December 2022 | VOL. 21, NO. 12| www.McGowan.pitt.edu
Life After Weight Loss Program

After undergoing bariatric surgery, losing a large amount of weight is a major feat. But it might leave you with excess skin that can affect your self-esteem and remind you of your past weight. UPMC’s Life After Weight Loss program can help with a focus on plastic surgery for people after massive weight loss. The program is nationally and internationally known for its cutting-edge body contouring surgical techniques. In fact, many of the methods used by plastic surgeons around the world were pioneered by UPMC surgeons.
Ashley Welch, writer for WebMD.com, covered the process of this program with McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD (pictured), Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh.
During body contouring procedures, the patient is put under general anesthesia, and a surgeon trims excess skin and fat. Remaining skin is sutured together.
“These surgeries can really be done from head to toe,” says Dr. Rubin.
Surgeons focus on areas of the body that patients find most bothersome. Dr. Rubin says one of the most common contouring procedures is a panniculectomy, also called an abdominoplasty or tummy tuck.
Other procedures include arm lifts, female breast reshaping, male breast reductions, thigh and buttock lifts and reshaping, face and neck lifts, and liposuction.
People must meet certain clinical criteria before they get body contouring, including maintaining their goal weight for at least 6 months.
“You don’t want to take someone to the operating room who’s actively losing weight,” Dr. Rubin says.
Generally, a plastic surgeon will perform these procedures only 12 to 18 months after bariatric surgery.
The cost can range between $5,000 and $10,000 per procedure, Dr. Rubin says. Sometimes, insurance companies cover the bill, depending on the state and individual plans.
“After undergoing body contouring procedures, people should expect to be out of work for 2 to 4 weeks and should hold off on strenuous activities for at least 6 weeks,” Dr. Rubin says.
Risks from body contouring procedures are the same as with any major surgery, and include fluid buildup, infections, and bleeding.
Another thing to consider is that skin removal surgeries leave scars, which vary from person to person but can be extensive.
For more information on this procedure, please phone for a consultation at 412-641-3960 or 1-877-639-9688.
RESOURCES AT THE MCGOWAN INSTITUTE
January Histology Special – Frozen Sections
Safranin O staining is used for the detection of cartilage, mucin, and mast cell granules on formalin-fixed, paraffin-embedded tissue sections or frozen sections. The cartilage and mucin will be stained orange to red, and the nuclei will be stained black. The background is stained bluish green.
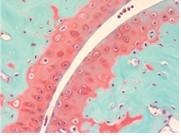
You’ll receive 30% off your Safranin O staining in January when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
UPCOMING EVENTS
Save the Date! 2023 McGowan Institute Scientific Retreat

The venue for the 2023 Retreat is the University Club, and the dates are March 6 & 7, 2023.
The program committee for the 2023 McGowan Institute Scientific Retreat, under the leadership of Bryan Brown, PhD, has begun to formulate the program and is requesting your suggestions for session topics.
The committee is also seeking to learn of possible interest by individuals in organizing a session. Please submit your program suggestions here.
SCIENTIFIC ADVANCES
FDA IND Received for Gene Therapy for the Treatment of Retinitis Pigmentosa

SparingVision is a biotechnology company focused on the discovery and development of innovative therapies for the treatment of blinding inherited retinal diseases (IRDs). SparingVision is developing SPVN06, a breakthrough gene therapy approach aimed at stopping or slowing disease progression in patients affected by IRDs and dry age-related macular degeneration (AMD), regardless of their genetic background. McGowan Institute for Regenerative Medicine affiliated faculty member José-Alain Sahel, MD (pictured), Chair of the Department of Ophthalmology at the University of Pittsburgh School of Medicine, Director of the UPMC Eye Center, the Eye and Ear Foundation Chair of Ophthalmology, and Director of the Institut de la Vision (Sorbonne Universite, Inserm, CNRS, Paris), is a co-founder of SparingVision along with Thierry Léveillard, PhD.
SparingVision announced the FDA has cleared the company’s Investigational New Drug application (IND) for SPVN06, its lead gene independent therapy for the treatment of retinitis pigmentosa (RP).
According to a news release, the company has also submitted a clinical trial authorization (CTA) application to the French regulator (ANSM), which is currently under review.
The company noted that the approval paves the way for the start of Promising Rod-Cone Dystrophy Gene Therapy (PRODIGY), a first-in-human (FIH) Phase I/II clinical trial. First safety data are anticipated in 2023 and the primary endpoint is expected to be reached in 2025.
PRODYGY trial design
The company noted that PRODYGY is a Phase I/II study to assess the safety, tolerability, efficacy, and quality of life following a single subretinal injection of SPVN06 in the worst-seeing eye of adult patients with RP due to a mutation in the RHO, PDE6A, or PDE6B gene. The study will recruit up to a total of 33 patients, in two steps:
Step One: open-label, dose-escalation phase including three cohorts of three subjects with advanced forms of RP, to determine two recommended highest tolerated doses for Step Two. The decision to initiate cohorts 2 and 3 will be based on the review by a Data Safety Monitoring Board (DSMB) of all available safety data from all subjects of the previous cohort(s).
Step Two: controlled, double-masked randomized phase including 24 patients with advanced intermediate RP, divided into three cohorts: six untreated patients and 18 patients receiving either the low or high dose determined through Step One.
The primary endpoint of PRODYGY is the safety and tolerability of SPVN06, 12 months after administration of a single injection of the gene therapy. Secondary objectives include notably preliminary efficacy and quality of life data. In addition, the long-term follow-up of the study will focus on safety and tolerability for a total of five years after treatment administration.
Daniel Chung, MD, chief medical officer of SparingVision, noted that RP is a highly prevalent eye disease leading inevitably to blindness with no treatment available for the vast majority of patients.
“The unique neuroprotective mechanism of action of SPVN06 has the potential to change the course of the natural history of the disease, independently of the genetic background of patients and of the time of disease diagnosis,” he said in the news release. “With this Phase I/II trial we will be looking to demonstrate SPVN06’s safety and tolerability as well as identify preliminary signs of efficacy through several structural, functional, and quality of life endpoints.”
SPVN06 is a breakthrough gene therapy approach aimed at stopping or slowing disease progression in patients affected by IRDs and dry age-related macular degeneration (AMD), regardless of their genetic background. SparingVision is initially focused on mid-stage RP, one of the leading causes of blindness globally that affects two million patients. SPVN06 counteracts the degeneration of cone photoreceptors by restoring RdCVF, a neurotrophic factor naturally produced by functioning rods in the retina; and by promoting RdCVFL, a potent antioxidant which protects cones against oxidative stress.
Moreover, the company noted that the DNA of the two distinct isoforms (RdCVF and RdCVFL) of the NXNL1 gene are supplied via an Adeno-associated virus (AAV), the viral vector of choice for retinal gene therapy. The treatment is delivered by subretinal injection, a route of administration that has already been proven safe and efficacious for the delivery of gene therapy.
Stéphane Boissel, president and CEO of SparingVision, lauded the FDA’s clearance.
“Securing the IND clearance is a testament to the incredible efforts of everyone at SparingVision, but also to the strength of the science, which encompasses more than 20 years of leading research by our scientific founders, including Professor José Sahel, chair of the Ophthalmology Department at the University of Pittsburgh Medical Center,” Mr. Boissel explained. “With over 80 genes involved in RP, each with numerous causative mutations, we need to go beyond the gene-by-gene treatment approach. SPVN06 has the potential to become the universal therapeutic solution that patients need, and we are excited for the next phase of development.”
HeadSMART II Clinical Study of BRAINBox TBI Concussion Diagnostic and Prognostic Test

BRAINBox Solutions has enrolled the 1,000th patient in the pivotal HeadSMART II study of its concussion diagnostic and prognostic test, BRAINBox TBI (Traumatic Brain Injury). The multi-national, multi-site trial is designed to support an application for regulatory clearance by the U.S. FDA.
McGowan Institute for Regenerative Medicine affiliated faculty member David Okonkwo, MD, PhD (pictured), Professor at the University of Pittsburgh Medical Center and Director, Neurotrauma Clinical Trials Center, Director, Scoliosis and Spinal Deformity Program, and Special Advisor, UPMC Enterprises, is the Chair of the Scientific Advisory Board (SAB) and has been active in the study design and oversight.
“We are developing the BRAINBox TBI test as the first objective test to aid in the diagnosis of concussion and to provide an assessment of the risk of post-concussive symptoms,” said Donna Edmonds, BRAINBox Solutions’ CEO. “We are now in the home stretch of this very important study for the field. We believe the test has great potential to change clinical practice in the diagnosis and management of concussion and will define for the first time, objective diagnostic criteria for this condition, termed Acute Traumatic Encephalopathy (ATE). As such, the trial has been rigorously designed in collaboration with leading experts in traumatic brain injury and emergency medicine. The study evaluates patients in multiple settings and includes state-of-the-art expert adjudication panels.”
She added that the current study is being conducted in adults (18 years old or older). A separate National Institutes of Health-supported, multi-center study in geriatric subjects is expected to begin enrollment in late 2022 to establish a geriatric specific test for diagnosis and prognosis. Additionally, the company is engaged a Pilot Study for a Pediatric Test for individuals under the age of 18, aimed at the same claims as the adult study.
The BRAINBox TBI multi-modal test combines clinical data, neurocognitive testing, symptom reporting and blood-based biomarkers, with proprietary AI algorithms to generate an objective score for diagnosis up to 96 hours from the time of injury. In addition, a prognosis report is generated, providing the likelihood of injury-related symptoms occurring at 30 days and up to three months after the event. The clinical study sites were selected to reflect real world practice and include Level I Trauma Centers, Emergency Departments, and Urgent Care settings in systems and community-based hospitals.
The HeadSMART II study’s primary endpoints include ATE diagnosis, as determined by the BRAINBox TBI test, compared with the Gold Standard diagnosis and the test’s ability to predict persistent symptoms up to 90 days after an ATE diagnosis.
The Gold Standard for diagnosis is determined by an adjudication committee of clinical experts blinded to the test results. Two randomly selected experts each make a diagnostic determination based on evaluation of the clinical information, including imaging and neurological assessments. If there is discordance, a third expert performs an independent review and serves as the tie breaker. There is a similar expert adjudication process for neuroimaging data from participants who receive a head CT scan or MRI as part of standard of care.
Adjudicated TBI patients are stratified as having high- or low-risk for post-concussive symptoms in each symptom category, based on biomarker and neurocognitive testing.
The design and oversight of the study is being conducted by leading experts in emergency medicine and neurotrauma, including: W. Franklin Peacock MD FACEP, Principal Investigator, and Professor of Emergency Medicine and Vice Chair for Research in the Department of Emergency Medicine at Baylor College of Medicine; Damon Kuehl, MD, Vice Chair for Research in the Department of Emergency Medicine for Carilion Clinic and Vice Chair of the Virginia Tech Carilion School of Medicine’s Department of Emergency Medicine; and Ramon Diaz-Arrastia, MD, PhD, Professor of Neurology, Director, Clinical TBI Research Center, University of Pennsylvania, Perelman School of Medicine, all of whom are enrolling subjects in the study. Drs. Peacock, Kuehl, and Diaz-Arrastia, also serve on the BRAINBox Solutions SAB. An additional SAB member who has been active in the study design and oversight is Alan Wu, Professor Laboratory Medicine, Chief, Clinical Chemistry Laboratory, SFGH, and Chief, Clinical Pharmacogenomics Laboratory, UCSF.
Pitt’s Healthy Home Lab Receives Funding to Make Homes Safer for Older Adults

Natalie Baney reports for Inside UPMC that Pitt is one of seven institutions to receive a portion of a $5.7 million research grant from the U.S. Department of Housing and Urban Development, or HUD. Pitt’s Schools of Health and Rehabilitation Sciences (SHRS), Public Health and Engineering, and community partners Women for a Healthy Environment (WHE) and the Allegheny County Area Agency on Aging (AAA) received a $918,709 grant to develop assessment tools and interventions to make homes safer for seniors, people with disabilities, and other vulnerable populations.
“We are excited to work with HUD and two terrific community partners to improve housing conditions for better health,” said co-principal investigator Dan Ding, PhD (pictured), associate professor at SHRS and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “Pitt recently established the Healthy Home Laboratory, a community laboratory based in a 105-year-old Pittsburgh home. This will be the perfect testbed for the HUD project.”
Through HUD’s grant, Pitt’s multi-disciplinary team will build a new residential environmental hazards assessment module to be added to existing home health assessments.
“The current tools used in Medicare and Medicaid Home and Community Based Services often fail to assess the ‘health’ of the home itself,” said Dr. Ding.
According to Sarah Haig, PhD, co-investigator and assistant professor in Pitt’s Swanson School of Engineering’s Department of Civil and Environmental Engineering, indoor air quality and the presence of mold are also important health considerations.
“Mold in the home may pose a major health concern, and accurately quantifying and assessing mold risk can be a complex, expensive and time-consuming task. Our goal is to develop a simpler and less expensive mold assessment method that can provide near-real-time risk assessment,” said Dr. Haig.
“HUD notes that a ‘home is a determinant of health.’ This is especially true for older adults and people with chronic conditions and disabilities,” said co-principal investigator Steven M. Albert, PhD, professor and Hallen Chair, Department of Behavioral and Community Health Sciences, Pitt’s School of Public Health. “Some home environments support health while others cause excess morbidity. The highly vulnerable populations receiving these services face substantial risk from unmeasured home environment hazards that may interact with their health conditions.”
Demand for services in the home continue to increase. In 2021, over 160,000 Pennsylvanians with physical and age-associated disabilities received in-home services through Pennsylvania OPTIONS and the new Medicaid Community Health Choices program, including many residents of Allegheny County.
Jon Pearlman, PhD, chair of the Department of Rehabilitation Science and Technology in SHRS, said that Pitt’s Healthy Home Lab will be the key research site.
Dr. Pearlman, who will also serve as the technical director of the Healthy Home Lab, explained that the lab will be used to develop real-world simulations of environmental hazards, including low temperatures, high humidity, and poor indoor air quality. Researchers will use these simulations to develop an assessment tool that will be piloted in the community. The team will test the usability of the tool with AAA and WHE home health assessors and prepare training materials with these partner organizations.
By 2050, the U.S. population over 65 will almost double to reach 83.7 million. The majority of seniors and people with disabilities prefer to remain in their homes as they age. However, according to the U.S. Census Bureau, only about 10% of homes are “aging-ready.”
“HUD is a leader in addressing the health aspects of home environments and has made major gains in identifying and remediating conditions, such as lead and asbestos, that are linked to significant health risks,” Dr. Pearlman said. “One of the important aspects of partnering with HUD is that the agency shares our interest in developing near-term practical solutions and then supporting their implementation through community partners. This aligns perfectly with the goals of our Healthy Home Lab to develop scalable solutions to make houses safer and more age friendly.”
Dr. Roberto Mota Alvidrez Receives FOCUS Funding

A new sub-award for research to be conducted by McGowan Institute for Regenerative Medicine affiliated faculty member Roberto Mota Alvidrez, MD (pictured), has been approved for funding. Dr. Mota Alvidrez is the principal investigator on the project, “Role of Cytosolic Phospholipase A2 in Vascular Wall Lipid Accumulation in Atherosclerosis,” which will be funded by the University of California (UC), San Diego Future Faculty of Cardiovascular Sciences (FOCUS) program. The specific objectives of the UC San Diego FOCUS program are to enhance the academic skills and research programs of underrepresented early career academics in cardiovascular sciences and success in obtaining NIH or equivalent funding. Underrepresented early career junior faculty and transitioning postdoctoral fellows who have cardiovascular scientific expertise maximally benefit from the program.
Dr. Mota Alvidrez is a Research Assistant Professor, Department of Surgery, University of Pittsburgh Medical Center. His research program identifies timely and accurate preclinical diagnostic modalities that can be translated to the clinic in diabetic vasculopathy. The program also focuses on identifying therapeutic targets and vascular drug delivery and immunotherapies in the still unresolved and important biomedical problem of diabetic vasculopathy with three innovative and timely approaches: 1) use of tissue specific knockout animal models with a focus in cardiovascular pathophysiology using translational small animal surgical approaches to mimic human conditions, 2) discovery of novel preventive and diagnostic cardiovascular serologic biomarkers of disease to translate preclinical point of care approaches in clinical pathology and laboratory medicine, and 3) implementation of molecular imaging and immunobiology assays and techniques for parallel in vivo cardiovascular morphology/function and protein/mRNA assays ex vivo using the state-of-the-art metabolomics and lipidomic assessment in genetically modified small animal models.
Congratulations, Dr. Mota Alvidrez!
Bringing New Hope to Patients Suffering from Lung Fibrogenesis

The NIH National Heart, Lung, and Blood Institute funded research entitled “Discovering Extracellular Modulators of Lung Fibrogenesis by Profiling Newly Synthesized Extracellular Matrix,” which will markedly advance the understanding of how extracellular matrix dysregulation leads to lung fibrosis (interstitial lung disease). The work will promote extracellular targeted therapeutic development that is expected to complement and boost the efficacy of existing cell-centric therapies in treating lung fibrosis. Thus, knowledge derived from this research will bring new hope to patients suffering from this devastating, currently irreversible respiratory condition.
McGowan Institute for Regenerative Medicine affiliated faculty member Xi Ren, PhD (pictured), Assistant Professor in the Department of Biomedical Engineering at Carnegie Mellon University, is the project principal investigator of this 1-year effort. Work began on September 20, 2022.
The abstract of the project follows:
Interstitial lung diseases (ILD) are devastating disorders causing progressive scarring (i.e., fibrosis) of lung tissue, and result from reciprocal interactions of cellular abnormalities and extracellular matrix (ECM) dysregulation. Current ILD treatment primarily targets cellular signaling and remains unable to halt or reverse fibrogenesis. Despite being a key pathological hallmark of ILD, the ECM has rarely been directly targeted for therapeutic intervention. Fundamentally, the extracellular mechanism underlying lung fibrosis progression remains elusive. To bridge this gap, our objective is to develop an innovative approach for selective profiling of newly synthesized ECM (newsECM) along lung fibrogenesis. The proposed technology will selectively label newsECM produced over defined short time spans by incorporating chemoselective azido-tags via post-translational glycosylation. This will enable enrichment of newsECM free from the pre-existing ECM, and thereby enables sensitive proteomic detection of the dynamic ECM synthesis with unprecedented daily temporal resolution, irrespective of the abundant pre-existing ECM background. Our proposed approach has an inherent preference to ECM proteins, the majority of which are glycosylated. The proposed newsECM profiling will address a major technical barrier in conventional, non-selective mass spectrometry analysis, which suffers from limited sensitivity in detecting the dynamic new ECM deposition that is usually in low abundance. The proposed newsECM profiling technology is versatile and will be implemented in three lung fibrosis models, including in vivo mouse fibrosis models, an ex vivo donor lung perfusion model, and an in vitro synthetic fibrosis model. We intend to pursue the following specific aims. Aim 1 will track newsECM dynamics in vivo during the progression and resolution of lung fibrogenesis, which is expected to reveal pro- and anti-fibrotic ECM factors. Aim 2 will establish the correlation between the in vivo and ex vivo newsECM profiles using murine lungs as a model and apply the resulting optimized newsECM profiling condition and algorithm to the ex vivo lung perfusion (EVLP) of donor human lungs bearing idiopathic pulmonary fibrosis (IPF), the most common form of ILD, to reveal human-specific pathogenic ECM mechanism. Finally, Aim 3 will establish a synthetic lung fibrosis model tissue-engineered combining fibroblast, epithelium, endothelium and macrophage within decellularized native lung ECM scaffold, and use it as an experimentally tractable system to further decode fibrogenic lung cell-ECM interaction. Furthermore, combining newsECM labeling and native ECM biomaterial engineering, we will offer a chemoselective platform for effective functional evaluation of candidate fibrosis-modulating ECM factors in a biomimetic, ECM-associated manner. In summary, this research will facilitate a paradigm shift in ILD treatment by promoting ECM-targeted therapeutic development and by enabling combined therapeutic interventions aiming at both cellular and extracellular targets to bring new hope for the impacted patients.
Congratulations, Dr. Ren!
Improving Survival During ECMO

The NIH National Heart, Lung, and Blood Institute recently funded a project which will examine a means of providing potent anticoagulation within extracorporeal membrane oxygenation (ECMO) circuits without limiting normal coagulation in a patient’s tissues. To accomplish this, principal investigator Keith Cook, PhD (pictured), Professor and Chair of the Department of Biomedical Engineering at Carnegie Mellon University and affiliated faculty member of the McGowan Institute for Regenerative Medicine, and his team propose combining polycarboxybetaine surface coatings with a highly selective bicyclic peptide Factor XIIa inhibitor. Together, these should improve survival during ECMO and enable long-term respiratory support outside the intensive care unit.
The almost 4-year project entitled “Combined Use of Polycarboxybetaine Coatings with a Selective FXIIa Inhibitor to Create Potent Biomaterial Anticoagulation Without Bleeding During Extracorporeal Life Support,” began on September 15, 2022.
The abstract of this project follows:
Over 190,000 people suffer from acute respiratory distress syndrome in the US each year, with mortality rates from 30-40% with the best treatment. In addition, there are over 12 million patients with chronic lung disease, 6.9 million emergency room visits, and over 180,000 deaths. When mechanical ventilation is insufficient to support these patients, extra-corporeal membrane oxygenation (ECMO) is used as a bridge-to-recovery or bridge-to-transplantation. Unfortunately, ECMO is plagued by bleeding and thrombotic complications that reduce patient survival by approximately 40 and 33%, respectively. The cause of coagulation is primarily surface adsorption of plasma proteins, subsequent activation of the intrinsic branch of the coagulation cascade, and platelet binding to adsorbed fibrinogen. This is combated using systemic, intravenous heparin, but this inhibits both biomaterial-induced coagulation in the ECMO circuit and tissue-factor-induced coagulation in the patient’s tissues, resulting in bleeding complications. To eliminate both of these problems simultaneously, we propose to combine two means of selectively inhibiting coagulation at the blood-biomaterial interface while leaving tissue-based coagulation intact. The first is biomaterial surface coating with zwitterionic polycarboxybetaine (PCB). Our initial results demonstrate that the PCB coating dramatically decreases protein adsorption and platelet binding in vitro and long-term clot formation during sheep ECMO. The second is FXII900, a potent, highly-selective bicyclic peptide FXIIa inhibitor. FXII900 inhibits surface-induced activation of coagulation at nanomolar concentrations without affecting the tissue-based extrinsic branch or common branch of the coagulation cascade. In our preliminary, short-term rabbit ECMO studies, we demonstrate a 94% reduction in clot formation vs. standard clinical heparin anticoagulation. At the same time, FXII900 plus PCB maintained a normal bleeding time, while the heparin increased the bleeding time to 2.9 times normal. The goals of this proposal are to extend this technology toward clinical applications by i) proving the effectiveness of combined PCB plus FXII900 anticoagulation during 5-day in vivo extracorporeal life support and ii) developing long-acting FXII900 formulations that enable bolus dosing every 8 or 12 hours rather than a continuous intravenous drip. If successful, these studies would lead to a clinical anticoagulation strategy that i) reduces bleeding and thrombotic complications during ECMO, ii) reduces ECMO mortality, and iii) simplifies clinical application of ECMO. These benefits, when combined, might also allow safe long-term ECMO outside the intensive care unit.
Congratulations, Dr. Cook!
Study Reveals Dual Roles Wielded by Type of Cell Death Involved in Fighting Cancer

The immune system is the body’s army against bacteria, viruses or any foreign pathogen that can cause disease. This innate defense system also protects the body against irregularities, such as cancer cells, by marking them as “dangerous” and targeted for destruction.
Inducing cell death has been a significant area of interest for cancer therapy, with one notable pathway of cell death gaining traction since its discovery a decade ago: ferroptosis. New research published in Nature reveals that ferroptosis is doing more than marking cells for death – it’s also sending signals that influence the immune response. Lisa Hong reports on these new findings for Inside UPMC.
“Ferroptosis is an exploding field,” said study co-author Valerian Kagan, PhD, DSc (pictured), professor in the University of Pittsburgh School of Public Health’s Department of Environmental and Occupational Health and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “Understanding exactly how it works in not only destroying tumor cells, but also in helping immune cells function correctly to fight cancer will show doctors when to induce ferroptosis and when to inhibit it.”
Ferroptosis is a specific type of programmed cell death that is dependent on iron (“ferro” meaning iron in Latin), as this element participates in electron-transfer reactions, also known as redox reactions, that can damage cells.
Ferroptosis-inducing drugs are effective at killing cancer cells in a dish. These drugs also greatly reduce tumors in immunodeficient mice with cancer. However, mice that have working immune systems do not respond as well to ferroptosis-inducing drugs.
The newly published, National Institutes of Health-funded study investigates this discrepancy.
Dr. Kagan and Dmitry Gabrilovich, MD, PhD, chief scientist of cancer immunology at AstraZeneca, found that pathologically activated neutrophils, unlike normal neutrophils that inhibit cancer, actually suppress anti-tumor immune response and are associated with poor clinical outcomes in cancer. In the tumor microenvironment, ferroptosis spontaneously kills pathologically activated neutrophils, suggesting that inducing ferroptosis could be a therapeutic target in cancer therapy. However, the new study found that ferroptosis also causes the release of immunosuppressive molecules from these neutrophils, which inhibit another important class of cancer-fighting cells called T cells. As such, the researchers found that inducing ferroptosis actually promoted tumor growth in mice with functional immune systems.
“The word ferroptosis initially meant only death – a ferroptotic type of death,” said Dr. Kagan. “Now this has to be revised because we’ve shown ferroptosis is a much broader response of cells to dysregulation of redox reactions.”
Future research will need to characterize the cascading signals set off by the redox reactions that instruct cells to undertake various actions, Dr. Kagan said. By understanding this process, doctors may someday be able to incorporate a precision medicine approach to using ferroptosis to treat cancer, inhibiting or inducing it depending on whether the patient’s immune system is functional.
Ms. Hong is a PhD student in the University of Pittsburgh School of Medicine’s Molecular Pharmacology Program. She is participating in the UPMC Science Writing Mentorship Program.
AWARDS AND RECOGNITION
2022 NAI Fellows Named: Drs. James Antaki and Joseph Glorioso III

The National Academy of Inventors (NAI) has named the induction of 169 distinguished inventors to be NAI Fellows. Election as an Academy Fellow is the highest professional distinction awarded to academic inventors. Two McGowan Institute for Regenerative Medicine affiliated faculty members are a part of this distinguished group. They are:
James Antaki, PhD (pictured top): At Cornell University, Dr. Antaki is the Susan K. McAdam Professor of Heart Assist Technology in the Meinig School of Biomedical Engineering. He holds an Adjunct Professor position at the University of Pittsburgh in the Department of Bioengineering. The majority of Dr. Antaki’s professional career has been devoted to the development of blood-wetted medical devices. Equally important as the devices themselves, his research focuses on the methodology by which they are designed and implemented. Dr. Antaki has contributed to the development of several heart-assist devices used clinically, including the Heartmate-II, Novacor, Ventracor, TandemHeart, and Levacor. In 1997, he directed a multidisciplinary team that produced the Streamliner heart-assist device – the world’s first magnetically levitated rotodynamic blood pump to be tested in-vivo.
Joseph Glorioso III, PhD (pictured bottom): Dr. Glorioso is a Professor in the Department of Microbiology and Molecular Genetics at the University of Pittsburgh School of Medicine and holds a secondary appointment in the Department of Human Genetics, University of Pittsburgh Graduate School of Public Health. Dr. Glorioso has established over a 40-year history of research related to the basic biology and genetics of herpes simplex virus (HSV). His contributions to the field include defining antiviral immune responses to infection, the genetics of viral pathogenesis and latency, and mechanisms of viral infection. Furthermore, he has been a pioneer in the design and application of HSV gene vectors for the treatment of nervous system diseases such as peripheral neuropathies, chronic pain, and brain tumors.
The 2022 Fellow class hails from 110 research universities, governmental and non-profit research institutions worldwide. They collectively hold over 5,000 issued U.S. patents. Among the new class of Fellows are members of the National Academy of Sciences, Engineering and Medicine; Fellows of AAAS; and other prestigious organizations; Nobel Laureates; other honors and distinctions as well as senior leadership from universities and research institutions. Their body of research and entrepreneurship covers a broad range of scientific disciplines involved with technology transfer of their inventions for the benefit of society.
The 2022 class of Fellows will be honored and presented their medals at the 12th Annual Meeting of the National Academy of Inventors on June 27th, 2023, in Washington, DC.
Congratulations, Drs. Antaki and Glorioso!
Dr. Peter Wearden Named 2022 Finest Doctor in Orlando
McGowan Institute for Regenerative Medicine affiliated faculty member Peter Wearden, MD, PhD (pictured), was named one of Orlando’s 2022 Finest Doctors in the category of Pediatric Cardiac Surgery. Dr. Wearden is the Director Nemours Cardiac Center—Florida, Chair, Department of Cardiovascular Surgery, and Chief, Cardiothoracic Surgery, at the Nemours Children’s Hospital, Orlando, Florida.
Dr. Wearden graduated from Georgetown University with honors and then received his MD from West Virginia University School of Medicine, and then his PhD also from West Virginia University in Pharmacology and Toxicology. During his post-graduate studies, he held positions which included being a Resident in the Cardiothoracic Surgery Department at the University of Pittsburgh Medical Center and held several positions at West Virginia University School of Medicine which included Chief Resident in Surgery, Senior Surgical Resident, Research Associate, Intern and Junior Surgical Resident, and Research Assistant. In 2005 he completed a clinical fellowship in pediatric cardiac surgery at The Hospital for Sick Children, Toronto, Canada. Prior to joining Nemours, Dr. Wearden was an Associate Professor in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine. At Children’s Hospital of Pittsburgh of UPMC, Dr. Wearden served as the Surgical Director of Heart, Lung, and Heart/Lung Transplantation and the Director of Mechanical Cardiopulmonary Support.
Orlando Magazine Premier Doctors list was assembled by DataJoe Research, a software and research company specializing in data collection and verification that conducts various nominations across the United States on behalf of publishers. DataJoe Research used an online peer-voting process and reached out to medical groups and individual doctors to facilitate participation, while also referencing other government and Internet sources to establish credibility/eligibility of doctors. DataJoe then tallied the results to score doctors in each category and confirmed that each winning candidate had a current, active license status with the state regulatory board.
Congratulations, Dr. Wearden!
Dr. Burhan Gharaibeh Receives dB-SERC Leader Award
McGowan Institute for Regenerative Medicine affiliated faculty member Burhan Gharaibeh, PhD (pictured), faculty at the Department of Biological Sciences, and an adjunct faculty member at the Department of Bioengineering at the University of Pittsburgh, was one of the fourteen teaching members in the natural sciences departments in University of Pittsburgh’s Dietrich School of Arts and Sciences who were recently awarded the Discipline-based Science Education Research (dB-SERC) Leader Award. This annual award recognizes contributions to the dB-SERC faculty learning community and active participation in many dB-SERC events during the last academic year. The dB-SERC promotes and supports evidence-based approaches to teaching and learning in the natural sciences departments at Pitt.
Prior to his current appointment, Dr. Gharaibeh worked as a Senior Researcher at Biopatterning and Tissue Engineering Laboratory, the Institute for Complex Engineered Systems, Carnegie Mellon University (CMU), and the Stem Cell Research Center at the McGowan Institute.
Dr. Gharaibeh received his PhD in Zoology from Texas Tech University in 1997. He also has an MSc in Zoology and a BSc in Biological Sciences, both from Yarmouk University.
Dr. Gharaibeh is a member of the Human Anatomy and Physiology Society. He also participates in community outreach by presenting popular scientific lectures on the topic of stem cells, bioethics, and regenerative medicine to various high schools and adult education programs in the area.
Congratulations, Dr. Gharaibeh!
Dr. Zachary Freyberg Achieves Faculty Promotion
McGowan Institute for Regenerative Medicine affiliated faculty member Zachary Freyberg, MD, PhD (pictured), has been promoted to Associate Professor of Psychiatry by the University of Pittsburgh School of Medicine.
Dr. Freyberg received his MD and his PhD in Developmental & Molecular Biology from Albert Einstein College of Medicine. At New York Presbyterian Hospital he completed his internship in internal medicine, neurology, and psychiatry, and residency in psychiatry. He then conducted postdoctoral research training and a clinical fellowship at Columbia University. Dr. Freyberg joined the Pitt Department of Psychiatry in 2016 at the rank of assistant professor.
Dr. Freyberg’s research focuses on improving our understanding of how the mechanisms of dopaminergic neurotransmission are associated with disorders such as addiction, schizophrenia, and Parkinson’s disease. His research has provided important new discoveries that elucidate the mechanisms of cell plasticity.
He currently leads a National Institute of Diabetes and Digestive and Kidney Diseases R01 grant investigating dopaminergic signaling mechanisms in the endocrine pancreas that may be altered by anti-psychotic drugs. Dr. Freyberg is also principal investigator (PI) of a National Institute on Aging R21 focused on mechanisms for preserving neurons in Alzheimer’s disease-related dementias across fly and mouse models, as well as contact PI on a multiple principal investigator (MPI) National Institute on Drug Abuse Cutting-Edge Basic Research Awards (CEBRA) R21 grant.
As MPI of an R21 from the National Institute on Alcohol Abuse and Alcoholism, Dr. Freyberg investigates age- and sex-specific effects of glutamatergic modulation of alcohol reinforcement and motivation. He is also PI of a Department of Defense award looking at a new in situ cryo-electron microscopy approach for direct visualization of mutations in mitochondrial disease, and of a Pennsylvania Department of Health grant to conduct Alzheimer’s disease research.
Dr. Freyberg’s expertise has been recognized nationally through honors including his election as an associate member of the American College of Neuropsychopharmacology, and as a member of the American Society for Clinical Investigation. He is additionally a past recipient the Society of Biological Psychiatry’s A.E. Bennett Award.
An outstanding educator, Dr. Freyberg has mentored undergraduates, graduate and medical students, residents, and postdoctoral scholars. He currently serves as associate director of the Department’s Psychiatry Research Pathway for residents.
“Dr. Freyberg is a gifted physician-scientist with a sustained record of innovative research on intercellular signaling using state-of-the-art approaches, including genetic, pharmacology, and light and electron microscopy. He is also highly valued as a research collaborator, and is an excellent teacher and mentor,” said David Lewis, MD (Chair, Department of Psychiatry).
Congratulations, Dr. Freyberg!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#240 –– Ms. Julia Hart discusses her work as the manager of the McGowan Institute’s histology lab.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Tianmeng Chen, Matthew J Delano, Kong Chen, Jason L Sperry, Rami A Namas, Ashley J Lamparello, Meihong Deng, Julia Conroy, Lyle L Moldawer, Philip A Efron, Patricia Loughran, Christopher Seymour, Derek C Angus, Yoram Vodovotz, Wei Chen, Timothy R Billiar
Title: A road map from single-cell transcriptome to patient classification for the immune response to trauma
Summary: Immune dysfunction is an important factor driving mortality and adverse outcomes after trauma but remains poorly understood, especially at the cellular level. To deconvolute the trauma-induced immune response, we applied single-cell RNA sequencing to circulating and bone marrow mononuclear cells in injured mice and circulating mononuclear cells in trauma patients. In mice, the greatest changes in gene expression were seen in monocytes across both compartments. After systemic injury, the gene expression pattern of monocytes markedly deviated from steady state with corresponding changes in critical transcription factors, which can be traced back to myeloid progenitors. These changes were largely recapitulated in the human single-cell analysis. We generalized the major changes in human CD14+ monocytes into 6 signatures, which further defined 2 trauma patient subtypes (SG1 vs. SG2) identified in the whole-blood leukocyte transcriptome in the initial 12 hours after injury. Compared with SG2, SG1 patients exhibited delayed recovery, more severe organ dysfunction, and a higher incidence of infection and noninfectious complications. The 2 patient subtypes were also recapitulated in burn and sepsis patients, revealing a shared pattern of immune response across critical illness. Our data will be broadly useful to further explore the immune response to inflammatory diseases and critical illness.
Source: JCI Insight. 2021 Jan 25;6(2):e145108.
GRANT OF THE MONTH
PI: Stephen Badylak
Title: IPA#A. – Development and Evaluation of Xenografts for Soft Tissue Reconstruction; IPA#15 – Development and/or Evaluation of Synthetic Materials, Synthetic/Biologic Material Composites, surgical hemostats/sealants/adhesives and/or Methods for Improving the Host Tissue Response to Such Materials; IPA#B – Development and/or Refinement of In Vitro Methods which would Characterize and/or Predict the Host Response to a Test Article; and IPA#C – Development / Refinement of Preclinical Models and Ex-Vivo Test Methods
Description: Continuation of three grants.
Source: CR Bard
Term: 3 years
Amount: $800,000/year



