February 2017 | VOL. 16, NO. 2| www.McGowan.pitt.edu
Publication Named a NIEHS Top Paper of 2016

The National Institutes of Health, National Institute of Environmental Health Sciences (NIEHS), recently honored its 2016 Papers of the Year—the top 25 of 2700 publications. In that elite group, McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, MPT, Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh, and her team were recognized for their paper entitled “Arsenic promotes NF-kB-mediated fibroblast dysfunction and matrix remodeling to impair muscle stem cell function,” published in the journal Stem Cells. McGowan Institute faculty member Donna Stolz, PhD, Associate Director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and an Associate Professor in the Departments of Cell Biology and Pathology at the University of Pittsburgh, is a co-author on the study.
The paper’s abstract reads:
Arsenic is a global health hazard that impacts over 140 million individuals worldwide. Epidemiological studies reveal prominent muscle dysfunction and mobility declines following arsenic exposure; yet, mechanisms underlying such declines are unknown. The objective of this study was to test the novel hypothesis that arsenic drives a maladaptive fibroblast phenotype to promote pathogenic myomatrix remodeling and compromise the muscle stem (satellite) cell (MuSC) niche. Mice were exposed to environmentally relevant levels of arsenic in drinking water before receiving a local muscle injury. Arsenic-exposed muscles displayed pathogenic matrix remodeling, defective myofiber regeneration and impaired functional recovery, relative to controls. When naïve human MuSCs were seeded onto three-dimensional decellularized muscle constructs derived from arsenic-exposed muscles, cells displayed an increased fibrogenic conversion and decreased myogenicity, compared with cells seeded onto control constructs. Consistent with myomatrix alterations, fibroblasts isolated from arsenic-exposed muscle displayed sustained expression of matrix remodeling genes, the majority of which were mediated by NF-κB. Inhibition of NF-κB during arsenic exposure preserved normal myofiber structure and functional recovery after injury, suggesting that NF-κB signaling serves as an important mechanism of action for the deleterious effects of arsenic on tissue healing. Taken together, the results from this study implicate myomatrix biophysical and/or biochemical characteristics as culprits in arsenic-induced MuSC dysfunction and impaired muscle regeneration. It is anticipated that these findings may aid in the development of strategies to prevent or revert the effects of arsenic on tissue healing and, more broadly, provide insight into the influence of the native myomatrix on stem cell behavior.
Congratulations, all!
Read more…
Abstract (Arsenic promotes NF-Κb-mediated fibroblast dysfunction and matrix remodeling to impair muscle stem cell function. Zhang C, Ferrari R, Beezhold K, Stearns-Reider K, D’Amore A, Haschak M, Stolz D, Robbins PD, Barchowsky A, Ambrosio F. Stem Cells; 2016 Mar;34(3):732-42.)
RESOURCES AT THE MCGOWAN INSTITUTE
March Histology Special
Come and see what the McGowan Histology Core has to offer. From Optic nerves to whole eye sectioning, we can give you what you’re looking for. Bring your nerve tissue to the lab for Silver Staining for nerve fibers, Luxol Fast Blue for myelin, Cresyl Echt Violet stain for Nissl Substance, Tunel staining for apoptosis.
For the entire month of March you will receive 25% your entire bill when you bring your nervous system studies for histologic processing to the McGowan Histology Lab.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
Molecular Medicine Features Work of McGowan Institute Affiliated Faculty Members

The work of McGowan Institute for Regenerative Medicine affiliated faculty members Ruben Zamora, PhD, Research Associate Professor at the University of Pittsburgh in the Department of Surgery, and Yoram Vodovotz, PhD, Professor in the Department of Surgery with secondary appointments in the Department of Computational & Systems Biology, the Department of Bioengineering, the Department of Immunology, the Department of Communication Science and Disorders (of the School of Health and Rehabilitation Science), and the Clinical and Translational Science Institute, and also the Director of the Center for Inflammation and Regenerative Modeling at the McGowan Institute, is featured in the November 2016 issue of the publication, Molecular Medicine.
Drs. Zamora and Vodovotz’s article is entitled “Data-Driven Modeling for Precision Medicine in Pediatric Acute Liver Failure.” The abstract reads:
Absence of early outcome biomarkers for Pediatric Acute Liver Failure (PALF) hinders medical and liver transplant decisions. We sought to define dynamic interactions among circulating inflammatory mediators to gain insights into PALF outcome sub-groups. Serum samples from 101 participants in the PALF study, collected over the first 7 days following enrollment, were assayed for 27 inflammatory mediators. Outcomes (Spontaneous survivors [S, n=61], Non-survivors [NS, n=12], and liver transplant patients [LTx, n=28]) were assessed at 21 days post-enrollment. Dynamic interrelations among mediators were defined using data-driven algorithms. Dynamic Bayesian Network inference identified a common network motif with HMGB1 as a central node in all patient sub-groups. The networks in S and LTx were similar, and differed from NS. Dynamic Network Analysis suggested similar dynamic connectivity in S and LTx, but a more highly-interconnected network in NS that increased with time. A Dynamic Robustness Index calculated to quantify how inflammatory network connectivity changes as a function of correlation stringency differentiated all three patient sub-groups. Our results suggest that increasing inflammatory network connectivity is associated with non-survival in PALF, and may ultimately lead to better patient outcome stratification.
Read more…
Abstract (Data-Driven Modeling for Precision Medicine in Pediatric Acute Liver Failure. Zamora R, Vodovotz Y, Mi Q, Barclay D, Yin J, Horslen S, Rudnick D, Loomes KM, Squires RH. Molecular Medicine; 2016 Nov 23;22.)
McGowan Institute Affiliated Faculty Receive 2017 Chancellor’s Innovation Commercialization Funds from the Innovation Institute

The University of Pittsburgh Innovation Institute has awarded funding to four University of Pittsburgh Innovator teams to help them move their discoveries towards commercialization, where they can make a positive impact on society. The teams were selected by a panel of judges from a pool of two dozen applicants that was narrowed into a group of 10 finalists. Two of the recognized teams include affiliated faculty leadership from the McGowan Institute for Regenerative Medicine.
“We are thrilled to be able to provide these funds to entrepreneurial Pitt faculty and graduate students to help expedite their commercialization journey,” said Marc Malandro, PhD, Founding Director of the Innovation Institute. “Often the most difficult hurdle to climb for commercializing University research is providing so-called ‘gap’ funding that can bridge the space between a promising idea and a marketable product.”
The winning projects of $35,000 each include:
Thermoresponsive Hydrogel for Orbital Volume Augmentation
Morgan Fedorchak, PhD, Assistant Professor, Department of Ophthalmology, Chemical Engineering, and Clinical and Translational Science, and Jenny Yu, MD, Assistant Professor and Vice Chair, Clinical Operations Department of Ophthalmology, have discovered a non-degradable hydrogel material that can be injected into the orbit of the eye following ocular trauma or as a treatment for genetic eye disorders. The material can also be used to administer anti-inflammatory or antibiotic medications.
The funding will be used to provide proof-of-concept studies. Data from the successful completion of the studies will better position the innovation for application to the Department of Defense for funding to explore the therapeutic potential of the technology. Also, matching funds for this effort will come from the University of Pittsburgh Center for Military Medicine Research, whose mission is to address combat-related injuries.
Reactive Extraction of Water: Desalination Without Membranes or Distillation
Eric Beckman, PhD, Distinguished Service Professor of Chemical Engineering, has developed a chemical method for desalinating water that requires less energy than the longstanding existing methods such as reverse osmosis or flash distillation. The award will fund testing to validate the technology.
The Chancellor’s Innovation Commercialization Funds were established to provide support for promising early-stage Pitt innovations to assist in reducing the technical and/or market risk associated with the innovations and make them more attractive to investors or potential licensees. One of the paths for identifying funding opportunities is through a request for proposal program that was launched in November of 2016 and recently culminated in these awards.
Congratulations, all!
Meditation and Mindfulness Practices: Heart Patient’s Treatment Plan Options

Cardiovascular disease is the leading cause of death worldwide. Per the American Heart Association, practicing meditation and mindfulness may reduce death, heart attack, and stroke in heart patients. Meditation is a practice — often using deep breathing, quiet contemplation, or sustained focus on something benign, such as a color, phrase, or sound — that helps you let go of stress and feel peaceful and maintain a relaxed state of mind. Mindfulness is a mental state achieved by focusing one’s awareness on the present moment, while calmly acknowledging and accepting one’s feelings, thoughts, and bodily sensations, used as a therapeutic technique.
Recently interviewed by Jeena Cho, a Forbes contributor, McGowan Institute for Regenerative Medicine affiliated faculty member Joon Sup Lee, MD, Associate Professor of Medicine at the University of Pittsburgh, Division Chief of Cardiology, and Co-Director Heart and Vascular Institute at the University of Pittsburgh School of Medicine, explained the benefits of these practices when included in a heart patient’s treatment plan.
“The link between chronic stress and progression of coronary artery disease is now well accepted by a large majority of cardiologists. Although elimination of stress is impossible as long as we are alive, a reduction of stress and more adaptive response to stress should be an important part of any cardiovascular health regimen,” said Dr. Lee.
It is not the stress in life, but the reaction to stress that is so potentially harmful to our health. Meditation and mindfulness can reverse the physiological manifestations of stress.
“There are well documented studies that show meditation reverses the physiologic manifestations of stress such as elevated blood pressure and heart rate. In addition, there is now more gathering evidence that stress can have harmful effects on the functioning of our immune system. An abnormally activated immune system may actually trigger heart attacks and worsen coronary artery disease,” continued Dr. Lee.
Illustration: University of Pittsburgh Medical Center.
Medical Milestone: 2000 Lung Transplants

On December 6, 2016, UPMC marked a major medical milestone for the UPMC Lung Transplant Program: its 2000th lung transplant procedure. The Lung Transplant Program was established in 1982 and has become one of the largest and most experienced centers in the world for lung and combined heart-lung transplantation.
“We’re one of only a few programs in the United States that has achieved this volume, while maintaining outcomes that are on par with national averages,” said McGowan Institute for Regenerative Medicine affiliated faculty member Jonathan D’Cunha, MD, PhD, UPMC’s head of lung transplantation. “Reaching our 2000th lung transplant is a reflection of the clinical excellence found at UPMC.”
Dr. D’Cunha said reaching the 2,000-transplant mark — and being the first transplant center nationwide to do so — means that “when you come here, there is nothing we haven’t seen at this point. We use a team-based approach to navigate and get the best outcomes for the most complex patients.”
One of the first patients to receive a double lung transplant at UPMC was Paul McGuinness in November of 1988.
The Avalon, Pennsylvania, resident lived with cystic fibrosis while waiting 15 months before receiving his transplant and was told at the time that he had only a 10 percent chance of living 5 years. He’s now 65 years old.
“Of course I wanted to do the transplant,” said Mr. McGuinness. “When the surgeon took out my lungs, he said they were the worst he had ever seen. This transplant was a gift, and I cherish that gift. I’m lucky to be here.”
“One of the greatest things we do in medicine and surgery is giving breath again,” Dr. D’Cunha said. “These are patients carrying oxygen tanks — and not the person at the grocery store with an oxygen tank, but people who can’t leave the house or even get up without turning blue. They are very sick patients, and transplantation gives them hope for the future.”
Correcting Metabolic Deficiencies May Improve Depression Symptoms

Identifying and treating metabolic deficiencies in patients with treatment-resistant depression can improve symptoms and in some cases even lead to remission, according to new research from the University of Pittsburgh School of Medicine published in the American Journal of Psychiatry. McGowan Institute for Regenerative Medicine affiliated faculty member David Finegold, MD, professor of pediatrics, medicine, and human genetics at the University of Pittsburgh, is a co-author on the study.
This research is funded through a 2014 Pitt Innovation Challenge Award from Pitt’s Clinical and Translational Science Institute.
“What’s really promising about these new findings is that they indicate that there may be physiological mechanisms underlying depression that we can use to improve the quality of life in patients with this disabling illness,” said David Lewis, MD, Thomas Detre professor and chair of Pitt’s Department of Psychiatry.
Major depressive disorder, also referred to simply as depression, affects nearly 15 million American adults and is one of the most common mental disorders. Unfortunately, at least 15 percent of patients don’t find relief from conventional treatments such as antidepressant medications and psychotherapy, explained lead study investigator Lisa Pan, MD, professor of psychiatry, and clinical and translational science, Pitt School of Medicine. Depression also is the cause of more than two-thirds of suicides that occur annually.
The groundwork for the current study was laid 5 years ago when Dr. Pan and David Brent, MD, endowed chair in suicide studies at Pitt, treated a teen with a history of suicide attempts and long-standing depression. “Over a period of years, we tried every treatment available to help this patient, and yet he still found no relief from his depression symptoms,” she explained.
Searching for answers, Dr. Pan contacted Jerry Vockley, MD, PhD, chair of genetics, Children’s Hospital of Pittsburgh of UPMC, and Dr. Finegold, and through a series of biochemical tests, the three discovered that the patient had a cerebrospinal fluid deficiency in biopterin, a protein involved in the synthesis of several brain signaling chemicals called neurotransmitters.
After receiving an analogue of biopterin to correct the deficiency, the patient’s depression symptoms largely disappeared and today he is a thriving college student.
The success prompted the researchers to examine other young adults with depression who were not responding to treatment, explained Dr. Pan.
In the published trial, the researchers looked for metabolic abnormalities in 33 adolescents and young adults with treatment-resistant depression and 16 controls. Although the specific metabolites affected differed among patients, the researchers found that 64 percent of the patients had a deficiency in neurotransmitter metabolism, compared with none of the controls.
In almost all of these patients, treating the underlying deficiency improved their depression symptoms, and some patients even experienced complete remission. In addition, the further along the patients’ progress in the treatment, the better they are getting, Dr. Pan added.
“It’s really exciting that we now have another avenue to pursue for patients for whom our currently available treatments have failed. This is a potentially transformative finding for certain groups of people with depression,” said Dr. Pan.
This research also was supported by funding from the American Foundation for Suicide Prevention and a Brain and Behavior Research Foundation NARSAD Young Investigator Award. Additional funding from the Beck and Lohman families through the Children’s Hospital of Pittsburgh Foundation is supporting the testing and follow-up of additional patients with treatment-resistant depression and suicidal behavior, and allowing the researchers to begin to understand the underlying biological changes.
Abstract (Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Lisa A. Pan, MD, Petra Martin, BS, Thomas Zimmer, BS, Anna Maria Segreti, BS, Sivan Kassiff, BS, Brian W. McKain, RN, MSN, Cynthia A. Baca, RN, MSN, Manivel Rengasamy, MD, Keith Hyland, PhD, Nicolette Walano, MS, Robert Steinfeld, MD, Marion Hughes, MD, Steven K. Dobrowolski, PhD, Michele Pasquino, BS, Rasim Diler, MD, James Perel, PhD, David N. Finegold, MD, David G. Peters, PhD, Robert K. Naviaux, MD, PhD, David A. Brent, MD, Jerry Vockley, MD, PhD. The American Journal of Psychiatry; 2016.)
AWARDS AND RECOGNITION
MEBot Wins 2016 Blackwood Design Award

University of Pittsburgh’s Human Engineering Research Laboratory’s (HERL’s) Mobility Enhancement Robotic Wheelchair (MEBot) won the prestigious 2016 Blackwood Design Award for Best New Concept. The award judging panel was very impressed not only with the functionality of the design but the level of research involved as well as the fact that this was not looking for a problem to solve. It is intended for real life situations and comes from the experiences of the designers and engineers, several of whom use powerchairs.
McGowan Institute for Regenerative Medicine affiliated faculty member and principal investigator on the project Rory Cooper, PhD, FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, Professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh, and the Founding Director and VA Senior Research Career Scientist of HERL, says winning this recognition “is pretty exciting. It was a nice validation of our work and our design. Everybody is very happy.”
The Mobility Enhancement Robotic Wheelchair (MEBot) will tackle both curbs and challenging terrains. The large center driving wheels can reposition themselves to simulate front-, mid-, or rear-wheel driving. The four smaller caster wheels are controlled with compressed air and move up and down freely and independently. For climbing curbs, the front caster wheels lift up onto the curb, and then the driving wheels lift themselves up and forward onto the curb which lifts the chair onto the curb. This is done automatically, whenever MEBot senses a curb or step. The ultimate goal is for MEBot to climb a set of stairs.
The same general function is used to operate on icy or slippery surfaces. A traditional power wheelchair can get stuck on this kind of terrain. MEBot, however, uses its front and rear caster wheels to inch forward on the slick surface by extending its front casters, moving the seat forward, bringing the rear casters forward, and then repeating the process. Meanwhile, the seat stabilization system keeps the driver safely upright.
Watch the MEBot video here.
Blackwood is a pioneer in accessible housing, having built its first house in Dundee, Scotland, in 1972. Its founder, Dr. Margaret Blackwood, was the leading voice in Scotland in the ongoing campaign to improve the lives of people with disabilities in terms of housing, accessibility, and benefits, to name just a few. Today, the Blackwood vision is to be able to offer people beautiful, accessible, and affordable homes.
Congratulations, all!
Illustration: HERL.
Forbes Names Two Pitt Engineering Alumni “30 Under 30” in Manufacturing & Industry

With the new year comes a new class of honorees for Forbes’ 30 Under 30 ranking of young innovators and visionaries, and this year University of Pittsburgh Swanson School of Engineering alumni Noah Snyder, PhD, and Kasey Catt, PhD, were recognized for their work as founders of a coating and surface treatment startup. However, as with most entrepreneurs, they found that the trials and tribulations to bring one idea to fruition would actually serve as a lesson to bring a more successful product to market.
The idea for their company, Interphase Materials, started while Drs. Snyder and Catt were doctoral candidates in Pitt’s bioengineering program. They worked in the Neural Tissue Engineering Lab (NTE Lab) under advisor Tracy Cui, PhD, William Kepler Whiteford Professor of Bioengineering at Pitt and McGowan Institute for Regenerative Medicine faculty member.
“At the NTE Lab we investigate interactions between neural tissue and smart biomaterials,” explains Dr. Cui. “We research new tools to improve the performance of neural recording devices when implanted in tissue. Noah and Kasey, who we are proud to have as lab alumni, had a great impact on our research, but they had aspirations to take concepts from the lab and apply them directly to patients or other people in need.”
After participation in Pitt’s Coulter Translational Research Partners II Program, Dr. Snyder caught the entrepreneurial bug. He invited Dr. Catt to join him in the Innovation Institute’s Startup Pittblitz—a weekend-long dash for Pitt students to take a new business or product and make it ready to pitch to investors by Sunday afternoon. The two came up with an idea to apply a technology from their lab to the development of anti-microbial brain implants.
“The scientific approach of collecting lots of data and analyzing every detail differs greatly from the entrepreneurial mentality,” says Dr. Snyder. “When I started participating in some of the entrepreneurial programs offered at Pitt, I knew I wanted to take what we were working on in the lab and find a way to make it marketable, even if it meant making a lot of assumptions and discovering new things along the way.”
Their experience with Pittblitz encouraged them to enter the Randall Family Big Idea Competition, an annual startup competition helping Pitt students commercialize their ideas. Drs. Snyder and Catt tweaked their business plan to focus on dental implants and won the competition’s $25,000 top prize. They then entered Blast Furnace, a business accelerator for Pitt students, and won another first prize at the Wells Competition, both of which are offered by the Innovation Institute. At this time, they decided to make a critical pivot to the business.
“We realized registering dental implants with the Food and Drug Administration would be a long and difficult process. We also didn’t want to give away parts of the company to investors, so we knew we had to come up with something that would be self-sustaining in a short period of time. We wanted to make an impact on the world in two years, not 20,” says Dr. Snyder.
Drs. Snyder and Catt believed the technology behind the anti-microbial implants could also be used to develop a biochemical additive to prevent things like algae, mold, and fungus from contaminating a wide variety of surfaces. They turned their eyes toward industry solutions and were accepted into Alphalab Gear, an early-stage seed investment fund supported by the state. They officially launched Interphase Materials with Dr. Snyder serving as CEO and president and Dr. Catt as the CTO.
From Inside the Brain to Outside the Box
Interphase Materials began promoting an industrial coating that protected pipelines, bridges, and boats from contamination by marine life. According to Dr. Snyder, they quickly found a large potential market in tube and pipe coatings used for cooling power plants. They attracted the attention of construction and manufacturing companies, but their reputation didn’t stop there. The United States Navy offered them a contract to develop coating solutions for nuclear submarines.
Although they are trying to balance all of the possibilities for Interphase Materials with a focused business model, Dr. Snyder says he’s happy where the business is right now—in terms of both growth and geographical location.
“Kasey and I both have roots in western Pennsylvania, and we wanted to keep the company in the region,” explains Dr. Snyder. “Pittsburgh is one of the best places to be for the coating industry. PPG Industries, the largest coating company in the world, is headquartered here. Four of the top five largest coating companies internationally are located in Pennsylvania and Ohio. There is a huge talent pool. It’s like the Silicon Valley of advanced materials.”
The Forbes 30 Under 30 list comprises 20 industries, ranging from science and technology to art and entertainment, and seeks to “embrace the optimism, inventiveness and boldness of youth.” Tasked with investigating more than 15,000 applicants, a team of 80 judges and 50 staff reporters and editors made the final decisions about the honorees.
“After submitting the application to Forbes, I noticed my LinkedIn profile was getting a lot more views by people associated with the magazine,” Dr. Snyder says. “I think they were initially interested in us because we started out with brain implants and ended up working on nuclear subs, but all of the information about our business online helped. The University did a good job of supporting us and showcasing us along the way, which also helped us to realize that we could succeed. A lot of people were counting on us at Pitt. Now we have a whole new set of expectations we want to live up to.”
Drs. Snyder and Catt continue to collaborate with researchers at the University of Pittsburgh on the development of medical implants that are more compatible with the body and the immune system; however, their primary focus, Dr. Snyder admits, is the success of Interphase Materials.
Illustration: University of Pittsburgh Swanson School of Engineering.
Dr. Bryan Brown Lab Student Wins C. William Hall Scholarship

Ms. Meghan Wyatt, Senior in the Bioengineering Department at the University of Pittsburgh, Undergraduate Research Assistant at the McGowan Institute for Regenerative Medicine in the laboratory of faculty member Bryan Brown, PhD, and University Honors College Student Ambassador, is this year’s winner of the C. William Hall Scholarship from the Society for Biomaterials.
This award honors the memory of the Society’s first president, C. William Hall, MD. Dr. Hall was internationally recognized as a leader and pioneer in biomaterials and artificial organs research.
The scholarship is awarded to a junior or senior undergraduate pursuing a bachelor’s degree in bioengineering or a related discipline. During the year (Jan-Dec) of the scholarship award, it is required that the student participate in undergraduate research in biomaterials. The C. William Hall Scholarship applicants submit brief essays on their planned research and career objectives, as well as obtain a letter from their advisor/mentor confirming their status and role in the research.
Ms. Wyatt will have all of her expenses paid for participation in the 2017 Society for Biomaterials Annual Meeting and Exposition in Minneapolis, Minnesota (April 5-8, 2017).
The Society for Biomaterials is a multidisciplinary society of academic, healthcare, governmental, and business professionals dedicated to promoting advancements in all aspects of biomaterial science, education, and professional standards to enhance human health and quality of life.
Congratulations, Ms. Wyatt!
Illustration: Meghan Wyatt Twitter.
University of Pittsburgh Cancer Institute Academy Expands Summer Scholar Program to Include College Students

The University of Pittsburgh Cancer Institute (UPCI) Academy, now in its 9th year, will expand its summer medical research program for high school students to include additional research experiences for academy alumni currently attending college. Three McGowan Institute for Regenerative Medicine affiliated faculty members will participate as mentors in the 2017 program:
- Deborah Galson, PhD, Associate Professor of Medicine, Division of Hematology/Oncology and of Microbiology & Molecular Genetics, University of Pittsburgh School of Medicine, at the Cancer Biology Site
- Andrew Duncan, PhD, Assistant Professor in the Department of Pathology at the University of Pittsburgh, at the Cancer Environment, Bioengineering, Imaging and Genetics Site
- Steffi Oesterreich, PhD, Professor of Pharmacology and Chemical Biology at UPCI, at the Women’s Cancer Research Center Site at Magee-Womens Research Institute
The expansion is funded, in part, by the Doris Duke Charitable Foundation (DDCF), which launched the Clinical Research Continuum: High School to College program to provide funds for eight institutions, including the UPCI Academy. The goal is to increase the diversity of the biomedical research workforce by providing hands-on experience for students, particularly from minority groups, who are underrepresented in medicine.
Over the course of its history, the UPCI Academy has become an award-winning science, technology, engineering and mathematics program that prepares college-bound teenagers and those just beginning college for successful careers in science and medicine.
“It is well known that there is a lack of diversity in the biomedical workforce,” said David Boone, PhD, executive director of the UPCI Academy. “This program allows us to provide hands-on experience for high-achieving students who may not otherwise have access. They must meet strict standards and demonstrate commitment and excitement to science to be selected.”
Scholars in the UPCI Academy engage in cutting-edge cancer research by working alongside scientists and cancer clinicians in laboratories at six sites across the University of Pittsburgh campus. Since 2009, the program has grown from five participants to an expected 60 in 2017. This year, for the first time, the program also will include four UPCI Academy alumni currently enrolled in college along with the high school juniors and seniors.
“We are empowering these students to move forward with careers in the biomedical field. UPCI Academy has helped students publish research papers in peer-reviewed journals, and several also have presented research findings at national symposia,” said Dr. Boone.
In addition to the McGowan Institute affiliated faculty members, the students will work alongside the following UPCI researchers during their term at UPCI Academy at these different sites: Joseph Ayoob, PhD, The Drug Discovery, Systems and Computational Biology Site; Greg M. Delgoffe, PhD, and Tullia Bruno, PhD, Tumor Immunology Site; Gary Thomas, PhD, and Carolyn Anderson, PhD, The Cancer Environment, Bioengineering, Imaging and Genetics Site; David Boone, PhD, The Computer Science, Biology and Biomedical Informatics Site.
In addition to the Doris Duke Charitable Foundation (DDCF), the UPCI Academy, founded by Michael Lotze, MD, professor in the Pitt Department of Surgery, also receives funding from the National Cancer Institute CURE Program, Jack Kent Cooke Foundation, and UPMC, as well as support from UPCI, Pitt’s Department of Biomedical Informatics, and donations from grateful parents and patients.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#168 –– Dr. Rory Cooper is FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh. He is also Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories, a VA Rehabilitation R&D Center of Excellence in partnership with Pitt, as well as the Co-Director of the NSF Quality of Life Technology Engineering Research Center. Dr. Cooper discusses his work in rehabilitation engineering applied to mobility and manipulation.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
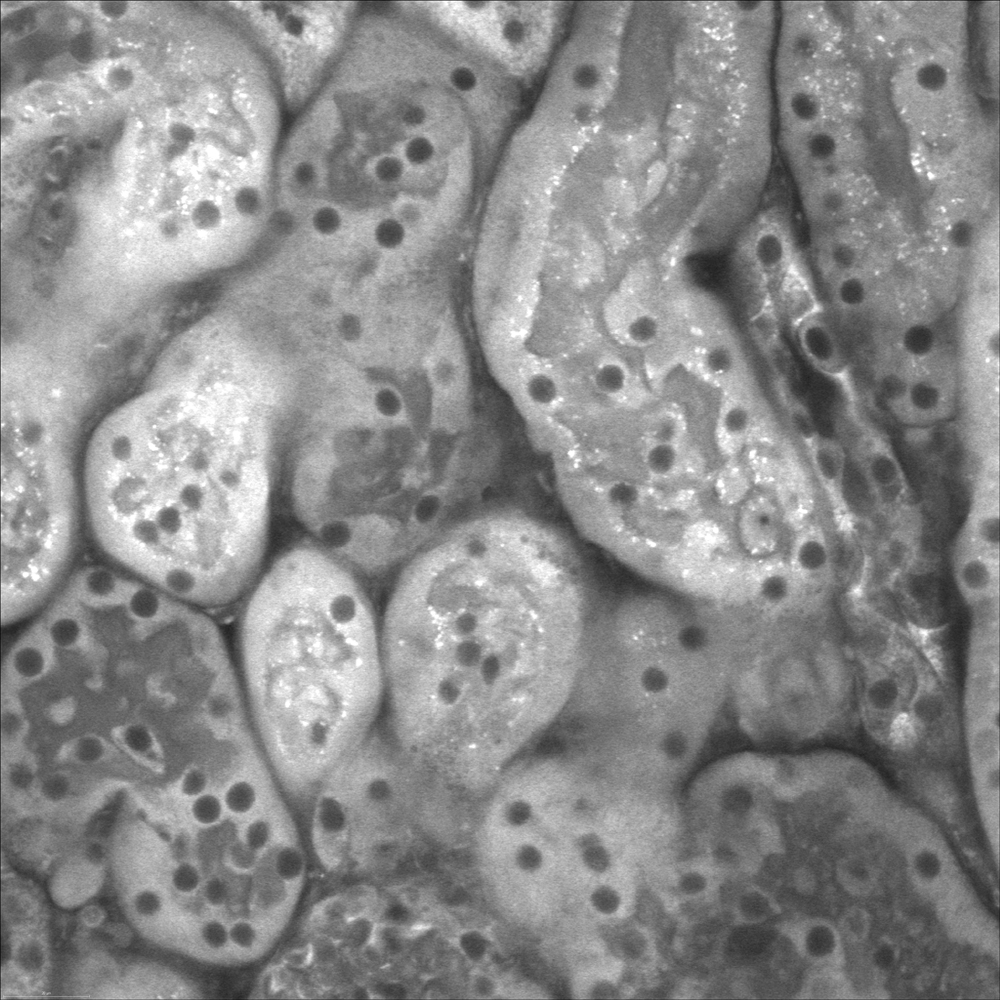
Live cell confocal imaging of ex vivo mouse kidney slice. Mitochondria stained with MitoTracker Red CMXRos, 40X objective, 1.1 NA.
Attribution: Catherine Baty DVM, PhD, Renal Electrolyte Division, Dept of Medicine.
PUBLICATION OF THE MONTH
Author: Zhang C, Ferrari R, Beezhold K, Stearns-Reider K, D’Amore A, Haschak M, Stolz D, Robbins PD, Barchowsky A, Ambrosio F
Title: Arsenic Promotes NF-Κb-Mediated Fibroblast Dysfunction and Matrix Remodeling to Impair Muscle Stem Cell Function
Summary: Arsenic is a global health hazard that impacts over 140 million individuals worldwide. Epidemiological studies reveal prominent muscle dysfunction and mobility declines following arsenic exposure; yet, mechanisms underlying such declines are unknown. The objective of this study was to test the novel hypothesis that arsenic drives a maladaptive fibroblast phenotype to promote pathogenic myomatrix remodeling and compromise the muscle stem (satellite) cell (MuSC) niche. Mice were exposed to environmentally relevant levels of arsenic in drinking water before receiving a local muscle injury. Arsenic-exposed muscles displayed pathogenic matrix remodeling, defective myofiber regeneration and impaired functional recovery, relative to controls. When naïve human MuSCs were seeded onto three-dimensional decellularized muscle constructs derived from arsenic-exposed muscles, cells displayed an increased fibrogenic conversion and decreased myogenicity, compared with cells seeded onto control constructs. Consistent with myomatrix alterations, fibroblasts isolated from arsenic-exposed muscle displayed sustained expression of matrix remodeling genes, the majority of which were mediated by NF-κB. Inhibition of NF-κB during arsenic exposure preserved normal myofiber structure and functional recovery after injury, suggesting that NF-κB signaling serves as an important mechanism of action for the deleterious effects of arsenic on tissue healing. Taken together, the results from this study implicate myomatrix biophysical and/or biochemical characteristics as culprits in arsenic-induced MuSC dysfunction and impaired muscle regeneration. It is anticipated that these findings may aid in the development of strategies to prevent or revert the effects of arsenic on tissue healing and, more broadly, provide insight into the influence of the native myomatrix on stem cell behavior.
Source: Stem Cells. 2016 Mar;34(3):732-42. Epub 2016 Jan 8.
GRANT OF THE MONTH
PI: Bryan Brown
Co-I: Pamela Moalli and Stephen Badylak
Title: Assessing the Impact of Macrophage Polarization Upon the Success of Biomaterial Implants
Description: There are several criteria by which the success or failure of implantable materials can be measured; but, without question, the manner in which the host responds to the implanted material will be a critical determinant of outcome. Largely, the interaction of immune cells with implanted materials has been considered a precursor to the foreign body reaction with associated negative impacts upon functionality. Recently, the role of the innate immune system, particularly that of macrophages, in the host response to biomaterials has received renewed attention. It has now been shown that macrophages, depending upon highly plastic and context- dependent polarization profiles (i.e. M1 pro-inflammatory vs. M2 anti-inflammatory/regulatory), are also capable of affecting positive outcomes following biomaterial implantation. This emerging understanding of the essential constructive and regulatory roles of macrophages in positive outcomes represents a significant departure from the classical paradigms of host biomaterial interactions. It now appears desirable that emerging biomaterials-based approaches to tissue reconstruction should not only accommodate but also promote involvement of the immune system to facilitate positive outcomes. However, such approaches cannot be developed without a detailed understanding of both the contributions of individual macrophage subtypes to tissue remodeling and integration and the context in which host encounters the implant. The present proposal seeks to develop a comprehensive and integrated approach to broadening the current understanding of how macrophages, their functional subsets, and host characteristics affect the host response to biomaterials. The proposed work builds upon our previous studies demonstrating that macrophage M1/M2 polarization at early time points is predictive of downstream integration outcomes and that the implantation microenvironment (i.e. tissue type, age, disease status) strongly affects the host response and subsequent tissue remodeling outcomes. Additionally, we have developed a number of model systems for assessing the specific contributions of M1 and M2 macrophages in the host response to biomaterials and novel methods for the modulation of the host response at the biomaterial surface. Collectively, these studies will provide crucial insight into the use of biomaterials as implants and provide methods for promoting improved outcomes associated with their use.
Biomaterial implants are used ubiquitously throughout medicine to save and improve the quality of life for patients. There are several criteria by which the success or failure of implantable materials can be measured; but, without question, the manner in which the host responds to the implanted material will be a critical determinant of outcome. The present proposal seeks to develop a comprehensive and integrated approach to broadening the current understanding of how macrophages, their phenotypic subsets, and host characteristics affect the host response to biomaterials and develop methods for modulating the host-biomaterial interface to improve outcomes associated with biomaterial implantation.
Source: National Institutes of Health
Term: 5 years
Amount: $1.54 million
