January 2020 | VOL. 19, NO. 1| www.McGowan.pitt.edu
Researchers Regrow Damaged Nerves with Polymer and Protein

University of Pittsburgh School of Medicine researchers have created a biodegradable nerve guide — a polymer tube — filled with growth-promoting protein that can regenerate long sections of damaged nerves, without the need for transplanting stem cells or a donor nerve.
So far, the technology has been tested in monkeys, and the results of those experiments appeared in Science Translational Medicine.
McGowan Institute for Regenerative Medicine affiliated faculty members who are co-authors on this work include
- Kacey Marra, PhD, Professor in the Departments of Plastic Surgery in the School of Medicine (primary) and Bioengineering in the School of Engineering (secondary) at the University of Pittsburgh and the Vice Chair of Research for the Department of Plastic Surgery
- Douglas Weber, PhD, Associate Professor in the Department of Bioengineering at the University of Pittsburgh with secondary appointments in the Department of Physical Medicine and Rehabilitation, the Department of Rehabilitation Science and Technology, and the Center for the Neural Basis of Cognition
“We’re the first to show a nerve guide without any cells was able to bridge a large, 2-inch gap between the nerve stump and its target muscle,” said senior author Dr. Marra. “Our guide was comparable to, and in some ways better than, a nerve graft.” Watch Dr. Marra explain this technology.
Half of wounded American soldiers return home with injuries to their arms and legs, which aren’t well protected by body armor, often resulting in damaged nerves and disability. Among civilians, car crashes, machinery accidents, cancer treatment, diabetes and even birth trauma can cause significant nerve damage, affecting more than 20 million Americans.
Peripheral nerves can regrow up to a third of an inch on their own, but if the damaged section is longer than that, the nerve can’t find its target. Often, the disoriented nerve gets knotted into a painful ball called a neuroma.
The most common treatment for longer segments of nerve damage is to remove a skinny sensory nerve at the back of the leg — which causes numbness in the leg and other complications, but has the least chance of being missed — chop it into thirds, bundle the pieces together and then sew them to the end of the damaged motor nerve, usually in the arm. But only about 40 to 60% of the motor function typically returns.
“It’s like you’re replacing a piece of linguini with a bundle of angel hair pasta,” Dr. Marra said. “It just doesn’t work as well.”
Dr. Marra’s nerve guide returned about 80% of fine motor control in the thumbs of four monkeys, each with a 2-inch nerve gap in the forearm.
The guide is made of the same material as dissolvable sutures and peppered with a growth-promoting protein — the same one delivered to the brain in a recent Parkinson’s trial — which releases slowly over the course of months.
The experiment had two controls: an empty polymer tube and a nerve graft. Since monkeys’ legs are relatively short, the usual clinical procedure of removing and dicing a leg nerve wouldn’t work. So, the scientists removed a 2-inch segment of nerve from the forearm, flipped it around and sewed it into place, replacing linguini with linguini, and setting a high bar for the nerve guide to match.
Functional recovery was just as good with Dr. Marra’s guide as it was with this best-case-scenario graft, and the guide outperformed the graft when it came to restoring nerve conduction and replenishing Schwann cells — the insulating layer around nerves that boosts electrical signals and supports regeneration. In both scenarios, it took a year for the nerve to regrow. The empty guide performed significantly worse all around.
With these promising results in monkeys, Dr. Marra wants to bring her nerve guide to human patients. She’s working with the Food and Drug Administration (FDA) on a first-in-human clinical trial and spinning out a startup company, AxoMax Technologies Inc.
“There are no hollow tubes on the market that are approved by the FDA for nerve gaps greater than an inch. Once you get past that, no off-the-shelf tube has been shown to work,” Dr. Marra said. “That’s what’s amazing here.”
Additional authors on the study include Neil Fadia, Jacqueline Bliley, Gabriella DiBernardo, Donald Crammond, PhD, Benjamin Schilling, Wesley Sivak, MD, PhD, Alexander Spiess, MD, Kia Washington, MD, Matthias Waldner, MD, Liao Han Tsung, PhD, Isaac James, MD, Danielle Minteer, PhD, Casey Tompkins-Rhoades, Deok-Yeol Kim, Riccardo Schweizer, MD, Debra Bourne, MD, Adam Cottrill, George Panagis, Asher Schusterman, MD, Francesco Egro, MD, Insiyah Campwala, Tyler Simpson, MS, Trent Gause, MD, Jack Brooker, Tvisha Josyula, Astrid Guevara, Alexander Repko and Christopher Mahoney, all of Pitt.
This study was funded by the Armed Forces Institute of Regenerative Medicine (award number W81XWH-14-2-0003). MedGenesis Therapeutix Inc. supplied the growth-promoting protein. AxoMax Technologies was formed after the experiments were completed.
Illustration: Lauren Kokai, PhD (left), developed the technology used for this project when she was a graduate studying under Kacey Marra, PhD (right). Credit: UPMC.
RESOURCES AT THE MCGOWAN INSTITUTE
February Histology Special
The McGowan Institute Histology Core wants to be your Valentine this month with big savings that will warm your heart.
Bring your cardiac tissue to the McGowan Histology Core and receive 25% off your entire order in the month of February, when you mention this ad.
From our hearts to yours, you won’t beat these prices, or our rapid turnaround times. Race in today for top quality results!
Contact Julia at the McGowan Core Histology Lab and ask about our staining specials. Email Hartj5@upmc.edu or call 412-624-5265
Save the Date!
McGowan Institute Annual Retreat
 Registration is now open for the 2020 McGowan Institute Scientific Retreat. The retreat will be held March 9-10, 2020 at Oglebay Resort in Wheeling, WV.
Registration is now open for the 2020 McGowan Institute Scientific Retreat. The retreat will be held March 9-10, 2020 at Oglebay Resort in Wheeling, WV.
Program highlights include:
- Regenerative Medicine Technology Pitch Competition
- Team Formation to Address Grand Challenges in Regenerative Medicine
- Screening of Burden of Genius
- Fireside Chat with Local Entrepreneurs
- Commercialization Office Hours
- Invention Disclosure Sprints
- Emerging Themes in Regenerative Medicine
- Poster Teasers and Poster Presentations
The program committee under the leadership of Julie Phillippi, PhD is developing an exciting program. For more information and to register, please click here.
AWARDS AND RECOGNITION
Dr. William Wagner Named a Pittsburgh “Power 100” Honoree

In October, the Pittsburgh Business Times announced the establishment of their inaugural “Power 100” List which they describe as individuals who represent “Pittsburgh’s most influential movers and shakers. These are the people who have defined and are redefining the region, who are controlling the agenda right now, and who are leading and shaping the industries they are in.” McGowan Institute for Regenerative Medicine director William Wagner, PhD, Distinguished Professor of Surgery, Bioengineering and Chemical Engineering at the University of Pittsburgh, was selected as a “Power 100” honoree as a Pittsburgh driving force and motivator.
Under Dr. Wagner’s leadership, the McGowan Institute has advanced its mission to develop therapies that reestablish tissue and organ function impaired by disease, trauma, or congenital abnormalities. Dr. Wagner is internationally recognized for his personal advances to science and clinical care (his personal area of research relates to cardiac care), and under his leadership, the scientists and clinicians at the McGowan Institute have developed and translated technologies that have treated more than ten million patients…and this is only a “tip of the iceberg” as there are a multitude of emerging technologies in various stages of maturity in the McGowan Institute Labs.
Dr. Wagner has also led the development of a growing commercial community –with over 30 spin-out companies based on technologies developed by McGowan Institute affiliated faculty.
Success in the McGowan Institute’s mission impacts patients’ lives, brings economic benefit to the region, serves to train the next generation of researchers, and advances the expertise of our faculty in the basic sciences, engineering and clinical sciences. Dr. Wagner is known around the world for leadership in regenerative medicine-based science and the success in making those innovations available to patients in need.
“Our philosophy is, if it does not get to the patient, if it does not impact patients’ lives, we’ve not fulfilled our mission,” said Dr. Wagner during a Q&A session with Pitt’s Innovation Institute.
Congratulations, Dr. Wagner!
Illustration: Pittsburgh Business Times
Read more… (subscription required)
SCIENTIFIC ADVANCES
Dr. Fabrisia Ambrosio Named Co-PI on $3.8M R01 Grant

The National Institutes of Health recently awarded $3.8 million to the R01 Grant Project entitled “Physical exercise and blood-brain communication: Exosomes, Klotho and the Choroid Plexus.” The project’s co-principal investigators include:
- Radosveta Koldamova, MD, PhD, Professor, Environmental and Occupational Health, University of Pittsburgh
- Fabrisia Ambrosio, MPT, PhD, Associate Professor, Physical Medicine & Rehabilitation, University of Pittsburgh
Dr. Ambrosio is a faculty member of the McGowan Institute for Regenerative Medicine and the Director of Rehabilitation for UPMC International.
From the award’s abstract:
Aging is the major risk factor for Alzheimer’s disease (AD). Numerous studies have confirmed that physical exercise has positive effects in patients with AD and other neurodegenerative disorders. The majority of the studies examining the effect of physical exercise in animal models of neurodegeneration have reported neuroprotection, improved memory and cognitive performance. The molecular mechanisms of the interactions between the non-neuronal systems involved in the physical/aerobic exercise and brain, however, remain poorly understood.
The antiaging protein, a-Klotho, has well-known neuroprotective activity, and recent studies demonstrated that systemic elevation of a-Klotho protein in transgenic mice or injection of soluble α-Klotho fragment, enhanced cognition and neural resilience in young, aging, and a murine disease model. Recent reports have suggested that a-Klotho levels decline in brain of animal models of AD. It has been recently shown that physical aerobic exercise increases the circulating levels of a-Klotho, and we have found that direct muscle contraction via neuromuscular electrical stimulation significantly enhanced a-Klotho expression in the hippocampus.
These findings raised the novel hypothesis that skeletal muscle may be a regulator of circulating a-Klotho. We posit that muscle-induced stimulation of a-Klotho may play a role in the beneficial effect of exercise on cognitive outcomes. Importantly, it has been established that signals from periphery to the central nervous system (CNS) are transmitted through mechanisms highly specific to choroid plexus (CP) epithelium, and physical exercise increases the release and amount of extracellular vesicles into the circulation. Our preliminary data demonstrate that a-Klotho is detectable at high levels in exosomes isolated from plasma, and that muscle contractile activity increases the release and amount of a-Klotho-containing exosomes in circulation.
We also show that the exosomal cargo can transmit a signal to cells in vitro, thus affecting the expression level of intracellular proteins. We hypothesize that the effects of physical exercise on CNS are results of signals generated in peripheral muscles and transmitted to the brain via the CP epithelium. The signals are associated with and depend on increased circulating levels of anti-aging protein, a-Klotho, released by muscles within exosomes. This interdisciplinary research will integrate the expertise of AD researchers experienced with AD animal models, analysis of AD-like pathology and -omix approaches (R. Koldamova and I. Lefterov), established researchers in biology of a-Klotho, rehabilitation, and aging (F. Ambrosio) and cell biology (C. St. Croix).
The goal of this proposal is to further our understanding of the interactions between a-Klotho expression in skeletal muscles, physical activity and brain, and to elucidate the relationship of age-related changes in skeletal muscle and progression of AD. The work includes the following aims:
- Aim 1: To determine if the effects of physical exercise on phenotype and gene expression in the hippocampus and cortex are mediated by a-Klotho.
- Aim 2: To reveal the effect of muscle training on exosomal cargo in plasma and cerebrospinal fluid (CSF), and to integrate their proteomic and miRNA profiles with the phenotype and brain transcriptomes.
- Aim 3: To elucidate the role of Choroid Plexus and a-Klotho in communicating signals from peripheral muscles to CNS.
Congratulations, all!
Illustration: University of Pittsburgh and McGowan Institute for Regenerative Medicine.
UPMC First in the U.S. to Implant Wireless Retinal Device

UPMC has implanted the first patient in the United States with a new wireless retinal device as part of a clinical trial aimed at restoring partial sight to patients with advanced age-related macular degeneration (AMD), a disease that leads to permanent blindness.
“Vision research has advanced dramatically in the recent past and UPMC is at the forefront of this revolution. This is the first of many such breakthroughs led by UPMC and Pitt that will benefit patients with vision loss in our community and around the world,” said José-Alain Sahel, MD, director of the UPMC Eye Center, Eye and Ear Foundation chair of ophthalmology and distinguished professor at the University of Pittsburgh School of Medicine, and McGowan Institute for Regenerative Medicine affiliated faculty member who initiated the trial at UPMC. “We are proud to be the first center in the United States to test this next generation retinal implant that could help treat an incurable disease like AMD.”
The system, called PRIMA, is designed to restore sight in patients blinded by retinal degeneration. It consists of a 2×2 millimeter, 30-micron thick miniaturized wireless photovoltaic chip, placed under the damaged retina and works in tandem with augmented reality glasses that have a built-in miniaturized camera and infrared projector.
The chip acts like a tiny artificial retina, made up of 378 tiny electrodes that convert infrared light from the glasses to electrical signals that are carried by the optic nerve to the brain. After receiving the implant, patients undergo an intensive rehabilitation program that trains their brains to understand and interpret the signals from the implant in combination with their remaining natural vision. Compared to earlier-generation implants, PRIMA is wireless and has significantly more electrodes, which allows for the transmission of more visual information.
“This is an incredibly exciting first for us at UPMC and I’m honored to be a part of it,” said Joseph Martel, MD, the implanting surgeon at the UPMC Eye Center and the Pitt School of Medicine, and the principal investigator of the trial at UPMC. “I’m grateful to our patients who have volunteered to participate in this trial, without whom this would not be possible.”
AMD is the leading cause of vision loss in people older than 50. Today, it affects approximately 14 million people in the United States, and the prevalence is expected to rise as the baby boomers age. As AMD progresses, the center of vision becomes increasingly blurry. “Atrophic” AMD, which accounts for a large proportion of advanced cases, has no curative treatment available.
The UPMC feasibility trial is running in parallel with the first-in-human trial in France, which involves five patients with advanced AMD, who now have been followed for more than a year. The 12-month results from the French study demonstrated the ability of most patients to identify sequences of letters and there were no device-related serious adverse effects.
“We are working with a great sense of urgency because the aging population of the United States, especially the western Pennsylvania region we live in, will see a significant rise in the number of patients at risk for vision loss through diseases like age-related macular degeneration, glaucoma and vascular eye disease, as well as earlier onset genetic conditions such as retinitis pigmentosa,” said Dr. Sahel. “This is why our physicians and researchers at UPMC and Pitt, in collaboration with our U.S. and international colleagues — especially at the Paris Vision Institute at Sorbonne University — are taking a multi-pronged effort to treat and rehabilitate patients with vision impairments.”
In March 2019, UPMC broke ground on the UPMC Vision and Rehabilitation Tower at UPMC Mercy, which when completed, will provide advanced specialty clinical care and innovative programs for visually impaired patients. It also will be the home for the vision research program at Pitt and UPMC.
The PRIMA implant was invented by Daniel Palanker, PhD, professor of ophthalmology at Stanford University, and licensed and developed by Pixium Vision, a spin-off from the Paris Vision Institute. Dr. Sahel is a co-founder of Pixium and holds shares in the company.
Illustration: José-Alain Sahel, MD, holding the PRIMA implant. Credit: UPMC.
Salama Lab Study Reveals How Relaxin Targets Cardiovascular Disease
As a healthy heart ages, it becomes more susceptible to cardiovascular diseases. Though researchers have discovered that relaxin, an insulin-like hormone, suppresses atrial fibrillation (AF), inflammation, and fibrosis in aged rats, the underlying mechanisms of these benefits are still unknown. In a recent Scientific Reports paper, a University of Pittsburgh research team discusses how relaxin interacts with the body’s signaling processes to produce a fundamental mechanism that may have great therapeutic potential.
The study, “Relaxin reverses maladaptive remodeling of the aged heart through Wnt-signaling,” was led by McGowan Institute for Regenerative Medicine affiliated faculty member Guy Salama, PhD, professor of medicine at Pitt, and Brian Martin, a graduate student researcher from the Swanson School of Engineering’s Department of Bioengineering.
“Relaxin is a reproductive hormone discovered in the early 20th century that has been shown to suppress cardiovascular disease symptoms,” said Mr. Martin. “In this paper, we show that relaxin treatment reverses electrical remodeling in animal models by activating canonical Wnt signaling – a discovery that reveals a fundamental underlying mechanism behind relaxin’s benefits.”
A better understanding of how relaxin interacts with the body may improve its efficacy as a therapy to treat cardiovascular disease in humans. As the U.S. population ages, the rates of these age-associated diseases are expected to rise, requiring better treatment for this leading cause of death. According to a report from the American Heart Association, the total direct medical costs of cardiovascular disease are projected to increase to $749 billion in 2035.
“A common problem in age-associated cardiovascular disease is altered electrical signaling required for proper heart contraction,” Mr. Martin explained. “When ions in the heart and their associated channels to enter or exit the heart are disrupted, complications occur.”
“Natural, healthy aging has been shown to be accompanied by changes in structure and function,” Dr. Salama added. “For example, aged cardiomyocytes start to express embryonic contractile proteins and fewer voltage-gated Na+ channels by unknown mechanisms. The reversal of some aspects of the aging process by relaxin is mediated by the reactivation of Wnt canonical signaling which may partly explain mechanisms of the aging process.”
The group’s study found that relaxin upregulated the prominent sodium channel, Nav1.5, in cells of heart tissue via a mechanism inhibited by the Wnt pathway inhibitor Dickkopf-1.
“Wnt signaling is thought to be active primarily in the developing heart and inactive later in life,” Mr. Martin said. “However, we show that relaxin can reactivate Wnt signaling in a beneficial way to increase Nav1.5.”
Increased Nav1.5 is associated with better electrical signaling in the heart may reduce susceptibility to cardiac rhythm disorders.
“Further, we show that relaxin can also reverse the age-associated reduction in cell adhesion molecules and cell-cell communication proteins,” he continued. “In summary, relaxin appears to reverse problematic reductions or pathological reorganization of vital cardiac signaling proteins.”
While these data provide new insight into relaxin’s mechanisms of action, further work is needed to understand the precise steps required for relaxin to alter Wnt signaling and if steps can be taken to directly alter Wnt signaling to provide its beneficial effects.
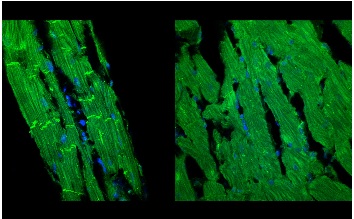
Illustration: “Left ventricular tissue sections (7-µm thick) from aged rat hearts (24 months old) were labeled with the nuclear stain (DAPI-blue) and an antibody against β-catenin (green). Rats were treated with Relaxin (0.4 mg/kg/day for 2-weeks) (left panel) or with the control vehicle (sodium acetate) (right panel) and the tissue sections were imaged by confocal microscopy (600X magnification). Relaxin treatment (left) produced a marked positive remodeling of aged ventricles with a reduction of cell hypertrophy, improved organization of myofibrils and cell membrane compared to untreated, control aged hearts (right).” Credit: Dr. Guillermo Romero.
Heart Transplants from Donors with Hepatitis C May Be Safe

Transplants from donors with hepatitis C may be a safe option for those awaiting new hearts – a major development in curbing the nationwide organ shortage, according to new research.
Patients who received heart transplants from donors who had hepatitis C saw similar outcomes a year after surgery as those whose donors did not have hepatitis C. Researchers compared one-year survival, organ rejection, dialysis and incidence of stroke.
The study, published in the Journal of the American Heart Association, examined 7,889 U.S. adults who received heart transplants between 2016 and 2018. Slightly more than 4%, or 343, received heart transplants from donors with hepatitis C.
“We are encouraged by these results and believe this is a landmark change in our ability to better meet the demand for heart transplantation by increasing the donor supply,” McGowan Institute for Regenerative Medicine affiliated faculty member Arman Kilic, MD, lead study author, said in a news release. He is an Assistant Professor of Cardiothoracic Surgery, the Director, Surgical Quality and Analytics, Cardiac Surgery, and Co-Director, Center for Cardiovascular Outcomes and Innovation, in the Department of Cardiothoracic Surgery at the UPMC Heart and Vascular Institute. “It is our hope that more centers will use hepatitis C-positive donors for heart transplantation.”
McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Gleason, MD, the Ronald V. Pellegrini Endowed Professor of Cardiothoracic Surgery and Chief, Division of Cardiac Surgery of the University of Pittsburgh School of Medicine, and the Co-Director of the Heart and Vascular Institute at UPMC, is a co-author on the study. He is also Director of the Center for Thoracic Aortic Disease and Co-Director of the Center for Heart Valve Disease, and he is a member of the Center for Vascular Remodeling and Regeneration at Pitt.
Hepatitis C, though curable in most cases, can be a debilitating illness. It is a viral liver infection that spreads through contact with contaminated blood, by way of shared needles and from mother to infant during pregnancy and delivery. Because it can be treated, there has been an increase in organ donors with hepatitis C as the need for heart transplants continues to exceed the supply.
In the new study, researchers found similar survival rates regardless of whether patients received a heart from a donor with or without hepatitis C – 90% compared to 91%, respectively. There was also little difference between the two groups when comparing the rates of stroke, drug-treated organ rejection, and kidney dialysis to remove toxins from the blood.
More than 6 million people in the U.S. have heart failure and more than 900,000 new cases are diagnosed each year. It occurs when other types of heart disease weaken the heart until it is unable to pump blood effectively throughout the body. Although lifestyle changes and medications can help manage mild heart failure, severe cases may require a heart transplant.
The study did have limitations. For example, researchers did not collect information about the type of hepatitis C infection each donor had or past treatments they received. The study also did not report whether transplant recipients developed the infection.
Dr. Takashi Kozai Receives $1.6M NIH Award to Develop an Innovative Wireless Neural Device for Long-Term and Precise Stimulation
Neural stimulation is a pioneering technology that can be used to recover function and improve the quality of life for individuals who suffer from brain injury or disease. It serves an integral role in modern neuroscience research and human neuroprosthetics, including advancements in prosthetic limbs and brain-computer interfaces. A challenge that remains with this technology is achieving long-term and precise stimulation of a specific group of neurons.
McGowan Institute for Regenerative Medicine affiliated faculty member Takashi Kozai, PhD, assistant professor of bioengineering at the University of Pittsburgh, recently received a $1,652,844 award from the National Institutes of Health (1R01NS105691-01A1) to develop an innovative solution to address these limitations.
“Implantation of these devices causes a reactive tissue response which degrades the functional performance over time, thus limiting device capabilities,” Dr. Kozai explained. “Current electrical stimulation implants are tethered to the skull, which leads to mechanical strain in the tissue, and in turn, causes chronic inflammation and increases the possibility of an infection.”
Dr. Kozai, who leads the Bio-Integrating Optoelectric Neural Interface Cybernetics Lab in the Swanson School of Engineering, will use the NIH award to develop a wireless in vivo stimulation technology that will enable precise neural circuit probing while minimizing tissue damage. In this design, the electrode will be implanted in the brain and activated by light – via the photoelectric effect – with a far-red or infrared laser source, which can sit outside of the brain.
“This use of photostimulation removes the mechanical requirements necessary in traditional microstimulation technology and improves spatial selectivity of activated neurons for stable, long-term electrical stimulation,” Dr. Kozai said.
His group found that photostimulation drives a more localized population of neurons when compared to electrical stimulation under similar conditions. When used, the activated cells were closer to the electrode, which indicates increased spatial precision. The proposed technology will be smaller than traditional photovoltaic devices but larger than nanoparticles to improve device longevity.
“With this project, we hope to develop advanced neural probes that are capable of activating specific neurons for long periods of time and with great precision,” Dr. Kozai said. “This technology could significantly impact neuroscience research and ultimately the treatment of neurological injury and disease in humans.”
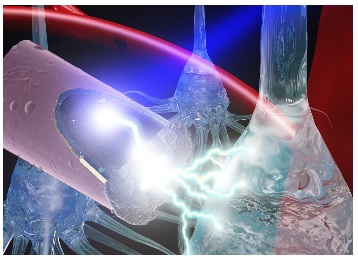
Illustration: A laser shining onto an untethered, ultrasmall carbon fiber electrode to stimulate neurons via the photoelectric effect. Photo credit: J. Mater. Chem. B, 2015,3, 4965-4978 – Reproduced by permission of The Royal Society of Chemistry.
Nerve-Healing Startup Renerva Joins McGovern Incubator

Renerva, a medical startup that is developing an injectable gel to speed the healing of damaged nerves and creating an off-the-shelf nerve-graft product that may spare patients life-long disability, has joined Cornell University’s McGovern Center life sciences business incubator.
The company was formed concurrently at Cornell and the University of Pittsburgh.
“We’ve developed a novel way of manipulating nerve repair and improving the recovery of nerves,” said McGowan Institute for Regenerative Medicine affiliated faculty member Jonathan Cheetham, VetMB, Diplomate ACVS, PhD, associate professor in Cornell’s College of Veterinary Medicine and chair of Renerva’s scientific advisory board.
“Other technology has focused on the late stages of nerve healing,” Dr. Cheetham said. “What we’re doing is trying to influence the early part of nerve healing that may lead to improved nerve function outcomes for patients down the road.”
Renerva’s first product, Peripheral Nerve Matrix, is an injectable hydrogel derived from porcine tissue that acts like a scaffold. It supports nerve-cell growth and tissue formation, said McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, Renerva’s chief technology officer and an associate professor of bioengineering at the University of Pittsburgh. He is a former Cornell postdoctoral researcher.
Millions of people in the U.S. suffer from peripheral nerve injuries or problems, and peripheral nerve regeneration is slow and not always complete, Dr. Brown said.
“Acute nerve injuries don’t heal well, so more than half of the patients have unsatisfactory outcomes,” he said. “This is a material that can be injected into the nerve – after a surgical repair – to speed and enhance the recovery, improve a patient’s quality of life and help them heal faster.”
Renerva’s second product, which could offer surgeons an “off-the-shelf” nerve autograft that restores motor and sensory function, is in development. It could spare patients complications, discomfort and loss of function associated with nerve autografts, according to Lorenzo Soletti, PhD, MBA, Renerva’s president and chief executive officer.
“Renerva epitomizes the research nature of Cornell,” said Lou Walcer, MBA, director of the McGovern Center. “The company uses University research and technology, and it is extending that technology to help patients alleviate problems and pain. This is quite exciting.”
In 2018, the U.S. Department of Defense provided $2.4 million through a Medical Technology Enterprise Consortium award to Renerva to complete a preclinical program and begin human trials. Additionally, the National Science Foundation and the National Institutes of Health awarded $500,000 to accelerate development of the company’s products.
Peripheral Nerve Matrix is expected to enter clinical trials this year.
Illustration: Lorenzo Soletti, left, Jon Cheetham, Lou Walcer, and Bryan Brown pose with the key to the McGovern Center. Cornell University.
Abbott Gets FDA Approval for Less-Invasive Heart Pump Implant Procedure

Abbott Laboratories, the Abbott Park, Illinois-based health care giant, has won approval from the U.S. Food and Drug Administration (FDA) for a less invasive surgical procedure that allows surgeons to implant the company’s heart pump without a patient undergoing open heart surgery.
The newly approved alternative allows Abbott’s HeartMate 3 left ventricular assist device, or LVAD, heart pump to be implanted through an incision made between the patient’s ribs, giving access to the heart. Historically, heart pumps are implanted through open heart surgery, but this method can result in less bleeding during surgery and shorter recovery time for patients, according to Abbott, which ran two trial studies on the alternative procedure.
The FDA approved Abbott’s HeartMate 3 in 2017. It was designed for patients with advanced heart failure who are awaiting transplantation or are not candidates for heart transplantation. The medical device pumps blood through the body in people whose heart is too weak to do so on its own.
“We continue to focus on advancing our heart failure devices and techniques to make life better for the patients we serve,” McGowan Institute for Regenerative Medicine affiliated faculty member Robert Kormos, MD, FAHA, FRCS(C), FACS, currently the Division Vice President Global Medical Affairs Heart Failure at Abbott Laboratories, said in a statement. “The first approved LVAD – HeartMate I – was approved more than 25 years ago. Since that time, the technology has evolved immensely. Today’s HeartMate 3 device, including its design and size, allows physicians to successfully implant it without having to perform open heart surgery and offers survival rates, as demonstrated in the MOMENTUM 3 clinical trial, at two-years that are comparable to heart transplants.”
In 2018, HeartMate 3 was approved as a destination therapy for those individuals who need a new heart but are not eligible for a transplant.
In 2019, Abbott (NYSE: ABT) was ranked No. 103 on the Fortune 500 list, with revenue of more than $30 billion, up more than 11% from a year earlier.
Dr. Kormos is a former McGowan Institute for Regenerative Medicine deputy director and the past director of UPMC’s Artificial Heart Program. He has been part of the education of over 100 residents and 60 Cardiothoracic Transplant Fellows.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#205 –– Dr. Michel Modo discusses his research in the development of new treatments for stroke.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Neil B. Fadia, Jacqueline M. Bliley, Gabriella A. DiBernardo, Donald J. Crammond, Benjamin K. Schilling, Wesley N. Sivak, Alexander M. Spiess, Kia M. Washington, Matthias Waldner, Han-Tsung Liao, Isaac B. James, Danielle M. Minteer, Casey Tompkins-Rhoades, Adam R. Cottrill, Deok-Yeol Kim, Riccardo Schweizer, Debra A. Bourne, George E. Panagis, M. Asher Schusterman II, Francesco M. Egro, Insiyah K. Campwala, Tyler Simpson, Douglas J. Weber, Trent Gause II, Jack E. Brooker, Tvisha Josyula, Astrid A. Guevara, Alexander J. Repko, Christopher M. Mahoney and Kacey G. Marra
Title: Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates
Summary: Severe injuries to peripheral nerves are challenging to repair. Standard-of-care treatment for nerve gaps >2 to 3 centimeters is autografting; however, autografting can result in neuroma formation, loss of sensory function at the donor site, and increased operative time. To address the need for a synthetic nerve conduit to treat large nerve gaps, we investigated a biodegradable poly(caprolactone) (PCL) conduit with embedded double-walled polymeric microspheres encapsulating glial cell line–derived neurotrophic factor (GDNF) capable of providing a sustained release of GDNF for >50 days in a 5-centimeter nerve defect in a rhesus macaque model. The GDNF-eluting conduit (PCL/GDNF) was compared to a median nerve autograft and a PCL conduit containing empty microspheres (PCL/Empty). Functional testing demonstrated similar functional recovery between the PCL/GDNF-treated group (75.64 ± 10.28%) and the autograft-treated group (77.49 ± 19.28%); both groups were statistically improved compared to PCL/Empty-treated group (44.95 ± 26.94%). Nerve conduction velocity 1 year after surgery was increased in the PCL/GDNF-treated macaques (31.41 ± 15.34 meters/second) compared to autograft (25.45 ± 3.96 meters/second) and PCL/Empty (12.60 ± 3.89 meters/second) treatment. Histological analyses included assessment of Schwann cell presence, myelination of axons, nerve fiber density, and g-ratio. PCL/GDNF group exhibited a statistically greater average area occupied by individual Schwann cells at the distal nerve (11.60 ± 33.01 μm2) compared to autograft (4.62 ± 3.99 μm2) and PCL/Empty (4.52 ± 5.16 μm2) treatment groups. This study demonstrates the efficacious bridging of a long peripheral nerve gap in a nonhuman primate model using an acellular, biodegradable nerve conduit.
Source: Science Translational Medicine. 22 Jan 2020: Vol. 12, Issue 527, eaav7753
GRANT OF THE MONTH
PI: Radosveta Koldamova
Co-PI: Fabrisia Ambrosio
Title: Physical exercise and blood-brain communication: Exosomes, Klotho and the Choroid Plexus
Description: Aging is the major risk factor for Alzheimer’s disease (AD). Numerous studies have confirmed that physical exercise has positive effects in patients with AD and other neurodegenerative disorders. The majority of the studies examining the effect of physical exercise in animal models of neurodegeneration have reported neuroprotection, improved memory and cognitive performance. The molecular mechanisms of the interactions between the non-neuronal systems involved in the physical/aerobic exercise and brain, however, remain poorly understood. The antiaging protein, a-Klotho, has well-known neuroprotective activity, and recent studies demonstrated that systemic elevation of a-Klotho protein in transgenic mice or injection of soluble α-Klotho fragment, enhanced cognition and neural resilience in young, aging, and a murine disease model. Recent reports have suggested that a-Klotho levels decline in brain of animal models of AD. It has been recently shown that physical aerobic exercise increases the circulating levels of a-Klotho, and we have found that direct muscle contraction via neuromuscular electrical stimulation significantly enhanced a-Klotho expression in the hippocampus. These findings raised the novel hypothesis that skeletal muscle may be a regulator of circulating a-Klotho. We posit that muscle-induced stimulation of a-Klotho may play a role in the beneficial effect of exercise on cognitive outcomes. Importantly, it has been established that signals from periphery to the CNS are transmitted through mechanisms highly specific to choroid plexus (CP) epithelium, and physical exercise increases the release and amount of extracellular vesicles into the circulation. Our preliminary data demonstrate that a-Klotho is detectable at high levels in exosomes isolated from plasma, and that muscle contractile activity increases the release and amount of a-Klotho-containing exosomes in circulation. We also show that the exosomal cargo can transmit a signal to cells in vitro, thus affecting the expression level of intracellular proteins. We hypothesize that the effects of physical exercise on CNS are results of signals generated in peripheral muscles and transmitted to the brain via the CP epithelium. The signals are associated with and depend on increased circulating levels of anti-aging protein, a-Klotho, released by muscles within exosomes. This interdisciplinary research will integrate the expertise of AD researchers experienced with AD animal models, analysis of AD-like pathology and omix approaches (R. Koldamova & I. Lefterov), established researchers in biology of a-Klotho, rehabilitation, and aging (F. Ambrosio) and cell biology (C. St Croix). The goal of this proposal is to further our understanding of the interactions between a-Klotho expression in skeletal muscles, physical activity and brain, and to elucidate the relationship of age-related changes in skeletal muscle and progression of AD. Aim 1: To determine if the effects of physical exercise on phenotype and gene expression in the hippocampus and cortex are mediated by a-Klotho. Aim 2: To reveal the effect of muscle training on exosomal cargo in plasma and CSF, and to integrate their proteomic and miRNA profiles with the phenotype and brain transcriptomes. Aim 3: To elucidate the role of Choroid Plexus and a-Klotho in communicating signals from peripheral muscles to CNS.
Source: National Institute on Aging
Term: January 15, 2020 – December 31, 2024
Amount: $782,487 (2020)
