July 2018 | VOL. 17, NO. 7| www.McGowan.pitt.edu
Seeing a Brighter Future

In the Pitt Summer 2018 edition, author Jennie Dorris highlights the career paths of McGowan Institute for Regenerative Medicine affiliated faculty member James Funderburgh, PhD, Professor of Ophthalmology and Cell Biology & Physiology, University of Pittsburgh School of Medicine, and his wife, Martha Funderburgh, MPH, Lab Manager and Sr. Research Technician, Corneal Cell Biology Laboratory, Department of Ophthalmology, University of Pittsburgh School of Medicine. The story provides “an exciting outlook for a research team inspired by personal experience and driven by the desire to help others see a brighter future.”
In the Corneal Cell Biology Laboratory, the Funderburghs’ research focuses on the cornea, an organ that provides a visual portal to the world. A transparent outer layer that covers the iris and pupil, it’s responsible for helping the eye focus by bending, or refracting, light. The connective tissue of the cornea is extremely tough and transparent to light. It also presents a significant biological barrier to infection. In millions of individuals, worldwide, however the cornea has lost its transparency due to disease or trauma. Corneal scarring can disrupt vision permanently. The team’s work focuses on the biological processes that produce and maintain the unique connective tissue of the cornea as well as the pathological changes that occur during scarring. These involve studies of extracellular matrix molecules and how they influence cell behavior.
In the lab, work extends to studies designed to reverse the scarring process or replace scarred cornea with bioengineered cornea tissue. Over the past few years the researchers have explored the use of stem cells to restore corneal transparency. Using nanofiber scaffolding they found that stem cells produce a tissue identical to that of the transparent tissue of the cornea. They are developing this material to serve as replacement of corneal tissue in scarred eyes. In addition, the team has found that adult stem cells induce regeneration of corneal tissue, removing pathological tissue and replacing it with organized transparent new tissue. These adult cells can be obtained autologously, allowing individuals to be treated with their own cells. The lab is actively investigating the mechanism by which adult stem cells induce tissue regeneration and is developing an approach to clinical trials for using corneal stem cells to treat blindness.
It was in 2002, during a McGowan Institute seminar, the scientists learned that most tissues in the body harbor stem cells. A eureka moment! “We could grow these stem cells and then convert them into corneal cells,” Dr. Funderburgh remembers realizing.
In 2011, after Dr. Funderburgh presented his latest research findings at an international conference, he met Sayan Basu, MBBS, MS, an ophthalmologist and cornea specialist from the L V Prasad Eye Institute in Hyderabad, India. This meeting led to a collaborative effort to bring the Funderburghs’ research to the clinic. It was in 2014 in India the Funderburghs witnessed the first-ever procedure in a clinical trial to treat corneal damage in humans using stem cells applied in gel. The operation took mere minutes to complete. Over 70 patients have been treated with the technique so far, with no reported complications and a high rate of vision improvement. So far, the interim analysis of results, Dr. Basu says, is “extremely encouraging.”
Today, the researchers are exploring options of using either the stem cells or the products made by the cells in clinical trials in the United States. Though the process is slow and expensive, Dr. Funderburgh is hopeful that they could have a product ready for initial clinical trials within the next two years.
Illustration: Tom Altany/Pitt Visual Services.
RESOURCES AT THE MCGOWAN INSTITUTE
August Histology Special
Reticular fibers, or reticulin is a type of fiber in connective tissue, composed of type III collagen secreted by reticular cells. Reticular fibers crosslink to form a fine meshwork (reticulin). This network acts as a supporting mesh in soft tissues such as liver, bone marrow, and the tissues and organs of the lymphatic system.
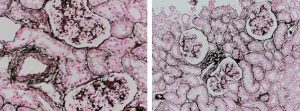
Jones Silver Stain demonstrates basement membrane and reticulin.
You’ll receive 25% off Jones Silver Stain in August when you mention this ad. Contact Lori at the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology in the lowest amount of time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
UPCOMING EVENTS
7th Annual Internal Symposium on Regenerative Rehabilitation Symposium

The Symposium on Regenerative Rehabilitation is the largest medical and scientific conference specific to Regenerative Rehabilitation in the world, bringing together two disparate disciplines, regenerative medicine and rehabilitative research, into one synergistic field of science. Innovative ideas, concepts and technologies were shared, deliberated and debated at this premier event. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
The Symposium will be held October 11-13, 2018 in Seattle, Washington.
SCIENTIFIC ADVANCES
Taking a Chance on the Unknown

When an institution encourages the free flow of ideas and doesn’t rigidly define the roles of its researchers, new and better solutions are the result. And free-thinking scientists are the sort of people who are attracted to a collaborative environment that encourages risk-taking. These are the environments where ideas are grown.
As highlighted in the June 1, 2018 edition of UMPC Next, the McGowan Institute for Regenerative Medicine has that type of environment and how that came to be is explained by Deputy Director Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery and Director of the Center for Pre-Clinical Tissue Engineering within the McGowan Institute.
“At the McGowan Institute, we look for the sort of people who aren’t afraid to take a different tack,” he says. “If you’re willing to be the one of the only scientists in your specialty here, you can approach your work from a standpoint that incorporates diversity of thought, and you’re already ahead.”
Dr. Badylak is a pioneer in his field of regenerative medicine and tissue engineering. His work on the use of biological scaffolds to support specialized structures goes back several decades. The idea of growing tissue from a matrix was new back in 1996, but now, about half of everything discussed at meetings for pioneers in regenerative medicine deals with this advancement. Approximately 90 related products are on the market now, mostly surgical mesh based on work that came from the Badylak Lab at the McGowan Institute. Millions of patients have benefited — people with ventral hernias, people with topical wounds. But the McGowan Institute has set its sights higher, on growing functional, enervated, vascularized skeletal muscle.
It makes sense that the technology that allows for the creation and use of surgical mesh would also enable the growth of skeletal muscle, but no physician-researchers thought to expand it in this way. After all, surgical mesh has been around for a while, and it’s very useful in its current capacity. And not many physician-researchers are faced with severe traumatic injuries, either. So why did researchers from the McGowan Institute think to apply and adapt the matrix to a much more complex purpose?
Dr. Badylak attributes the imaginative productivity of the McGowan Institute to its interdisciplinary nature.
“People who invest years in learning how to manipulate T cells, for example, naturally believe that the way to succeed is to surround themselves with other people who have studied the same thing. That’s where that type of science happens, and that’s how you increase your chances of success,” says Dr. Badylak. “But by shutting yourself off, you’re missing out on the perspective you can gain when you associate with other specialists. Together, people can help each other see the possibilities.”
The microJoint: Potential to Revolutionize the Treatment of Conditions Related to Joint Complications

The microJoint, a three-dimensional model that replicates a human joint on a small scale, is under development in Pittsburgh. Once completed, it could have the potential to revolutionize the treatment of conditions related to joint complications, such as osteoarthritis. A radically new way of thinking — and a culture that encourages risk-taking — were key to its development.
Leading the charge on this groundbreaking project is McGowan Institute for Regenerative Medicine affiliated faculty member and principal investigator Rocky Tuan, PhD, director of the Center for Cellular and Molecular Engineering in the Department of Orthopaedic Surgery at the University of Pittsburgh and a fellow of the National Academy of Inventors. He is also vice chancellor and president of the Chinese University of Hong Kong. He and his collaborators at Stanford University and Tulane University were recently awarded $1.75 million from the National Institutes of Health for their work, which has already shown incredible promise.
The microJoint is composed of human tissues, constructed in the laboratory and grown as microscale structures – usually referred to as tissue chips – and generated using three key components: adult mesenchymal stem cells as the cell source, biological factors to facilitate growth, and a light-polymerized gel that acts as a scaffold.
Engineered cartilage serves as the centerpiece of the model, sitting in a small cylindrical chamber within a microbioreactor built by Dr. Tuan’s team using 3D printing. The cartilage is connected to a series of similar chambers that house the key elements of the microJoint — including the synovium, macrophages, and an infrapatellar fat pad — by a precisely calibrated set of microtubes containing synovial fluid and other tissue fluids. The model may not look much like a human joint, but physical appearances aside, it is nearly identical.
“The only difference between the cartilage we’ve grown and the cartilage in your body is that ours was constructed from scratch as a tissue chip,” said Dr. Tuan. “It’s truly identical to human cartilage, meaning we’ll be able to use it to better understand joint diseases and test experimental treatments.”
Dr. Tuan and his team believe the microJoint could usher in the development of a new wave of effective, permanent treatments, in some cases even replacing options like opioid prescriptions and joint replacements.
Osteoarthritis is a leading cause of disability all around the world. It affects nearly one in six people in the United States, where more than $125 billion goes toward treatments to reduce pain or delay additional treatment. There is no cure; osteoarthritis is not preventable, and we are not sure what causes it to develop.
Dr. Shilpa Sant Receives 7-year, $2.7 million NIH R37 Award to Develop Three-Dimensional Organoid Models of Breast Cancer Progression

McGowan Institute for Regenerative Medicine affiliated faculty member Shilpa Sant, PhD, Assistant Professor of Pharmaceutical Sciences, has received a 7-year R37 MERIT Award from the National Cancer Institute (NCI), NIH, for her project entitled, “Three-dimensional organoid models to study breast cancer progression.” This grant is funded by the NCI under the Cancer Tissue Engineering Collaborative (TEC) Research Program that aims to support the development and characterization of state-of-the-art biomimetic tissue-engineered technologies for cancer research. The total funding for the first 5 years is $2.7 million.
The funded proposal aims to develop a three-dimensional in vitro organoid model that recapitulates key hallmarks of progression of non-invasive breast cancer to invasive disease. The goal is to discover key hypoxia-induced factors involved in initiation, maintenance, and spatial distribution of invasive breast cancer cells using 3D tumor organoid model and computational modeling approaches. Specifically, the proposal will use integrated experimental and computational approaches, coupled with deep learning-based image analysis, to delineate how hypoxia, tumor secretome, and intracellular signaling networks work together to induce the migratory phenotype and drive progression to invasive disease.
Currently, there are no prognostic biomarkers that can reliably predict the risk of progression from non-invasive to invasive breast cancer, leading to over-treatment of patients that are not at risk. The successful development of the proposed 3D organoid model will provide answers to two fundamental questions in the progression of invasive breast cancer:
- What causes some non-invasive breast cancer cells to become migratory and develop into invasive tumors?
- How and where does the migratory phenotype emerge?
The mechanistic understanding gained from these studies will improve diagnosis, prevent patient overtreatment, and lead to the development of treatment strategies to arrest invasion at the pre-invasive stage.
Co-principal investigators on the project include:
- Jianhua Xing, PhD, Associate Professor, Computational and Systems Biology
- Vera Donnenberg, PhD, Associate Professor of Cardiothoracic Surgery and Pharmaceutical Sciences, and McGowan Institute for Regenerative Medicine faculty member
- Simon Watkins, PhD, Distinguished Professor and Director of the Center for Biologic Imaging, and McGowan Institute for Regenerative Medicine affiliated faculty member
- Priscilla McAuliffe, MD, PhD, Assistant Professor of Surgery, University of Pittsburgh School of Medicine
Dr. Sant also holds appointments in the Department of Bioengineering and UPMC-Hillman Cancer Center.
Dr. Takashi Kozai Receives $390K NIH Grant to Develop Imaging Technology That May Improve Brain Implant Design

Chronic brain implants are long-term devices used to record brain activity or stimulate neurons with electrical pulses and are a crucial component of neuroprosthetics. The performance of these devices depends on the host tissue response, which is often inflammatory and results in device performance degradation. McGowan Institute for Regenerative Medicine affiliated faculty member Takashi Kozai, PhD, assistant professor of bioengineering at the University of Pittsburgh Swanson School of Engineering, was awarded an NIH R21 grant to improve device design by investigating the role of oligodendrocytes and oligodendrocyte progenitor cells in this process.
Dr. Kozai will work with Franca Cambi, MD, PhD, professor of neurology at Pitt, to develop in vivo imaging technology that will explore how these cells cause negative tissue response to chronic brain implants. Supported by the NIH’s National Institute of Neurological Disorders and Stroke, Drs. Kozai and Cambi received a two-year, $386,645 award for their research.
Dr. Kozai and his collaborators recently published work that reveals the importance of the brain’s glial cells. Oligodendrocytes and oligodendrocyte progenitor cells (OPCs) are a type of glia or connective tissue in the central nervous system that play an important role in brain injury and neuronal activity, including the body’s response to brain implants.
Oligodendrocytes are crucial for normal signaling in the brain. They produce proteins that help neurons grow, form synapses, and may even help neurons survive traumatic injuries. They play a key role in myelination, a process where oligodendrocytes wrap a fatty substance around the neuron’s axon to help insulate electrical signals and allow neural signals to move more rapidly.
“Oligodendrocytes, like neurons, consume enormous amounts of energy,” explained Dr. Kozai. “Neurons require the energy to maintain membrane potential, while oligodendrocytes require energy to maintain high production levels of protein and lipids. As a result, oligodendrocytes and neurons are one of the first cell types to die following brain injury.”
“Because the oligodendrocytes provide growth factors and support for neurons, the idea is maybe if we can help to oligodendrocytes to survive after injury, they can, in turn, help the neurons to survive,” said Dr. Kozai.
Drs. Kozai and Cambi hope to gain insight by getting a more detailed look at the life span of these cells using multiphoton imaging and neural engineering technology. Dr. Kozai said, “Much of the work on oligodendrocytes and OPCs has been carried out with post-mortem immunohistochemistry and molecular assays in disease models. As such, we only get a snapshot of the dead cells in their last moments, instead of seeing how and when they got there so that we can identify when and where to apply treatments and employ intervention strategies.”
By using in vivo imaging techniques like multiphoton imaging and pinpointing brain injury using neural engineering technology, Drs. Kozai and Cambi can map out the spatiotemporal relationships between oligodendrocyte loss, neuronal cell death, and OPC tissue repair and identify targets for intervention strategies, not just for brain implants, but also many neurodegenerative diseases.
Dr. Kurt Weiss Receives Pitt Seed Grant

Mario Cattabiani III, The Pitt News, recently reported on University of Pittsburgh’s first-ever Seed Grants. Pitt received 171 applications and from that group selected 23 projects for awards, granting a total of approximately $1 million. One project selected was submitted by McGowan Institute for Regenerative Medicine affiliated faculty member, Kurt Weiss, MD, Associate Professor of Orthopaedic Surgery in the University of Pittsburgh School of Medicine, Department of Orthopaedic Surgery, Division of Musculoskeletal Oncology, who received a $50,000 grant to fund the Pittsburgh Sarcoma Research Collaborative, or PSaRC — a project that will aim to develop new therapeutic strategies and eventually improve sarcoma patient care and outcomes.
“The Pitt Seed grant will help us on our goal to make the University of Pittsburgh a destination center for excellence for sarcoma research and treatment,” Dr. Weiss said.
PSaRC is a multidisciplinary team of researchers and physicians which focuses on sarcomas — rare cancerous tumors that afflict both children and adults. Treatment strategies and resulting outcomes for adult and pediatric sarcoma patients have not changed in the last 30 years — making them especially dangerous.
Congratulations, Dr. Weiss!
Fight-or-Flight Response Triggers White Blood Cells, Increases Heart Attack Risk in People with Diabetes
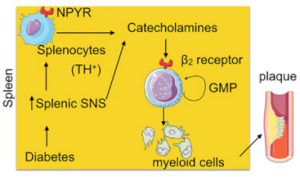
Stress hormones can save lives or take them. Released during an emergency or in pressure situations, stress hormones can gang up with white blood cells to fight infections or lead to heart attacks.
New research published in the journal Immunity from members of the University of Pittsburgh School of Medicine including McGowan Institute for Regenerative Medicine affiliated faculty members Mauricio Rojas, MD, and Kang Kim, PhD, shows that white blood cells, which typically heal infections and injuries, can become overactive and cause inflammation in plaques in blood vessels, making them vulnerable to rupture and hemorrhage in people with diabetes.
“If the rupture occurs in the coronary artery, the person has a heart attack. If the rupture occurs in the carotid artery, it causes a stroke,” said Partha Dutta, DVM, PhD, assistant professor of medicine at Pitt’s School of Medicine.
The major cause of death in people with diabetes is heart attack, and nearly one-third of the U.S. population has diabetes or prediabetes.
Studying patients and mice with diabetes, Dr. Dutta and his team found that when the fight-or-flight response is triggered, it also triggers the over-production of fighting white blood cells, or leukocytes. They also showed for the first time that stress hormones, called catecholamines, are produced in the spleen by a sub-group of white blood cells. Previous studies have shown that they were produced only by the sympathetic nervous system.
In the spleen, stress hormones trigger the proliferation of granulocyte macrophage progenitor cells, which in turn create inflammatory myeloid cells. Large amounts of inflammatory myeloid cells, which are linked to heart attack, were found in plaque in blood vessels. Further, the granulocyte macrophage progenitor cells expressed high levels of beta 2 receptors for stress hormones. Interestingly, patients who were taking beta 2 blockers were found to have fewer inflammatory cells.
“The findings suggest that diabetic patients could be treated with beta 2 blockers to reduce the number of inflammatory myeloid cells that cause plaque to rupture,” said Dr. Dutta, the senior author of the study. “If patients are on beta 2 blockers, the spleen will still be making the myeloid cells necessary to fight infection, but in smaller amounts.
Although decreasing white blood cells may be a problem for people with diabetes who are prone to infection, we need to weigh the cost of preventing infections and preventing death.
Dr. Rojas is an Associate Professor and Scientific Director of the Simmons Center for Interstitial Lung Diseases at the University of Pittsburgh. Dr. Kim is an Associate Professor of Medicine and of Bioengineering at the University of Pittsburgh and the Heart and Vascular Institute, UPMC.
Illustration: Immunity.
The Biomechanics Aspects of Cell Injections into the Brain

The lab of McGowan Institute for Regenerative Medicine affiliated faculty member Michel Modo, PhD, Professor in the Department of Radiology at the University of Pittsburgh with secondary appointments in the Department of Bioengineering and the Center for Neural Basis of Cognition, recently published a paper in Scientific Reports that characterizes the biomechanics aspects of cell injections into the brain. Dr. Modo shares the paper with the regenerative medicine community as it conceptualizes the delivery of therapeutic products through a small-bore needle, an issue commonly neglected for these types of therapies. The abstract of the paper reads:
Intracerebral implantation of cell suspensions is finding its clinical translation with encouraging results in patients with stroke. However, the survival of cells in the brain remains poor. Although the biological potential of neural stem cells (NSCs) is widely documented, the biomechanical effects of delivering cells through a syringe-needle remain poorly understood. We here detailed the biomechanical forces (pressure, shear stress) that cells are exposed to during ejection through different sized needles (20G, 26G, 32G) and syringes (10, 50, 250 μL) at relevant flow rates (1, 5, 10 μL/min). A comparison of 3 vehicles, Phosphate Buffered Saline (PBS), Hypothermosol (HTS), and Pluronic, indicated that less viscous vehicles are favorable for suspension with a high cell volume fraction to minimize sedimentation. Higher suspension viscosity was associated with greater shear stress. Higher flow rates with viscous vehicle, such as HTS reduced viability by ~10% and also produced more apoptotic cells (28%). At 5 μL/min ejection using a 26G needle increased neuronal differentiation for PBS and HTS suspensions. These results reveal the biological impact of biomechanical forces in the cell delivery process. Appropriate engineering strategies can be considered to mitigate these effects to ensure the efficacious translation of this promising therapy.
Pediatric Sepsis Care Within an Hour Decreases Chance of Death, Largest Ever Analysis Finds

More than one in 10 children hospitalized with sepsis die, but when a series of clinical treatments and tests is completed within an hour of its detection, the chances of survival increase considerably, according to a new analysis led by the University of Pittsburgh School of Medicine and co-authored by McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH. Dr. Angus is Chair of the Department of Critical Care Medicine of both the University of Pittsburgh School of Medicine and the UPMC Healthcare System.
The results, published in the Journal of the American Medical Association, support an initially controversial New York mandate established after 12-year-old Rory Staunton died from undiagnosed sepsis in 2012 following an infection from a scrape. These results will likely encourage the mandate’s expansion to other states.
“No child should die from a treatable infection,” said senior author Christopher W. Seymour, MD, MSc, associate professor in Pitt’s Department of Critical Care Medicine and member of Pitt’s Clinical Research Investigation and Systems Modeling of Acute Illness (CRISMA) Center. “This is the best evidence to date that prompt identification and treatment of suspected sepsis leads to better outcomes in children.”
Sepsis is a condition that arises when the body’s response to an infection injures its own tissues and organs. Children with sepsis deteriorate particularly quickly – looking relatively healthy one moment and needing life support within hours.
Rory’s Regulations require New York hospitals to follow protocols regarding sepsis treatment and submit data on compliance and outcomes. The hospitals can tailor how they implement the protocols but must include a blood culture to test for infection, and administration of antibiotics and fluids within an hour to any child suspected of sepsis.
Dr. Seymour and his team analyzed the outcomes of 1,179 children with sepsis reported at 54 New York hospitals. The children had an average age of just over 7 years, and 44.5 percent were healthy before developing sepsis. A total of 139 patients died.
Completion of the sepsis protocol within one hour decreased the odds of death by 40 percent. When only parts of the protocol were completed within an hour – for example, giving fluids but not testing for infection or giving antibiotics – the patients did not fare better. The finding held only if the entire protocol was completed in an hour.
“It’s clear that completing the entire sepsis protocol within an hour is associated with lower mortality,” said lead author Idris V.R. Evans, MD, assistant professor in Pitt’s Department of Critical Care Medicine. “But the mechanism of benefit still requires more study. Does each element of the protocol contribute to specific biologic or physiologic changes that, when combined, improve outcomes? Or is it that completion within an hour may simply be an indication of greater awareness by doctors and nurses caring for the child? Or could it be something else entirely?”
The researchers note that testing the sepsis protocol in a future randomized clinical trial will be very difficult. Such work would require leaving off some protocol elements for some septic children but not others in a random fashion – a design which would not currently align with the standard of care. But if more states adopt rules and regulations similar to New York’s, and also mandate data reporting, future work could expand on these results.
The findings support the current recommendations from the American College of Critical Care Medicine for care and treatment of pediatric sepsis.
At the University of Pittsburgh, Dr. Angus holds the rank of Distinguished Professor and the Mitchell P. Fink Endowed Chair in Critical Care Medicine with secondary appointments in Medicine, Health Policy and Management, and Clinical and Translational Science and he directs the CRISMA Center. He also co-directs the UPMC ICU Service Center, responsible for the provision of ICU services across the 20-plus hospital system.
AWARDS AND RECOGNITION
Dr. Fabrisia Ambrosio and Colleagues Receive the Aging Cell Best Paper Prize
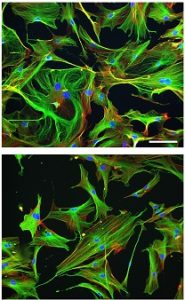
Aging Cell’s Best Paper Prize for 2017 honored the efforts of numerous McGowan Institute for Regenerative Medicine affiliated faculty members. The paper entitled “Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion,” appeared in the June 2017 issue of Aging Cell. The prize is awarded annually by the Anatomical Society on the recommendation of the Editor-in-Chiefs to the Lead Author and Co-Authors and is awarded to the paper considered to be the most outstanding publication in Aging Cell in that year by either a member or non-member of the Society.
The McGowan Institute affiliated faculty members (in alphabetical order) who are co-authors of this work include:
- Fabrisia Ambrosio, PhD, MPT, Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh with secondary appointments in the Departments of Physical Therapy, Bioengineering, Orthopaedic Surgery, and Microbiology & Molecular Genetics
- Antonio D’Amore, PhD, Research Assistant Professor in the Departments of Surgery and Bioengineering at the University of Pittsburgh
- Thomas Rando, MD, PhD, Professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, and the Director, Glenn Laboratories for the Biology of Aging, Stanford University, and the Deputy Director, Stanford Center on Longevity, also at Stanford University
- Rocky Tuan, PhD, Vice-Chancellor and President of The Chinese University of Hong Kong
- David Vorp, PhD, Associate Dean for Research, Swanson School of Engineering, University of Pittsburgh, and the John A. Swanson Professor of Bioengineering, with secondary appointments in the Departments of Cardiothoracic Surgery, Surgery, and the Clinical & Translational Sciences Institute at the University of Pittsburgh
- William Wagner, PhD, Director of the McGowan Institute for Regenerative Medicine as well as a Professor of Surgery, Bioengineering and Chemical Engineering at the University of Pittsburgh
Student authors include Drs. Kristen M. Stearns-Reider (formerly in Dr. Ambrosio’s lab and now at University of California, Los Angeles) and Alkiviadis Tsamis (formerly of Carnegie Mellon University and Pitt).
The paper summary reads:
Age‐related declines in skeletal muscle regeneration have been attributed to muscle stem cell (MuSC) dysfunction. Aged MuSCs display a fibrogenic conversion, leading to fibrosis and impaired recovery after injury. Although studies have demonstrated the influence of in vitro substrate characteristics on stem cell fate, whether and how aging of the extracellular matrix (ECM) affects stem cell behavior has not been investigated. Here, we investigated the direct effect of the aged muscle ECM on MuSC lineage specification. Quantification of ECM topology and muscle mechanical properties reveals decreased collagen tortuosity and muscle stiffening with increasing age. Age‐related ECM alterations directly disrupt MuSC responses, and MuSCs seeded ex vivo onto decellularized ECM constructs derived from aged muscle display increased expression of fibrogenic markers and decreased myogenicity, compared to MuSCs seeded onto young ECM. This fibrogenic conversion is recapitulated in vitro when MuSCs are seeded directly onto matrices elaborated by aged fibroblasts. When compared to young fibroblasts, fibroblasts isolated from aged muscle display increased nuclear levels of the mechanosensors, Yes‐associated protein (YAP)/transcriptional coactivator with PDZ‐binding motif (TAZ), consistent with exposure to a stiff microenvironment in vivo. Accordingly, preconditioning of young fibroblasts by seeding them onto a substrate engineered to mimic the stiffness of aged muscle increases YAP/TAZ nuclear translocation and promotes secretion of a matrix that favors MuSC fibrogenesis. The findings here suggest that an age‐related increase in muscle stiffness drives YAP/TAZ‐mediated pathogenic expression of matricellular proteins by fibroblasts, ultimately disrupting MuSC fate.
Congratulations to all!
Illustration: Cytoskeletal phenotype of young and old skeletal muscle fibroblasts. Aging Cell.
Federspiel Lab Student Receives Willem Kolff Award at ASAIO Annual Meeting

The American Society for Artificial Internal Organs (ASAIO) selected Alexandra May, a chemical engineering graduate student at the University of Pittsburgh, as a finalist for the Willem Kolff Award at its 64th annual meeting. The award, named after the late Dutch physician who invented the original artificial kidney, recognizes the top abstracts at each annual meeting.
Ms. May is a graduate student in the Swanson School of Engineering’s Cardiovascular Bioengineering Training Program and works in the Medical Devices Laboratory under the direction of McGowan Institute for Regenerative Medicine faculty member William Federspiel, PhD, a William Kepler Whiteford Professor of Bioengineering at Pitt. The lab develops clinically significant devices for the treatment of pulmonary and cardiovascular ailments by utilizing engineering principles of fluid flow and mass transfer.
Ms. May’s research focuses on the development of the Pittsburgh Pediatric Ambulatory Lung (P-PAL), an artificial lung device developed to bridge pediatric acute or chronic lung failure patients to transplant. The P-PAL integrates the blood pump and gas exchanging hollow fiber membrane bundle into a single compact unit and provides 70 percent to 90 percent of the patient’s oxygenation needs. The compact design of the P-PAL provides children with increased mobility pre-transplant, a factor which has been shown to improve post-transplant outcomes.
The ASAIO Annual Meeting was held June 13-16, 2018, in Washington, D.C. Ms. May’s abstract titled “Acute in vivo Performance of a Pediatric Ambulatory Artificial Lung” was awarded second place out of approximately 300 accepted abstracts, and she presented her work during the conference’s opening general session. Co-authors on the abstract include R. Orizondo, B. Frankowski, P. Wearden, and W. Federspiel. Peter Wearden, MD, PhD, congenital cardiothoracic surgeon and Department Chair, Division of Cardiovascular Surgery, Department of Cardiovascular Services at the Nemours Children’s Health System, Orlando, Florida, is an affiliated faculty member of the McGowan Institute.
“Alex deserves this recognition,” said Dr. Federspiel. “She is an extremely hard worker and devoutly dedicated to our mission of improving the lives of kids with respiratory failure.”
Congratulations, Ms. May!
Illustration: Alexandra May.
Work of Badylak Lab Students Highlighted on Cover of Nature Review Materials

McGowan Institute for Regenerative Medicine congratulates George Hussey, PhD, and Jenna Dziki, PhD student, on their newly published Nature Reviews Materials article entitled, “Extracellular matrix-based materials for regenerative medicine.” It is featured on the cover of the July 2018 issue of the journal with a 3D image of the matrix network by Visuals Unlimited, Inc./Dr. Edna Cukierman.
They worked very hard putting together what co-author and McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, believes is “the best manuscript written to date on biologic scaffolds for regenerative medicine. It will be highly cited in future years.”
Congratulations to all!
The abstract reads:
In tissue engineering and regenerative medicine, a biomaterial provides mechanical support and biochemical signals to encourage cell attachment and modulate cell behaviour. Nature’s template for a biomaterial is the extracellular matrix (ECM). The ECM contains intrinsic biochemical and mechanical cues that regulate cell phenotype and function in development, in homeostasis and in response to injury. The use of ECM-based materials in biomedical research has advanced from coating cell culture plates with purified ECM components to the design of ECM-mimicking biomaterials and the engineering of decellularized tissues aimed at recapitulating the dynamics, composition and structure of the ECM. In this Review, we highlight important matrix properties and functions in the context of tissue engineering and regenerative medicine, consider techniques such as proteomics for the investigation of matrix structure and composition and discuss different engineering strategies for the design of matrix-mimicking biomaterials. Tissue, whole organ and cell culture decellularization approaches are examined for their potential to preserve the tissue-specific biochemical composition and ultrastructure of the ECM and for the development of biomaterials that promote the formation of functional tissues in clinical applications. Finally, we investigate challenges and opportunities of ECM biomaterials for the design of organotypic models to study disease progression, for the ex vivo creation of engineered tissue and for the clinical translation of functional tissue reconstruction strategies in vivo.
Illustration: The extracellular matrix is nature’s template for an ideal biomaterial to guide tissue homeostasis and repair. In this Review, matrix-mimicking biomaterials and decellularized matrices are discussed for their potential to reconstruct and repair tissues in vitro and in vivo. Image: Visuals Unlimited, Inc./Dr. Edna Cukierman.
Dr. John Pollock Receives Presidential Award

Duquesne University professor John Pollock, PhD, has received the Presidential Award for Excellence in Science, Mathematics and Engineering Mentoring to honor his career in educating students from universities to grade schools. Dr. Pollock is an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
The White House Office of Science and Technology Policy (OSTP) and the National Science Foundation (NSF) announced that Dr. Pollock, along with 27 individuals and 14 organizations, was honored with presidential awards for their excellence in teaching or mentoring in science, technology, engineering and mathematics (STEM) fields. The award is the highest honor bestowed upon mentors who work to expand STEM talent, according to NSF.
The awards committee noted Dr. Pollock’s 36-year career in teaching university courses in neuroscience and biology, conducting research while mentoring high school and college students and his work in developing educational and multimedia resources for school children, including Emmy® Award winning educational apps.
“I’m honored to be selected for the presidential award,” said Dr. Pollock, biological sciences professor in Duquesne’s Bayer School of Natural and Environmental Sciences (BSNES). “More than ever, science is playing an increasingly important role in solving societal problems. It’s vital that we increase access to STEM education for all children so future generations can benefit from advances in health care and technology, to name just two areas. We need all hands on deck.”
Dr. Pollock has mentored more than 150 students, with about 25 percent from ethnic or racial groups who are underrepresented in STEM fields. Virtually all of his mentees have gone to pursue advanced degrees with many working in academia, industry and health care.
In the community, he helped to start science summer camps for children from underserved areas, reads with 4- and 5-year old children weekly and serves museums in Pittsburgh and beyond. Dr. Pollock has also produced a wide range of educational resources, including museum and traveling exhibits and interactive software and video games for the classroom with pre-college scholars, undergraduate students and post-graduate student mentees.
“John Pollock goes above and beyond to educate students about science and technology,” said Dr. Philip Reeder, dean of BSNES. “Through his work in the classroom, research labs and community, he has positively impacted thousands of students’ lives. I’m thrilled to see John receive this well-deserved honor.”
In addition to his teaching position, Dr. Pollock is co-director of Duquesne’s Chronic Pain Research Consortium and Director of the Partnership in Education, a STEM education and health literacy research group that has developed apps on topics including why we need sleep, sports-related concussions and health literacy for pediatric transplant patients.
During a visit to the nation’s capital, Dr. Pollock received a presidential citation at an awards ceremony and participated in discussions on STEM and STEM education priorities at the White House State-Federal STEM Summit led by OSTP and NSF. He will also receive $10,000 from NSF, which manages the award program on behalf of the White House.
Congratulations, Dr. Pollock!
Badylak Lab Student to Receive the 2018 Mary Ann Liebert, Inc. Outstanding Student Award

McGowan Institute for Regenerative Medicine is pleased to announce that Catalina Pineda Molina, a doctoral student within the Bioengineering Department at the University of Pittsburgh and a graduate researcher in the laboratory of McGowan Institute Deputy Director Stephen Badylak, DVM, PhD, MD, will be awarded the 2018 Mary Ann Liebert, Inc. Outstanding Student Award during the upcoming 2018 TERMIS World Congress. The 2018 TERMIS World Congress is scheduled for September 4-7, 2018, and will be held in Kyoto, Japan.
Ms. Molina’s award-winning manuscript will be published in the journal, Tissue Engineering, Part A. Her research interests are focused on the immune-modulatory effects of adipose derived stem cells used in combination with extracellular matrix scaffolds for regenerative medicine applications.
At the award presentation ceremony, Ms. Molina will be presented with a plaque recognizing her outstanding research accomplishments within the tissue engineering and regenerative medicine field.
Congratulations, Ms. Molina!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#185 –– Dr. David Vorp discusses his research in designing a small diameter tissue-engineered vascular graft to treat cardiovascular diseases.
#186 –– Dr. Lauren Black discusses his research in designing and developing new methods for repairing diseased or damaged myocardium. He also discuses his work with the Journal of Immunology and Regenerative Medicine.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Siddam AD, Gautier-Courteille C, Perez-Campos L, Anand D, Kakrana A, Dang CA, Legagneux V, Méreau A, Viet J, Gross JM, Paillard L, Lachke SA
Title: The RNA-binding protein Celf1 post-transcriptionally regulates p27Kip1 and Dnase2b to control fiber cell nuclear degradation in lens development
Summary: Opacification of the ocular lens, termed cataract, is a common cause of blindness. To become transparent, lens fiber cells undergo degradation of their organelles, including their nuclei, presenting a fundamental question: does signaling/transcription sufficiently explain differentiation of cells progressing toward compromised transcriptional potential? We report that a conserved RNA-binding protein Celf1 post-transcriptionally controls key genes to regulate lens fiber cell differentiation. Celf1-targeted knockout mice and celf1-knockdown zebrafish and Xenopus morphants have severe eye defects/cataract. Celf1 spatiotemporally down-regulates the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 by interacting with its 5′ UTR and mediating translation inhibition. Celf1 deficiency causes ectopic up-regulation of p21Cip1. Further, Celf1 directly binds to the mRNA of the nuclease Dnase2b to maintain its high levels. Together these events are necessary for Cdk1-mediated lamin A/C phosphorylation to initiate nuclear envelope breakdown and DNA degradation in fiber cells. Moreover, Celf1 controls alternative splicing of the membrane-organization factor beta-spectrin and regulates F-actin-crosslinking factor Actn2 mRNA levels, thereby controlling fiber cell morphology. Thus, we illustrate new Celf1-regulated molecular mechanisms in lens development, suggesting that post-transcriptional regulatory RNA-binding proteins have evolved conserved functions to control vertebrate oculogenesis.
Source: PLoS Genet. 2018 Mar 22;14(3):e1007278. eCollection 2018 Mar.
GRANT OF THE MONTH
PI: Shilpa Sant
Title: Three-dimensional organoid models to study breast cancer progression
Description: Approximately 20% of breast cancers detected through mammography are pre-invasive Ductal Carcinoma in situ (DCIS). If left untreated, approximately 20-50% of DCIS will progress to more deadly Invasive Ductal Carcinoma (IDC). No prognostic biomarkers can reliably predict the risk of progression from DCIS to IDC. Similar genomic profiles of matched pre-invasive DCIS and IDC suggests that the progression is not driven by genetic aberrations in DCIS cells, but microenvironmental factors, such as hypoxia and metabolic stress prevalent in DCIS, may drive the transition. We need innovative models to investigate how to halt steps of DCIS progression to invasive phenotypes and subsequent metastasis from the primary site. This proposal directly addresses this unmet need by developing a novel three-dimensional in vitro organoid model that recapitulates key hallmarks of DCIS to IDC progression: tumor-size induced hypoxia and metabolic stress, tumor heterogeneity and spontaneous emergence of migratory phenotype in the same parent cells without any additional stimulus. A tangible advantage of the proposed organoid models is the ability to precisely and reproducibly study how the hypoxic microenvironment induces tumor migration in real time and in isolation from non-tumor cells present in vivo, providing unique opportunity to define tumor-intrinsic mechanisms of DCIS to IDC progression. Our preliminary observations lead to central hypothesis that tumor size-induced hypoxia establishes a “hypoxic secretome”, which initiates the migratory phenotype; the hypoxic secretome then cooperate with intracellular signaling networks to independently maintain cell migration. We propose three independent but inter-related aims to link hypoxic secretome with the initiation, maintenance and spatial distribution of migratory phenotypes. Aim 1 will engineer size-controlled DCIS organoids (150-600 µm) with controlled hypoxic microenvironments to identify and examine how hypoxic secretome initiates migratory phenotype. We will combine experimental organoid models with time-lapse imaging and computational approaches to study organoid migration. Aim 2 will demonstrate that migratory cells can re-establish the secretome and maintain migratory phenotype independent of hypoxia. We will reconstruct an intracellular signaling network activated by the hypoxic secretome using microarray data. We will verify these gene expression signatures in sorted migratory and non-migratory cells, and validate them using secretome inhibition studies. Aim 3 will investigate, for the first time, the spatial distribution and origin of the migratory phenotype. We will use CRISPR-based gene knock-in (FP-labeling), automated image analyses, and a deep-learning algorithm to track and visualize the emergence of migratory phenotypes from the hypoxic core outward to the periphery or from the migratory front. The successful development of this 3D organoid model and completion of the proposed work will provide answers to two fundamental questions in the progression of invasive breast cancer: 1) What causes some DCIS cells to become migratory and develop into invasive tumors? 2) How and where does the migratory phenotype (IDC) emerge? The mechanistic understanding gained from these studies will improve diagnosis, lead to the development of treatment strategies to arrest invasion at the pre-malignant stage, and thus prevent patient overtreatment. It is straightforward to generalize our system to other tumor types, development of tumor/stromal co-culture, and drug screening.
Source: National Cancer Institute
Amount: $445,227 (2018)
Term: July 1, 2018 – June 30, 2023
