsatile flow is beneficial.
The key role of the INTERMACS Registry (Interagency Registry for Mechanically Assisted Circulatory Support) was noted. INTERMACS is a North American national registry for mechanical circulatory support devices (MCSDs) that are used to treat advanced heart failure. Dr. Kormos played a key role in the establishment of the registry.
The multidisciplinary approach that was created here—a combination of physicians and engineers—has led to the formation of Procirca, Inc. that supports circulatory support cases here and at other hospitals nationally and internationally.
The UPMC mechanical circulatory support program has done nearly 1,100 implants (~1,000 adults and 63 pediatric cases). While the primary application for circulatory support is to serve as a “bridge to a heart transplant”, today about 10% of the cases are “destination therapy”. There have been 21 cases of a “bridge to recovery” where the device is used to give a sick heart a rest, and when the heart recovers the pump is removed.
While significant progress has been made in understanding and utilizing engineering and biology to treat a sick heart, much remains to be done in both sectors.
AWARDS AND RECOGNITION
Marra Lab Student Receives NIH F31 Fellowship Award

Christopher Mahoney is a 4th year doctoral candidate in the Bioengineering Department at the Swanson School of Engineering and works in the laboratory of McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD, Associate Professor in the Departments of Plastic Surgery in the School of Medicine and Bioengineering in the School of Engineering at the University of Pittsburgh and also the Director of the Plastic Surgery Research Laboratory in the Department of Plastic Surgery in Pitt’s School of Medicine.
Mr. Mahoney is the awardee a Predoctoral Individual National Research Service Award Fellowship from the National Institutes of Health. The purpose of this program is to enhance the diversity of the health-related research workforce by supporting the research training of pre-doctoral students from population groups that have been shown to be underrepresented in the biomedical, behavioral, or clinical research workforce. The proposed mentored research training is expected to clearly enhance the individual’s potential to develop into a productive, independent research scientist.
Congratulations, Mr. Mahoney!
Chun Lab Student to Receive Society for Biomaterials Recognition

Puneeth Shridhar, MD, MS, is a student in the Medical Device Manufacturing Laboratory of McGowan Institute for Regenerative Medicine affiliated faculty member Youngjae Chun, PhD, Assistant Professor, Department of Industrial Engineering, Swanson School of Engineering, with a secondary appointment in the Department of Bioengineering at the University of Pittsburgh.
Dr. Shridhar’s paper entitled, The Rescue Stent for Non-Compressible Traumatic Hemorrhage, was nominated as an outstanding contribution to the Society for Biomaterials 2017 Annual Meeting. The Education and Professional Development Committee of the Society has awarded Dr. Shridhar with an Honorable Mention STAR (Student Travel Achievement Recognition).
Dr. Shridhar’s work is focused on developing a novel stent to manage firearm trauma happening in battlefield and the civilian environment. The medical device will help to achieve a 4-minute large vessel hemorrhage control at the trauma site versus a 20-minute timeframe which currently involves patient transport to a hybrid operating room. The device not only disrupts life threatening bleeding but also allows continued perfusion of organs.
Recently, Dr. Chun, along with McGowan Institute faculty member Bryan Tillman, MD, PhD, Assistant Professor in the Division of Vascular Surgery at University of Pittsburgh Medical Center, Sung Kwon Cho, PhD, and William Clark, PhD, received $2.5 million grant in funds from the U.S. Department of Defense to develop the technology.
“Puneeth and I have authored 20+ research papers, all within one year, focused on various medical devices that find critical applications from head-to-toe,” said his advisor Dr. Chun. “He is very passionate about next-gen devices, and a STAR recognition awarded to nurture a future leader in the biomaterial arena is a very positive sign.”
This year’s theme for Society for Biomaterials is Where Materials Meet Medicine. Major themes for 2017 included are translation, 3D printing, cells, drug discovery, immune response, and regulatory issues.
The STAR recognizes research excellence and develops future leaders within the Society. The Society for Biomaterials’ award program seeks to recognize significant contributions to the field of biomaterials science from industry, academia, regulatory agencies, and students.
Congratulations, Dr. Shridhar!
Dr. Rory Cooper Named Associate Dean for Inclusion, SHRS

University of Pittsburgh’s School of Health and Rehabilitation Sciences (SHRS) recently appointed McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, as its Associate Dean for Inclusion. Dr. Cooper, Distinguished Professor and FISA/Paralyzed Veterans of America Chair of the Department of Rehabilitation Science and Technology, also is Director of the Human Engineering Research Laboratories.
McGowan Institute affiliated faculty member Anthony Delitto, PhD, SHRS Dean, said: “Dr. Cooper is a most appropriate choice to fill the role of Associate Dean for Inclusion in our school. He has a personal and professional understanding of and appreciation for the needs of people with disabilities and has worked tirelessly to improve access to services, education, housing, and transportation and is a strong, visible advocate for the disabilities community.”
Dr. Cooper has 20 patents awarded or pending, has authored or co-authored over 300 peer-reviewed journal publications, and has co-authored two books and co-edited three others including Care of the Combat Amputee.
Dr. John Kellum Named to BioAegis Therapeutics Clinical Advisory Board

BioAegis Therapeutics Inc., a privately held biotechnology company exploiting plasma gelsolin’s (pGSN) role in immune function, announced that it has expanded its clinical advisory board to include McGowan Institute for Regenerative Medicine affiliated faculty member John Kellum, MD. Dr. Kellum is a Professor in the Departments of Critical Care Medicine (primary), Medicine, Bioengineering, and Clinical and Translational Science at the University of Pittsburgh. He is the Director of the Center for Critical Care Nephrology and the Vice-Chair for Research, both appointments in the Department of Critical Care Medicine, and Associate Director for Acute Illness in the Institute for Personalized Medicine at Pitt. Dr. Kellum also serves as an Intensivist in the Cardiothoracic ICU at UPMC.
Dr. Kellum’s research interests span various aspects of Critical Care Medicine, but center in critical care nephrology (including acid-base, and renal replacement therapy), sepsis and multi-organ failure (including blood purification), and clinical epidemiology. His research has received continuous funding from the National Institutes of Health since 2001, and he has active funding from multiple different NIH Institutes. Dr. Kellum has authored more than 300 publications and has also edited several major textbooks including Critical Care Nephrology and Stewart’s Textbook of Acid-Base.
UPMC Movement Disorders Expert Named to Parkinson Foundation Board of Directors

McGowan Institute for Regenerative Medicine affiliated faculty member Mark Richardson, MD, PhD, assistant professor of neurological surgery at the University of Pittsburgh School of Medicine and director of Epilepsy and Movement Disorders Surgery at UPMC, has been named to the board of directors of the Parkinson Foundation of Western Pennsylvania.
Parkinson’s disease is a brain disorder that leads to symptoms that include shaking and difficulty with walking, movement, and coordination. Richardson is a neurosurgeon-neuroscientist who specializes in the use of Deep Brain Stimulation (DBS) and gene therapy for movement dysfunctions commonly seen in patients with Parkinson’s disease.
“Mark [Richardson] is an outstanding clinician researcher, and a nationally recognized leader in the field of movement disorders,” said Robert Friedlander, MD, MA, Walter E. Dandy Professor and chair of neurological surgery at Pitt’s School of Medicine. “He is passionate about improving the lives of patients, and the Parkinson’s community will benefit greatly from his presence on the board of the foundation.”
Dr. Richardson started the interventional-MRI DBS program at UPMC, which involves a new method of performing DBS inside an MRI scanner while the patient is under anesthesia. This allows the surgeon to see real-time images of the brain that help guide the placement of the electrodes that are implanted in the area of the brain responsible for abnormal movement.
In February 2016, Dr. Richardson and his colleagues began testing the use of gene therapy to relieve the symptoms of tremor and mobility impairment in patients with Parkinson’s disease as part of a clinical trial. The technique shows promise in prolonging the effectiveness of levo-dopa, the mainstay treatment for the progressive neurodegenerative condition, by increasing production of a key enzyme essential to convert the drug into the neurotransmitter dopamine.
More recently, he was awarded a 3-year $3.3 million grant by the National Institutes of Health (NIH) to lead a multi-institutional research study to understand how speech is controlled in the brain. The study was funded as part of the NIH’s BRAIN Initiative launched by former President Barack Obama as a large-scale effort to understand the brain and apply the knowledge to treating a variety brain disorders.
Dr. Richardson received his undergraduate education at the University of Virginia. He completed his medical and doctoral education in the MD/PhD program at the Medical College of Virginia. Prior to joining the faculty at Pitt in 2011, Dr. Richardson completed his neurosurgical residency at the University of California, San Francisco, where he received specialized training in epilepsy neurosurgery, DBS, and brain mapping during awake craniotomies, and was awarded a NIH National Research Service Award to study gene therapy for Parkinson’s disease.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#169 –– Dr. Howard Edington is the System Chairman, Department of Surgery, and the Chief of Surgical Oncology at West Penn Allegheny Health System, in Pittsburgh. He is also a Professor of Surgery at Temple University School of Medicine and Drexel University School of Medicine. He is also an adjunct Professor of Biomedical Engineering at Carnegie Mellon University. Dr. Edington discusses his research in tissue engineering and skin cancer.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
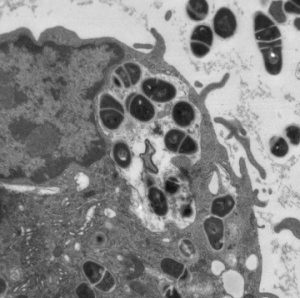
Mycobacterium tuberculosis being phagocytosed by a macrophage.
Transmission electron micrograph.
Center for Biologic Imaging.
PUBLICATION OF THE MONTH
Author: Ding Z, Joy M, Kameneva MV, Roy P
Title: Nanomolar concentration of blood-soluble drag reducing polymer inhibits experimental metastasis of human breast cancer cells
Summary: Metastasis is the leading cause of cancer mortality. Extravasation of cancer cells is a critical step of metastasis. We report a novel proof-of-concept study that investigated whether non-toxic blood-soluble chemical agents capable of rheological modification of the near-vessel-wall blood flow can reduce extravasation of tumor cells and subsequent development of metastasis. Using an experimental metastasis model, we demonstrated that systemic administration of nanomolar concentrations of so-called drag-reducing polymer dramatically impeded extravasation and development of pulmonary metastasis of breast cancer cells in mice. This is the first proof-of-principle study to directly demonstrate physical/rheological, as opposed to chemical, way to prevent cancer cells from extravasation and developing metastasis and, thus, it opens the possibility of a new direction of adjuvant interventional approach in cancer.
Source: Breast Cancer (Dove Med Press). 2017 Feb 24;9:61-65. eCollection 2017.
GRANT OF THE MONTH
PI: David H. Kohn, William V. Giannobile, David J. Mooney, Charles Sfeir, and William R. Wagner
Title: Michigan-Pittsburgh-Wyss Resource Center: Supporting Regenerative Medicine in Dental, Oral and Craniofacial Technologies
Description: Abstract The translation of innovative tissue engineering/regenerative medicine (TE/RM) technologies requires a new approach to bring TE/RM in dental, oral and craniofacial (DOC) technologies to clinical practice. To meet this need, an integrated, multidisciplinary Resource Center (RC) has been established as a partnership between University of Michigan, the University of Pittsburgh/McGowan Institute, Harvard University/Wyss Institute for Biologically Inspired Engineering, and clinical and industrial experts. This RC, named the Michigan-Pittsburgh- Wyss Resource Center: Supporting Regenerative Medicine in Dental, Oral and Craniofacial Technologies (MPWRM TechDOC), consists of leaders with clinical, basic science, engineering and business expertise, and an infrastructure to support navigation through the regulatory process and clinical trials. The goal of this RC is to translate TE/RM innovations that address the ongoing clinical need to restore or create healthy, functional DOC tissues. Central to our proposal is the use of a unique academic-industry assessment model, assembly of an outstanding team of experts, and a menu of innovative DOC technologies that are heavily focused on craniomaxillofacial and dental therapies. The vision of this RC is that the most promising technologies in TE/RM will be catalyzed, nurtured and expedited through a novel academic partnership to safely and effectively regenerate, reconstruct and restore functional DOC tissues. To achieve this vision, we will: 1) Implement the organizational structure of the consortium to prioritize and de-risk TE/RM technologies for translation to clinical practice. Support teams, subject matter experts (SMEs), and partnerships coordinated during our Planning Grants will be empowered to provide integrated assessment and translational pathways. 2) Implement processes to select regenerative technologies to translate to clinical practice, by utilizing a unique academic-industry assessment approach that emulates the industry proven Stage-Gate© model; execute technology validation, and implement plans to facilitate cGLP/cGMP activities for FDA submissions. Upon completion of the Stage II grant, we will have implemented the RC structure and operations, and provided training and education for our stakeholders to translate DOC technologies utilizing sound scientific and engineering, business, regulatory, and manufacturing principles. In doing so, the MPWRM TechDOC will be poised to advance TE/RM technologies through the translational pipeline in Phase III, leading to a transformation of patient care in dental, oral and craniofacial medicine.
Source: NIDCR
Term: 5 years
Amount: $3,911,029 FY Total Cost by IC
