July 2017 | VOL. 16, NO. 7| www.McGowan.pitt.edu
Nanoscale Forces Measured in Aortic Smooth Muscle Cells Tell Story of Disease
 Researchers from Virginia Tech and the University of Pittsburgh have collaborated to employ a novel nanoscale fibrous system that can measure the tiny forces exerted by and upon individual cells with extreme precision. The team hopes that this platform, which investigators call nanonet force microscopy (NFM), will provide new knowledge about smooth muscle cell biology that could have implications for treating cardiovascular disease, which is still a leading cause of death in the United States.
Researchers from Virginia Tech and the University of Pittsburgh have collaborated to employ a novel nanoscale fibrous system that can measure the tiny forces exerted by and upon individual cells with extreme precision. The team hopes that this platform, which investigators call nanonet force microscopy (NFM), will provide new knowledge about smooth muscle cell biology that could have implications for treating cardiovascular disease, which is still a leading cause of death in the United States.
The results of investigations on cells using this platform appear in the “Forces” issue of the journal Molecular Biology of the Cell, in the article “Nanonet Force Microscopy for Measuring Forces in Single Smooth Muscle Cells of Human Aorta.” (see “Publication of the Month” below for additional details)
The main goal of this current study, said McGowan Institute for Regenerative Medicine affiliated faculty member Julie Phillippi, PhD, assistant professor at the University of Pittsburgh Department of Cardiothoracic Surgery whose laboratory provided healthy human patient smooth muscle cells for the study, was to quantify forces that healthy cells experience in various conditions of stress. The fibrous nanonet itself was designed in the mechanical engineering laboratory of Amrinder Nain, PhD, associate professor at Virginia Tech and member of the American Society for Cell Biology. Forces measured using NFM, Dr. Nain said, include forces exerted by the cells themselves and forces exerted by the environment on the cells. “Everything in nature exerts and experiences a physical force,” said Dr. Nain. “This platform measures both simultaneously.”
Dr. Phillippi said that previous work tested the mechanical strength of whole aortic tissue and understanding the single cell biomechanics is vitally important. Single-cell studies provide insight into the proteins involved in the fleeting so-called focal adhesions that most cells make as they move around their microenvironment. The NFM assembly aims to mimic, in as physiologically relevant a way as possible, what cells endure within the collagen fibers of the extracellular matrix (ECM) — the matrix that supports cell growth in living things. Tweaking the artificial matrix by changing fiber diameter, density, and spacing in a controlled and repeatable manner, as well as using cells from diseased patients at different disease severities, will allow Drs. Phillippi and Nain to simulate the conditions experienced by cells in many realistic situations.
“We have looked very closely at how the collagen and elastin fibers in the ECM are arranged and the micro-architecture and everything points to these microstructural defects in the ECM contributing to the weakening of the aortic walls and the ballooning of the vessel,” said Dr. Phillippi. “What we don’t know is, are these ECM proteins arranged that way from birth or is it something that happens over time? Or is it both? What role do the cells play? This engineered platform will allow us to answer some of those questions.” Furthermore, Dr. Nain said, NFM could reveal the heterogeneity of cells taken from the same patient or from different patients with the same disease state down to the single-cell resolution.
Next steps for Drs. Phillippi and Nain include testing cells from the Pittsburgh team’s large repository of aortic specimens from patients, collected in collaboration with McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Gleason, MD, the Ronald V. Pellegrini Endowed Professor and Chair of Cardiothoracic Surgery and Chief of the Division of Cardiac Surgery, University of Pittsburgh School of Medicine, to establish a database of baseline forces for many types of cells that researchers and clinicians can use to diagnose and treat disease. “The platform gives us the ability to create in vitro disease models with multiple layers of sophistication,” said Dr. Phillippi.
In a broader context, the ability to achieve precise control on fiber diameter, spacing, and orientation to mimic native fibrous environments, will allow NFM to interrogate the push and pulls in a cell’s journey in developmental, disease, and repair biology.
RESOURCES AT THE MCGOWAN INSTITUTE
August Histology Special
Reticular fibers, or reticulin is a type of fiber in connective tissue, composed of type III collagen secreted by reticular cells. Reticular fibers crosslink to form a fine meshwork (reticulin). This network acts as a supporting mesh in soft tissues such as liver, bone marrow, and the tissues and organs of the lymphatic system.
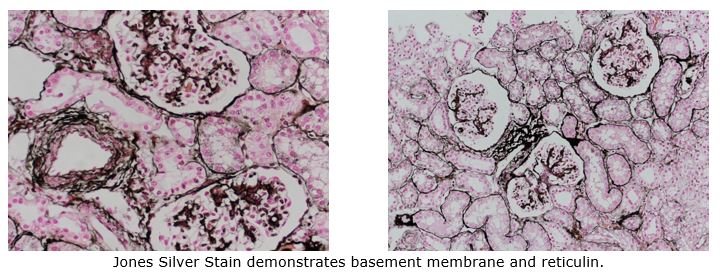
You’ll receive 25% off Jones Silver Stain in August when you mention this ad.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
Dr. Paulo Fontes and OrganEvac Receive Funding from Pitt and UPMC Liver Center
 The McGowan Institute for Regenerative Medicine congratulates deputy director Paulo Fontes, MD, Professor of Surgery in the Department of Surgery and director of the Machine Perfusion Program, a collaborative effort between the Starzl Transplantation Institute, Department of Surgery, and the McGowan Institute, on his award of $50,000 from the Coulter@Pitt TPII Program, the Center for Medical Innovation (CMI), and the University of Pittsburgh and UPMC Liver Center for a full year starting July 1, 2017 and ending June 30, 2018. Dr. Fontes is the Co-PI of this study.
The McGowan Institute for Regenerative Medicine congratulates deputy director Paulo Fontes, MD, Professor of Surgery in the Department of Surgery and director of the Machine Perfusion Program, a collaborative effort between the Starzl Transplantation Institute, Department of Surgery, and the McGowan Institute, on his award of $50,000 from the Coulter@Pitt TPII Program, the Center for Medical Innovation (CMI), and the University of Pittsburgh and UPMC Liver Center for a full year starting July 1, 2017 and ending June 30, 2018. Dr. Fontes is the Co-PI of this study.
Dr. Fontes and PI Christopher Hughes, MD, Associate Professor of Surgery at Pitt, demonstrated to the program’s Oversight Committee (OC) that OrganEvac satisfies a large unmet clinical need and that, in the OC’s judgement, there is likely a viable path to commercialization.
The Coulter Program’s mission is to help translate promising technologies to clinical practice through commercial products, with the goal of improving healthcare and patient outcomes. To do so requires tremendous effort on the part of the innovators and subsequent business teams to address the challenges of product development, clinical trials, regulatory approvals, raising capital, and a myriad of marketing and economic issues.
NIH Continues Pitt’s Cardiovascular Bioengineering Training Program
 The National Institutes of Health (NIH) has renewed funding for the University of Pittsburgh Department of Bioengineering’s Cardiovascular Bioengineering Training Program (CBTP). The program – which educates students who are interested in cardiovascular research and pursuing a PhD in bioengineering – will receive nearly $1.9 million over the next 5 years.
The National Institutes of Health (NIH) has renewed funding for the University of Pittsburgh Department of Bioengineering’s Cardiovascular Bioengineering Training Program (CBTP). The program – which educates students who are interested in cardiovascular research and pursuing a PhD in bioengineering – will receive nearly $1.9 million over the next 5 years.
McGowan Institute for Regenerative Medicine faculty member Sanjeev Shroff, PhD, the Distinguished Professor of and Gerald McGinnis Chair in Bioengineering at Pitt, established the CBTP in 2005 to train bioengineering doctoral students for careers in basic and/or translational cardiovascular research. By renewing the grant, the NIH has guaranteed funding until 2022.
“A unique feature of the program is that students are exposed first-hand to real-world clinical problems requiring bioengineering input for their solution,” said Dr. Shroff, who serves as principal investigator for the program. “The program is designed to provide students both breadth and depth in engineering and biological sciences and also includes a formal exposure to biostatistics, bioethics, and professional and career development issues. Upon completion, students are well-versed in both basic and clinical aspects of cardiovascular engineering and are well prepared for rewarding careers in a growing field.”
Student research within Pitt’s CBTP has focused on a variety of problems, ranging from basic science to novel biomedical technologies for the diagnosis and/or treatment of critical cardiovascular health issues. Examples of these research projects include:
- Regulation of cardiac muscle contraction by cardiac troponin-I phosphorylation
- Mechanical processes and pathways that underlie heart morphogenesis
- Molecular and cellular mechanisms underlying vaso-occlusion in Sickle Cell Disease
- Role of Profilin-1 in angiogenesis
- Externally regulated synthetic capillary system for promoting angiogenesis
- Rapidly degrading synthetic materials for tissue-engineered vascular grafts
- Extracellular matrix (ECM) scaffolds for heart tissue regeneration
- Adipose stem cell-based treatments for abdominal aortic aneurysms
- Improved biocompatibility of implanted cardiovascular devices to reduce rejection
- Coacervate-based controlled delivery of growth factors for cardiac repair
- Thermal strain imaging for non-invasive identification of vulnerable atherosclerotic plaques
The NIH National Heart, Lung, and Blood Institute provides funding for the program and has designated the grant a National Research Service Award. These awards are granted to training programs in disciplines that address the nation’s biomedical, behavioral, and clinical research needs with an emphasis on producing diverse pool of highly trained scientists.
Each student in the CBTP receives a monthly stipend, tuition scholarship, health insurance, and a travel budget.
About the Cardiovascular Bioengineering Training Program
The goal of the Cardiovascular Bioengineering Training Program (CBTP) is to provide a solid foundation upon which to build a productive independent career in cardiovascular bioengineering. This is accomplished via a highly coordinated and mentored interdisciplinary training program with a combination of core and elective courses, clinical internships, research activities, and specialized training opportunities to enhance professional and career development. There are three focus areas of this program:
- Basic understanding and quantitative characterization of native (normal and pathological conditions) and perturbed (i.e., with deployment of man-made devices or constructs) cardiovascular function at various levels of organization (cell, tissue, whole organ)
- Imaging for functional assessment at various levels of organization (cell, tissue, whole organ)
- Design and optimization of artificial devices and constructs (mechanical, tissue-engineered, and hybrid)
Introducing the Vascularized Composite Allotransplantation and Microsurgery (VCAM) Laboratory

From the desk of J. Peter Rubin, MD, FACS – UPMC Endowed Professor, Chair of Plastic Surgery, Professor of Bioengineering, and McGowan Institute faculty member:
I would like to take the opportunity to announce the renaming of our Vascularized Composite Allotransplantation (VCA) Laboratory to the “Vascularized Composite Allotransplantation and Microsurgery (VCAM) Laboratory.” This new name reflects not only the unique expertise in state-of-the-art VCA research, but also emphasizes the lab’s advanced microsurgical capabilities.
The VCAM Laboratory Co-Directors, Mario G. Solari, MD, and Kia M. Washington, MD, are exceptional leaders in VCA and both highly skilled reconstructive microsurgeons who can address the most difficult reconstructive problems in both the operating room and apply their skills to large and small animal models. They remain committed to advanced basic science and translational research, and are always happy to discuss collaborations both within our University research community and with investigators at other institutions. Notably, the advanced microsurgical capabilities can enable the completion of surgical models employed in research projects across numerous scientific disciplines. Our clinical VCA program remains active with the United Network for Organ Sharing (UNOS) and our commitment to translational research in VCA is stronger than ever.
The Vascularized Composite Allotransplantation and Microsurgery (VCAM) Laboratory capabilities include:
- Microsurgical transplantation of vascularized tissues, such as facial tissue, whole eye, limb, and the individual components of muscle, nerve, skin, and bone;
- State-of the-art immunological techniques including cellular assays, flow cytometry, rtPCR, and cytokine analysis;
- Nerve histomorphometery, conduction studies, and functional testing.
Key statistics of the lab include:
- Over $6 million in active funding from the Department of Defense and other sources including funding from the Joint Warfighter Medical Research Program for whole eye transplant research;
- Experienced team of 3 Principal investigators, 1 MD/PhD Transplant Immunologist, 5 microsurgeons, 3 postdoctoral fellows, and 1 Program Coordinator;
- Six operating microscopes, making this one of the best equipped microsurgical labs in the nation;
- Large and small animal surgery capabilities.
We now start a new era of scientific research, building and expanding upon the numerous successes of our VCAM Laboratory team. Dr. Solari and Dr. Washington look forward to continuing current collaborations and establishing new ones where their expertise in microsurgery and transplant immunology can be of value. I have no doubt that the Vascularized Composite Allotransplantation and Microsurgery (VCAM) Laboratory will flourish under their leadership.
Working the [Immune] System

As a rule, implants and the immune system don’t get along. The human body recognizes these materials as foreign substances and tries to fight them like a virus or bacteria. Although this response can cause trouble for doctors and patients, new research at the University of Pittsburgh suggests the immune system can assist the body in accepting implanted biomaterials.
The National Institute on Aging, one of the 27 Institutes and Centers of the National Institutes of Health (NIH), has awarded McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, assistant professor of bioengineering at Pitt’s Swanson School of Engineering, a 5-year, $1.57 million R01 grant to examine how aging affects implantable medical devices. This is the second R01 grant from the NIH Dr. Brown has received this year to support his research of implantable materials.
His study, “Assessing the Impacts of Aging upon the Macrophage Response to Implantable Materials,” will specifically address reactions to implantable medical devices by the aged body, including immunosenescence (a deterioration of the immune system brought on by aging), dysregulation of white blood cell function and polarization, and delayed resolution of acute immune responses.
“The impacts of aging on individuals with implants have never been investigated,” said Dr. Brown. “As Baby Boomers in particular age, the number of people over 65 will grow, and more than 75 percent of these individuals have at least one chronic condition, usually associated with inflammation. We need therefore a better understanding of how aging affects the immune system’s responses to implants.”
Dr. Brown will build upon earlier research in which he demonstrated that the host inflammatory response is critical to the success and function of implants. His study found that the first week of macrophage activity (a type of white blood cell) at the implant site could predict immune system response as far as 90 days down the road. By controlling the immune system response, Dr. Brown and his team are looking for the best way to lengthen the lifespan of implants and minimize the negative effects of implanting a foreign object into the body.
“Really, the challenges are not fully known,” explained Dr. Brown. “Many implants are used primarily in older individuals, so there is not always a point of comparison. However, in our previous work, we have demonstrated that the host inflammatory response is critical to success and function of implants. Therefore, we are trying to define changes in aged individuals to develop informed approaches to improving implant function in this population. With a projected two billion individuals over the age of 65 by 2050, optimizing the success of implants that can treat a wide range of illnesses could result in significant benefits for patients in their golden years.”
Illustration: Dr. Brown (middle) in the lab with Pitt BioE graduate students Alexis Nolfi (left) and Samuel LoPresti (right). University of Pittsburgh.
Phase 1 Clinical Trial for the Treatment of Severe Neurological Diseases Begins

Recently, a Phase 1 clinical trial for the treatment of severe neurological diseases began with its first patient with McGowan Institute for Regenerative Medicine affiliated faculty member Mark Richardson, MD, PhD, Director of Epilepsy and Movement Disorders Surgery at the University of Pittsburgh Medical Center and an investigator in the trial, performing the surgical delivery approach. The trial by Voyager Therapeutics, Inc., a clinical-stage gene therapy company developing life-changing treatments for severe neurological diseases, aims to further optimize the surgical delivery of VY-AADC01 for advanced Parkinson’s disease.
This planned Phase 1 trial explores a posterior (i.e., back of the head) surgical delivery approach, compared to Cohorts 1-3 from a separate, ongoing Phase 1b trial that used a transfrontal (i.e., top of the head) surgical delivery approach, into the putamen, a specific region of the brain targeted by Voyager’s gene therapy program. A posterior approach could better align the infusion of VY-AADC01 with the anatomical structure of the putamen to potentially reduce the total procedure time and increase the total coverage of the putamen. The administration of VY-AADC01 with this posterior approach was well-tolerated by the patient. No serious adverse events were reported and the patient was discharged from the hospital the day after surgery.
“We are excited to successfully dose the first patient with this new surgical delivery approach,” said Dr. Richardson. “Reducing the number of bilateral surgical infusions from two with the transfrontal approach to one with the posterior approach is likely to reduce the total procedure time for this innovative program to treat advanced Parkinson’s disease.”
“Along with the potential to reduce the procedure time, this approach may also increase the coverage of the brain region we are targeting with VY-AADC01,” said Bernard Ravina, MD, chief medical officer at Voyager. “Preliminary total procedure time and putaminal coverage data from this trial that continues to enroll will be available during the third quarter of 2017 and will help inform the design of the pivotal Phase 2-3 program planned to initiate during late 2017.”
The clinical trial approach with VY-AADC01 has the potential to durably enhance the conversion of levodopa to dopamine and provide clinically meaningful improvements in motor symptoms in Parkinson’s disease following a single administration.
Pitt School of Medicine Signs Collaborative Agreement with World-Renowned French Research Institutions

The University of Pittsburgh School of Medicine has entered into an agreement with three world-renowned French research institutions—the University Pierre et Marie Curie of the Sorbonne Universités in Paris, the Institut National de la Santé et de la Recherche Médicale (Inserm), and the Centre National de la Recherche Scientifique (CNRS)—to focus on collaborative research and education in the fields of medicine and biomedical sciences.
The agreement will enable researchers of all four institutions to cooperate on fundamental research, development of novel therapeutics, and clinical trials, with an initial focus on ophthalmology, vision, and neuroscience. Along with joint research, the agreement also emphasizes exchange of academic personnel, joint academic conferences, and exchange of scientific, educational, and scholarly materials.
The agreement, signed on July 12 at the French Embassy in Washington, DC, highlights an important partnership between Pitt and the French institutions that was spurred by the recent recruitment of José-Alain Sahel, MD, one of the world’s top experts in retinal diseases, as the chair of the Department of Ophthalmology at Pitt’s School of Medicine, director of the UPMC Eye Center, the Eye and Ear Foundation Chair of Ophthalmology, and McGowan Institute for Regenerative Medicine affiliated faculty member. Dr. Sahel retained his connections to Paris as the founder and director of the Institut de la Vision in Paris and as a professor at the Université Pierre-et-Marie-Curie of the Sorbonne Universités (which co-incidentally also is referred to by the acronym UPMC), a top ranked medical school and the largest scientific and medical complex in France.
Inserm, the French National Institute of Health and Medical Research, is the only public research institution solely focused on human health and medical research in France and a leading medical research agency worldwide; and CNRS, the French National Center for Scientific Research, is the largest governmental research organization in France and the largest fundamental science agency in Europe.
“This agreement will further strengthen the robust scientific and educational partnerships between Pittsburgh and Paris, bringing to bear our outstanding intellectual capacities to address some of the most significant diseases that lead to blindness and vision impairment through basic and translational research,” said Dr. Sahel
“Taking on an immense challenge like the quest to cure blindness requires that we not only have bold ideas, but also the brightest minds to work on them. The University of Pittsburgh is proud to be a part of this international partnership that will bring together the world-class scientific community at Pitt with researchers from France under the able leadership of Dr. Sahel,” said Arthur S. Levine, MD, Pitt’s senior vice chancellor for the health sciences and John and Gertrude Petersen Dean of Medicine.
Illustration: Signatories of the agreement between Pitt and the French research institutions pose for the cameras at the conclusion of the signing ceremony. (L to R) Dr. Sidney Wiener, Scientific Officer, International Affairs, CNRS; Dr. José-Alain Sahel, Professor and Chair of Ophthalmology, University of Pittsburgh School of Medicine; Gérard Araud, ambassador of France to the United States; Dr. Jean Chambaz, President, University Pierre et Marie Curie of the Sorbonne Universités; Dr. Yves Levy, President and CEO of Inserm; Dr. Arthur Levine, Pitt’s senior vice chancellor for the health sciences and John and Gertrude Petersen Dean of Medicine. UPMC/University of Pittsburgh Schools of the Health Sciences.
Earliest Molecular Events Leading to Organ Rejection Identified in Preclinical Studies

Researchers at the University of Pittsburgh School of Medicine and the University of Toronto have uncovered the first molecular steps that lead to immune system activation and eventual rejection of a transplanted organ. The findings, published recently in Science Immunology, may be used someday to create better donor-recipient matches and develop new ways to prevent rejection of transplanted tissues.
McGowan Institute for Regenerative Medicine affiliated faculty member Jeffrey Isenberg, MD, MPH, Associate Professor at the University of Pittsburgh in the Department of Medicine, with secondary appointments in the Department of Pharmacology and Chemical Biology, and the Department of Bioengineering in the Swanson School of Engineering, is a co-author on the study.
Approximately 50 percent of all transplanted organs are rejected within 10 to 12 years, so there is a great need for better ways to reduce or eliminate organ rejection, explained the study’s co-senior author Fadi Lakkis, MD, Frank & Athena Sarris Chair in Transplantation Biology and scientific director of Pitt’s Thomas E. Starzl Transplantation Institute (STI).
“For the first time, we have an insight into the earliest steps that start the rejection response,” Dr. Lakkis said. “Interrupting this first recognition of foreign tissues by the innate immune system would disrupt the rejection process at its earliest inception stage and could prevent the transplant from failing.”
UPMC has been a worldwide leader in organ transplantation for more than 35 years. Throughout its history, the University of Pittsburgh Transplantation Institute, renamed the STI in 1996 in honor of liver transplant pioneer Dr. Thomas E. Starzl, has been creating innovative transplantation strategies.
“This study is the latest example of the STI’s commitment to improving the lives of patients with organ failure,” Dr. Lakkis said.
The immune system is composed of innate and adaptive branches. The innate immune system is the first to detect foreign cells in the body and is required to activate the adaptive, or acquired, immune system. The mechanisms underlying this second phase of immune activation following organ transplantation are well studied, but the details of how innate immunity contributes to rejection have, until now, remained unknown.
In the new study, researchers used a classical genetic mapping approach to show that in mice a molecule called SIRP-alpha leads to innate immune system activation and differs between unrelated individuals. When the transplanted tissue SIRP-alpha is different from the host tissue SIRP-alpha, the transplant SIRP-alpha binds to a receptor called CD47 that is located on the recipient’s monocytes, a class of innate immune cells. This binding kicks off a series of cellular events that activate the innate and then eventually the adaptive immune system.
Like mice, humans also express SIRP-alpha, so sequencing the gene to identify donors and recipients with matched forms of the molecule hopefully will lead to lower organ rejection rates in the future, Dr. Lakkis said.
Blocking the interaction between SIRP-alpha and CD47 in mice prevented the monocyte activation, suggesting that disruption of this coupling could prevent recipient immune system activation. Future studies to examine how the interaction between SIRP-alpha and CD47 leads to monocyte activation could lead to new ways to prevent organ rejection.
Lauren Huckaby, MD Joins Gleason Lab Research Team

Dr. Lauren Huckaby has joined the Gleason Lab Research Team as a post-doctoral research fellow.
Lauren received her BS in Biochemistry from Brown University, and her MD from Temple University School of Medicine. She joined the University of Pittsburgh Medical Center in July 2014 as a resident in General Surgery. Her research interests include sex differences in thoracic aortic aneurysm in both bicuspid and tricuspid aortic valve patients. Dr. Huckaby’s research is currently funded through a Nina Starr Braunwald Research Fellowship Award from the Thoracic Surgery Foundation. Her post-doctoral research is also supported by an NIH T32 Vascular Surgery Research Training Program through the Department of Surgery.
AWARDS AND RECOGNITION
Dr. Freddie Fu Receives the Pioneer of Sports Medicine Award
 Gwinnett Medical Center (GMC), Lawrenceville, Georgia, recently awarded McGowan Institute for Regenerative Medicine affiliated faculty member Freddie Fu, MD, Distinguished Service Professor and the David Silver Professor of Orthopaedic Surgery and Chairman of the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine and University of Pittsburgh Medical Center, with its Pioneer of Sports Medicine Award. This award is an honor bestowed on a physician GMC determines to have made a “significant impact in the field of sports medicine and the treatment of athletes.” The award was presented to Dr. Fu for his extensive contributions to surgical techniques for patients.
Gwinnett Medical Center (GMC), Lawrenceville, Georgia, recently awarded McGowan Institute for Regenerative Medicine affiliated faculty member Freddie Fu, MD, Distinguished Service Professor and the David Silver Professor of Orthopaedic Surgery and Chairman of the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine and University of Pittsburgh Medical Center, with its Pioneer of Sports Medicine Award. This award is an honor bestowed on a physician GMC determines to have made a “significant impact in the field of sports medicine and the treatment of athletes.” The award was presented to Dr. Fu for his extensive contributions to surgical techniques for patients.
“Dr. Fu has built a global reputation for innovation in pioneering new surgical techniques for sports-related injuries,” said Gary Levengood, MD, chairman of GMC’s sports medicine program. “With this, we recognize a physician who has dedicated his life to improving patient care.”
“I am honored and humbled to be among the group of physicians who have received the Pioneer of Sports Medicine award over the years,” Dr. Fu said. “I’m deeply appreciative to GMC, who is helping advance sports medicine.”
Dr. Fu received his medical degree from the University of Pittsburgh School of Medicine where he also completed both a residency in orthopedic surgery and a fellowship in orthopedic research. He earned undergraduate and post graduate degrees from Dartmouth and Dartmouth’s Geisel School of Medicine.
In 1985, Dr. Fu helped establish the University of Pittsburgh School of Medicine Sports and Preventive Medicine Institute, which is now a preeminent center. Since 1986, he has served as the head team physician for the University of Pittsburgh Department of Athletics, and he established the school’s Sports Medicine Fellowship Program for physicians.
Dr. Fu received the award at a banquet held at the 1818 Club attended by orthopedic surgeons, residents, fellows and other sports medicine professionals from the metro Atlanta area.
Illustration: Gwinnett Medical Center.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#173 –– Dr. Sohail Husain is an Associate Professor of Pediatrics at the University of Pittsburgh and Director of the Exocrine Pancreas Research Program at the Children’s Hospital of Pittsburgh of UPMC. Dr. Husain discusses his research in pancreatic disorders.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
We encourage submissions of images that highlight your research. If you have images, or have an idea for an interesting image, please contact Donna Stolz, PhD at donna.stolz@pitt.edu.
PUBLICATION OF THE MONTH
Author: Hall A, Chan P, Sheets K, Apperson M, Delaughter C, Gleason TG, Phillippi JA, Nain A
Title: Nanonet force microscopy for measuring forces in single smooth muscle cells of the human aorta
Summary: A number of innovative methods exist to measure cell-matrix adhesive forces, but they have yet to accurately describe and quantify the intricate interplay of a cell and its fibrous extracellular matrix (ECM). In cardiovascular pathologies, such as aortic aneurysm, new knowledge on the involvement of cell-matrix forces could lead to elucidation of disease mechanisms. To better understand this dynamic, we measured primary human aortic single smooth muscle cell (SMC) forces using nanonet force microscopy in both inside-out (I-O intrinsic contractility) and outside-in (O-I external perturbation) modes. For SMC populations, we measured the I-O and O-I forces to be 12.9 ± 1.0 and 57.9 ± 2.5 nN, respectively. Exposure of cells to oxidative stress conditions caused a force decrease of 57 and 48% in I-O and O-I modes, respectively, and an increase in migration rate by 2.5-fold. Finally, in O-I mode, we cyclically perturbed cells at constant strain of varying duration to simulate in vivo conditions of the cardiac cycle and found that I-O forces decrease with increasing duration and O-I forces decreased by half at shorter cycle times. Thus our findings highlight the need to study forces exerted and felt by cells simultaneously to comprehensively understand force modulation in cardiovascular disease.
Source: Mol Biol Cell. 2017 Jul 7;28(14):1894-1900. doi: 10.1091/mbc.E17-01-0053. Epub 2017 Apr 27.
GRANT OF THE MONTH
PI: Christopher Hughes, MD
Co-PI: Paulo Fontes, MD
Title: Whole Organ Sonothrombolysis
Description: This technology is a new ex-vivo application for sonothrombolysis (SNT), which combines the use of ultrasound (US) probes and microbubbles timely infused through the arterial port of liver allografts being preserved by a machine perfusion (MP) system. The US probe pulses induces microbubble oscillation and bursting in a process called cavitation. This ex-vivo technology is intended to remove red blood cells (RBCs) plugs and cellular debris from the hepatic arterial peri-biliary plexus (PBP) prior to liver allograft implantation from organs obtained from donors after cardiac death (“DCD”) These patients experience extended periods of hypoperfusion under anoxic conditions prior to organ recovery. The use of DCD livers poses a significant risk for the subsequent development of ischemic cholangiopathy (IC) by the recipient in the post-operative period. IC is an irreversible complication stemming from prolonged ischemia to the PBP leading into recurrent biliary sepsis and subsequent liver allograft failure. This lethal condition requires mandatory retransplantation while yielding prolonged hospital stays and excessive post-operative costs. Previous attempts to prevent IC after DCD liver transplantation using different technologies have failed. IC is caused by progressive clotting of the small blood vessels supplying the PBP, which prevents blood and oxygen from reaching the biliary tree effectively once the liver is transplanted. The ex-vivo SNT technology was designed to remove the clots ex-vivo while enhancing the oxygenation of the bile duct system before the liver is transplanted. It can be used with all current MP systems currently being evaluated for liver preservation.
Source: Coulter@Pitt TPII Program, the Center for Medical Innovation (CMI), and the University of Pittsburgh and UPMC Liver Center
Term: July 1, 2017 – June 30, 2018
Amount: $50,000
