December 2017 | VOL. 16, NO. 12| www.McGowan.pitt.edu
Pittsburgh Business Times Recognizes the McGowan Institute and Affiliated Faculty for Innovative Achievements

Western Pennsylvania has long been a hub for innovation. This year the Pittsburgh Business Times (PBT) introduced its Innovation Awards, a program that recognizes those that made extraordinary advances in their respective fields, challenging conventional thinking. They are the disruptors, creating new products and developing new approaches that challenge traditional approaches. The McGowan Institute for Regenerative Medicine was selected as one of the winners of this inaugural award for its pioneering development and clinical implementation of regenerative medicine-based therapies. Also, the Human Engineering Research Laboratory under the leadership of McGowan Institute affiliated faculty member Rory Cooper, PhD, was recognized for the development of the PneuChair (TM) pneumatic wheelchair as a PBT Innovation Award winner. The third awardee that has a McGowan Institute relationship is Qrono, Inc. This Pittsburgh start-up was formed by McGowan Institute faculty member Steven Little, PhD, and his former graduate student Sam Rothstein, PhD.
McGowan Institute:
 Regenerative medicine seeks to reestablish tissue and organ function impaired by disease, trauma, or congenital abnormalities. To achieve these outcomes, the primary tools used are tissue engineering, cell-based therapy, and medical devices and artificial organs. The McGowan Institute has advanced the state-of-the-art in these areas. The McGowan Institute’s tag line is “Regeneration Through Innovation” (TM), and based on the advances realized the McGowan Institute affiliated faculty have demonstrated that the spirit of innovation is alive at the Institute. Accepting the award on behalf of the McGowan Institute was William Federspiel, PhD, William Kepler Whiteford Professor, Department of Bioengineering, and the Director of the Medical Devices Laboratory at the McGowan Institute.
Regenerative medicine seeks to reestablish tissue and organ function impaired by disease, trauma, or congenital abnormalities. To achieve these outcomes, the primary tools used are tissue engineering, cell-based therapy, and medical devices and artificial organs. The McGowan Institute has advanced the state-of-the-art in these areas. The McGowan Institute’s tag line is “Regeneration Through Innovation” (TM), and based on the advances realized the McGowan Institute affiliated faculty have demonstrated that the spirit of innovation is alive at the Institute. Accepting the award on behalf of the McGowan Institute was William Federspiel, PhD, William Kepler Whiteford Professor, Department of Bioengineering, and the Director of the Medical Devices Laboratory at the McGowan Institute.
The traditional standard of care primarily is focused on treating the symptoms of an insult to the body, whereas regenerative medicine seeks to reestablish tissue and organ function. The research conducted by McGowan Institute faculty benefits people who have a medical impairment, and secondarily, in many cases these technologies reduce the life-cycle costs to treat a chronic medical condition.
Human Engineering Research Laboratories (HERL):
 HERL was recognized by the PBT for the development of the PneuChair (TM) pneumatic wheelchair, which uses high-pressure air as an energy source instead of heavy batteries and electronics. The pneumatically powered chair also has the advantage that it is not affected by wet environments (like a splash park), and can be recharged in minutes vs. hours for a battery-powered chair. HERL is a joint effort between Pitt, the U.S. Department of Veterans Affairs, and UPMC. Accepting the award was Rory Cooper, PhD, Founding Director and VA Senior Research Career Scientist of HERL. Dr. Cooper is a McGowan Institute affiliated faculty member and the FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh.
HERL was recognized by the PBT for the development of the PneuChair (TM) pneumatic wheelchair, which uses high-pressure air as an energy source instead of heavy batteries and electronics. The pneumatically powered chair also has the advantage that it is not affected by wet environments (like a splash park), and can be recharged in minutes vs. hours for a battery-powered chair. HERL is a joint effort between Pitt, the U.S. Department of Veterans Affairs, and UPMC. Accepting the award was Rory Cooper, PhD, Founding Director and VA Senior Research Career Scientist of HERL. Dr. Cooper is a McGowan Institute affiliated faculty member and the FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh.
Qrono, Inc.
 This Pittsburgh start-up was formed by McGowan Institute faculty member Steven Little, PhD, Chairman of the Department of Chemical and Petroleum Engineering and the William Kepler Whiteford Endowed Professor in the Departments of Chemical and Petroleum Engineering, Bioengineering, Immunology, and Ophthalmology, and his former graduate student Sam Rothstein, PhD. Qrono, Inc. custom designs controlled release formulations in silico for pharmaceutical companies, academic laboratories, and agricultural companies.
This Pittsburgh start-up was formed by McGowan Institute faculty member Steven Little, PhD, Chairman of the Department of Chemical and Petroleum Engineering and the William Kepler Whiteford Endowed Professor in the Departments of Chemical and Petroleum Engineering, Bioengineering, Immunology, and Ophthalmology, and his former graduate student Sam Rothstein, PhD. Qrono, Inc. custom designs controlled release formulations in silico for pharmaceutical companies, academic laboratories, and agricultural companies.
Leading cancer therapies and most still under development target specific tumor receptor profiles or signaling pathways. Clinical data suggests that patients benefit most from these therapies when their tumors match particular molecular patterns. Qrono is bypassing molecular limitations with a new approach: targeting tumor and support cells through their innate behaviors. Qrono is targeting innate cell behaviors instead of niche signals and receptors. Accepting the award was Dr. Rothstein, CEO of Qrono.
Congratulations to all!
RESOURCES AT THE MCGOWAN INSTITUTE
January Histology Special
The McGowan Histology Core Laboratory is open to all university of Pittsburgh and outside the university, researchers. At the Histology laboratory you will find a friendly and knowledgeable staff. The Histology lab offers routine staining, special staining, and antibody work-up services. Lori Walton has worked for the University and UPMC, in histology for over 20 years, and is available to troubleshoot, or help to work up special circumstance staining for your individual needs. The lab utilizes many automated staining systems including an immuno-stainer, for uniform reproducible results.
The lab is located on Technology Drive with shuttle stops routinely. Check out PITTSHUTTLE for times.
Trichrome Stains are intended for use in the study of connective tissue, muscle and collagen fibers. Trichrome stains are used primarily for distinguishing collagen from muscle tissue.
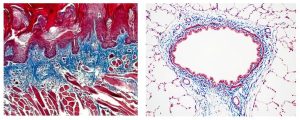
The McGowan Institute Histology Core Laboratory offers Masson Trichromes, automated staining results in one week or less, beautifully reproducible.
Through the whole month of January you will receive 25% off your trichrome stains, when you mention this ad.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
EVENTS
SAVE THE DATE-2018 McGowan Institute Retreat

SAVE THE DATE–March 5-6, 2018
Program Highlights:
- State of the Institute Address- William R. Wagner, PhD-Director
- McGowan Institute for Regenerative Medicine Distinguished Lecture, Dr. Molly Shoichet, University of Toronto: “Regenerative Strategies in the Central Nervous System”
New this Year!
NEW FORMAT for breakout sessions: Combination of lectures and audience engagement
- Topics Areas include: Cancer Immunotherapy, Reproductive Tissue Engineering, Additive Manufacturing, Drug Delivery, Craniomaxillofacial Tissue Engineering, Pediatric research-focused topics, and more
Pop-Up Workshops!
- Regenerative Cell and Tissue Therapies at UPMC- Organized by Dr. Kokai and Dr. Rubin
- Early Stage Commercial Translations- Organized by Don Taylor, PhD and the SciVelo Team
- Device Prototyping- Organized by SmithWise
Highlights Include:
- Pittsburgh Biomedical Breakfast
- Roundtable Lunch-Special Interest Topics *trainee focused
- Poster Competition and People’s Choice Award *trainee focused
- Rapid Fire Presentations *trainee focused
- Closing Banquet
Registration and abstract submission site will open January 15th, 2018; Poster session abstracts must be submitted by Febrary 1st to be eligible for a poster session award
Join us on March 5-6, 2018 at the University Club for the 17th Annual McGowan Institute for Regenerative Medicine Scientific Retreat. The program committee under the leadership of Julie Phillippi, PhD, Patrick Cantini, and Christopher Bettinger, PhD has designed an exciting program.
 Regenerative Medicine Summer School 2018
Regenerative Medicine Summer School 2018
Bryan Brown, PhD has announced the 5th Annual Regenerative Medicine Summer School- June 3-9, 2018
Objective: To provide national and regional students with a week-long didactic and experiential learning experience addressing the science and engineering related to the multidisciplinary field of regenerative medicine.
Target Audience: Undergraduates, enrolled in a science or engineering program that will have completed their 3rd year of study; exceptional candidates who will have completed their 2nd year of undergraduate study will be considered.
Venue: McGowan Institute-Pittsburgh, PA: June 3-9, 2018
Students who are interested in summer internships in addition to the summer school should apply by January 31, 2018.
SCIENTIFIC ADVANCES
Dr. Bryan Brown’s Nerve Repair Technology Moves Forward
 As reported by Dan Mohler for the University of Pittsburgh’s Innovation Institute, from the beginning, McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, Assistant Professor of Bioengineering, recognized that the nerve repair technology he was developing addressed an unmet clinical need.
As reported by Dan Mohler for the University of Pittsburgh’s Innovation Institute, from the beginning, McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, Assistant Professor of Bioengineering, recognized that the nerve repair technology he was developing addressed an unmet clinical need.
Taking advantage of available funding and mentoring opportunities throughout the University to move promising discoveries toward impact through commercialization, Dr. Brown, together with colleagues Paul Gardner, MD, Vice Chair, Department of Neurosurgery at Pitt, and McGowan Institute affiliated faculty member Jonathan Cheetham, VetMB, Diplomate ACVS, PhD, of Cornell University, pursued their mission to empower physicians with new technologies to help patients suffering peripheral nerve damage.
The last piece of the puzzle fit into place when Pitt Innovation Institute Entrepreneur in Residence (EIR) and project advisor Lorenzo Soletti, PhD, MBA, decided to join the team full time as the CEO of a new company, Renerva, formed around the new platform.
“I can say that unequivocally, without the EIR program and without Lorenzo Soletti in particular, there would be no Renerva,” said Dr. Brown. “As scientists, we are not trained to evaluate and develop the business aspects of the work which we are performing.”
Renerva is developing the Peripheral Nerve Matrix (PNM), an injectable material that accelerates and improves the functional recovery of injured peripheral nerves.
While there are specific methods utilized for treatment or reconstruction of injured peripheral nerves, they may vary by surgical specialty (e.g., plastic surgery, orthopedic surgery, neurosurgery), local practice, and individual preference. Currently, if residual nerve function is present, the patient is usually monitored for 3-6 months to allow natural recovery to take place, says Dr. Brown.
In the case of gap injuries, the standard of care is to use a sensory nerve autograft harvested from another location in the patient’s body to cross the tissue gap. While developments in surgical technique and the use of adjunct therapies have advanced over the last few decades, there remain limitations to their effectiveness. PNM is a gel derived from porcine nerves via proprietary processes. The result is a sterile, non-immunogenic, injectable solution that transforms into a supportive gel upon contact with the site of nerve injury and does not induce an immune response which may hinder healing.
The more he worked with Brown’s team as part of his portfolio at the Innovation Institute, the more Dr. Soletti was convinced of its commercial potential, so much so that he decided to create a company and as the CEO negotiate a license to take the technology out of the University.
Dr. Soletti said Renerva plans to initially target the traumatic peripheral nerve injuries market in the United States, and is working toward starting clinical trials by the end of 2018. Ideally, he said, the company will begin making U.S. sales in early 2020.
Dr. Brown said the experience in launching Renerva has been positive and rewarding.
“Our project and team received great guidance, funding, business development, and intellectual property development from the Innovation Institute. They also guided us to other resources, in particular, several business mentors, who helped in the early commercialization planning.”
Illustration: Innovation Institute.
Regional TV Highlights the McGowan Institute and Spin-Outs
 On December 17, 2017, “Our Region’s Business”, a Sunday-morning business affairs program co-produced by the Allegheny Conference on Community Development and WPXI-TV featured the biotechnology industry in Pittsburgh. The program is hosted by Bill Flanagan, who is Chief Corporate Relations Officer for the Allegheny Conference and its affiliated organizations. The program highlighted commercial startups whose core technologies came from local university research, including ALung, Inc., a spin-out from the McGowan Medical Devices Lab, and the research programs of the McGowan Institute. Also featured was Carmell Therapeutics whose founders include McGowan affiliated faculty Lee Weiss, PhD and Phil Campbell, PhD. Representing the Institute were Dr. Fabrisia Ambrosio, Director of Rehabilitation, UPMC International and Dr. William Wagner, Institute Director. The broadcast will be available on-line in the near future.
On December 17, 2017, “Our Region’s Business”, a Sunday-morning business affairs program co-produced by the Allegheny Conference on Community Development and WPXI-TV featured the biotechnology industry in Pittsburgh. The program is hosted by Bill Flanagan, who is Chief Corporate Relations Officer for the Allegheny Conference and its affiliated organizations. The program highlighted commercial startups whose core technologies came from local university research, including ALung, Inc., a spin-out from the McGowan Medical Devices Lab, and the research programs of the McGowan Institute. Also featured was Carmell Therapeutics whose founders include McGowan affiliated faculty Lee Weiss, PhD and Phil Campbell, PhD. Representing the Institute were Dr. Fabrisia Ambrosio, Director of Rehabilitation, UPMC International and Dr. William Wagner, Institute Director. The broadcast will be available on-line in the near future.
Severe Burns Show Dramatic Healing After Being Sprayed with Patients’ Own Stem Cells
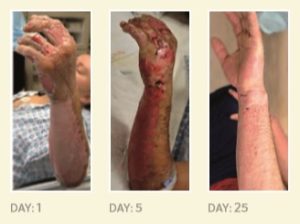
Highlighted in the University of Pittsburgh School of Medicine 2017 Annual Report, (Page 15) six men with severe second-degree burns caused by scalding water, hot tar, gasoline, a chemical explosion, contact with a live electrical wire, and ignition of paint-can fumes have become the latest patients treated at UPMC Mercy Hospital with an innovative skin regeneration technology.
To date, 45 people with burn injuries have undergone a procedure in which their own epidermal stem cells are isolated from healthy skin, then sprayed onto the burn to promote healing.
In an August 2016 on-line edition of Burns, the journal of the International Society for Burn Injuries, Jörg Gerlach, MD, PhD, director of the Bioreactor Group at the McGowan Institute for Regenerative Medicine and professor of surgery and of bioengineering, and colleagues report astonishing degrees of recovery and full return of burn-injury affected function in all six patients. McGowan Institute affiliated faculty members Alain Corcos, MD, Chief, Division of Multisystem Trauma, Department of Surgery, and Jenny Ziembicki, MD, Medical Director of the Burn Center, both at UPMC Mercy, are co-authors on the study.
The abstract of the paper reads:
Partial and deep partial-thickness burn wounds present a difficult diagnosis and prognosis that makes the planning for a conservative treatment versus mesh grafting problematic. A non-invasive treatment strategy avoiding mesh grafting is often chosen by practitioners based on their clinical and empirical evidence. However, a delayed re-epithelialization after conservative treatment may extend the patient’s hospitalization period, increase the risk of infection, and lead to poor functional and aesthetic outcome. Early spray grafting, using non-cultured autologous cells, is under discussion for partial and deep partial-thickness wounds to accelerate the re-epithelialization process, reducing the healing time in the hospital, and minimizing complications. To address planning for future clinical studies on this technology, suitable indications will be interesting. We present case information on severe second-degree injuries after gas, chemical, electrical, gasoline, hot water, and tar scalding burns showing one patient per indication. The treatment results with autologous non-cultured cells, support rapid, uncomplicated re-epithelialization with aesthetically and functionally satisfying outcomes. Hospital stays averaged 7.6±1.6 days. Early autologous cell-spray grafting does not preclude or prevent simultaneous or subsequent traditional mesh autografting when indicated on defined areas of full-thickness injury.
Illustration: Photographs of a patient’s burn injury taken before, five days after, and 25 days after being sprayed with a solution containing stem cells from his own skin. University of Pittsburgh School of Medicine 2017 Annual Report.
Abstract (Second-degree burns with six etiologies treated with autologous noncultured cell-spray grafting. Esteban-Vives R, Choi MS, Young MT, Over P, Ziembicki J, Corcos A, Gerlach JC. Burns; 2016 Nov, 42(7):e99-e106. Epub 2016 Aug 25.)
Dr. Rory Cooper and HERL to Work with Toyota on the Mobility Unlimited Challenge

McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, has been named an ambassador for the “Mobility Unlimited Challenge,” a $4 million global contest to change the lives of people with lower-limb paralysis, culminating in the unveiling of the winners in Tokyo in 2020. This effort will harness creative thinking from across the world to accelerate innovation and encourage collaboration with users to find winning devices to transform the world for people with lower-limb paralysis.
The Mobility Unlimited Challenge is run by the Toyota Mobility Foundation in partnership with Nesta’s Challenge Prize Centre.
The Mobility Unlimited Challenge is seeking teams around the world to create game-changing technology that will help radically improve the mobility and independence of paralyzed people.
The Mobility Unlimited Challenge aims to harness creative thinking from across the world to accelerate innovation and encourage collaboration with users to find winning devices to transform the world for people with lower-limb paralysis. The Challenge will reward the development of personal mobility devices incorporating intelligent systems.
The mobility solutions of the future could include anything from exoskeletons, to artificial intelligence and machine learning, from cloud computing to batteries.
Dr. Cooper is the FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh. Dr. Cooper is Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories, a VA Rehabilitation R&D Center of Excellence in partnership with Pitt.
Pitt’s Bioengineering’s BodyExplorer Research Featured at First Annual ACC Smithsonian Creativity and Innovation Festival

Virginia Tech and the Smithsonian’s National Museum of American History presented the first annual ACCelerate: ACC Smithsonian Creativity and Innovation Festival on October 13-15, 2017. The festival, programmed by Virginia Tech’s Institute for Creativity, Arts, and Technology and the Museum’s Lemelson Center for the Study of Invention and Innovation, was a 3-day celebration of creative exploration and research at the nexus of science, engineering, arts, and design (SEAD). Visitors to the festival interacted with innovators and experienced new interdisciplinary technologies developed to address global challenges. The event was free and open to the public.
The ACCelerate festival was an opportunity for all ACC schools in partnership with the Lemelson Center to showcase their work to the public, each other, students, alumni, companies, legislators, and invited guests from the nation’s capital. McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh, and the Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories (HERL), participated in the event showcasing the Mobility Enhancement Robotic Wheelchair (MEBot).
The MEBot tackles both curbs and challenging terrains. The large center driving wheels can reposition themselves to simulate front-, mid-, or rear-wheel driving. The four smaller caster wheels are controlled with compressed air and move up and down freely and independently.
For climbing curbs, the front caster wheels lift up onto the curb, then the driving wheels lift themselves up and forward onto the curb, which lifts the chair onto the curb. This is done automatically whenever MEBot senses a curb or step. The ultimate goal is for MEBot to climb a set of stairs. The same general function is used to operate on icy or slippery surfaces.
A traditional power wheelchair can get stuck on this kind of terrain. MEBot, however, uses its front and rear caster wheels to inch forward on the slick surface by extending its front casters, moving the seat forward, bringing the rear casters forward, and then repeating the process. Meanwhile, the seat stabilization system keeps the driver safely upright.
The research team includes Dr. Cooper, Brandon Daveler, PhD, Ben Gebrosky, Garrett Grindle, PhD, Andrea Sundaram, Hongwu Wang, PhD, and Jorge Candiotti.
Illustration: MEBot. HERL.
Florida Hospital for Children Partners with Children’s Hospital of Pittsburgh of UPMC to Develop Pediatric Liver Transplant Program in Florida

In order to make lifesaving liver transplants available throughout central and north Florida, Florida Hospital for Children is partnering with Children’s Hospital of Pittsburgh of UPMC to develop a comprehensive pediatric liver transplant program. This will be the first program of its kind in Orlando, the second in Florida, and is expected to start accepting patients in January.
Florida Hospital is one of the largest not-for-profit hospitals in the country. The organization’s range of nationally and internationally recognized services includes transplant, pediatrics, cardiology, and advanced surgical programs. Florida Hospital for Children’s flagship hospital in Orlando is the heart of a children’s network that includes primary care pediatricians, specialty clinics, emergency departments, and Kids Urgent Care.
“There is a critical need for children across our state to have access to a liver transplant program that is close to home,” said Regino Gonzalez-Peralta, MD, director of pediatric gastroenterology, hepatology, and liver transplantation with Florida Hospital for Children. “This partnership brings the experience of one the nation’s best pediatric liver transplant programs to central Florida. The Florida Hospital and Children’s Hospital of Pittsburgh of UPMC’s partnership is not only a win for our patients, but all of Florida.”
“We are grateful for this opportunity to now expand our services and expertise in pediatric liver transplantation to families in the Florida area,” said George Mazariegos, MD, chief of pediatric transplantation at Children’s and a McGowan Institute for Regenerative Medicine affiliated faculty member. “Our extension of expertise will provide the best possible care and make transplant a life-saving treatment for local families and help them to achieve a better quality of life.”
The teams will work in partnership with Florida Hospital’s Transplant Institute, which offers kidney, liver, kidney/pancreas, lung and heart transplants.
“Florida Hospital has been committed to saving lives through our transplant programs for more than 40 years, and it is our goal to provide the same level of advanced and compassionate care to infants and children in our community in need of liver transplants,” said Thomas Chin, MD, director of the Florida Hospital Transplant Institute’s liver transplant program. “We are honored to partner with Children’s Hospital of Pittsburgh of UPMC and bring our world-class programs together.”
In order to offer these transplants to families in the Florida area, the hospital will work with the Hillman Center for Pediatric Transplantation at Children’s Hospital of Pittsburgh of UPMC, which has performed more than 1,800 pediatric liver transplants – more than any other center in the United States, according to the United Network for Organ Sharing, with patient survival rates consistently higher than national averages.
In 1981, Children’s Hospital of Pittsburgh of UPMC opened the country’s first comprehensive pediatric transplant center under the guidance of transplant pioneer Thomas E. Starzl, MD, PhD. According to the 2017 data released by the Scientific Registry of Transplant Recipients, the pediatric liver transplant program at Children’s ranks number 1 out of 62 pediatric liver transplant centers in the United States for 1-year overall patient and graft survival when comparing hazard ratio estimates. The program remains at the leading edge of expertise, innovation, and patient- and family centered care for transplant patients from all over the world.
Members of the transplant team from Children’s Hospital of Pittsburgh of UPMC will participate in the management of patients in Florida. Transplant surgeons, medical specialists, and nurses from Florida and Children’s Hospital of Pittsburgh of UPMC will perform pediatric liver transplant surgeries together at Florida Hospital for Children.
The pediatric liver transplant partnership with Florida Hospital is the second program of its kind for Children’s Hospital of Pittsburgh of UPMC. In 2016, Children’s Hospital became the first and only pediatric liver transplant program to expand the geographic reach of its expertise through a partnership with the University of Virginia Children’s Hospital in Charlottesville. Today, Children’s pediatric liver transplant network extends from Pittsburgh to Virginia, and now Florida.
Illustration: Children’s Hospital of Pittsburgh of UPMC.
Dr. David Vorp to Present at International Meeting Celebrating 50 Years of Heart Transplantation

The first human heart transplant in the world was performed at Groote Schuur Hospital in Cape Town, South Africa, on December 3, 1967. After training over a decade in heart surgery, Christiaan Barnard, MD, PhD, Head of the Department of Experimental Surgery, accepted the risk of transplantation and successfully performed the operation. Many believe that Dr. Barnard’s courage to perform the human heart transplant, while others in the field hesitated, was his greatest contribution.
To commemorate this cross-disciplinary feat of “courage and innovation,” Groote Schuur Hospital and the University of Cape Town are hosting a 3-day program celebrating the “50th Anniversary of the 1st Heart Transplant.” The program is supported by scientific host societies across the world.
McGowan Institute for Regenerative Medicine faculty member David Vorp, PhD, Associate Dean for Research and John A. Swanson Professor of Bioengineering, will present on December 4, 2017. His session, hosted by the International Society for Applied Cardiovascular Biology (ISACB), is themed “ISACB 30 YEARS OLD: The Era of Repair, Replacement, and Regeneration.”
Dr. Vorp and John Curci, MD, Associate Professor of Surgery at Vanderbilt University Medical Center, will chair a morning session, “Aneurysmal Degeneration: The Interaction of Nature and Nurture,” and Dr. Vorp will also present a lecture on “Aortic Aneurysm Biomechanics.”
AWARDS AND RECOGNITION
Antonio D’Amore, PhD, Recognized by the Italian Scientists and Scholars in North America Foundation

McGowan Institute for Regenerative Medicine affiliated faculty member Antonio D’Amore, PhD, Research Assistant Professor in the Departments of Surgery and Bioengineering, and a member of the lab of McGowan Institute Director William Wagner, PhD, was recognized by his Italian colleagues for his scientific accomplishments.
Dr. D’Amore was a finalist for the Franco Strazzabosco Award for Young Engineers – ISSNAF (Italian Scientists and Scholars in North America Foundation), which recognized the top 3 Italian scientists in the U.S. under 40 years old. The recognition ceremony took place in Washington, DC, November 2017.
Most recently, Dr. D’Amore published two research papers. They are examples of his scientific excellence. The publications are
Heart valve scaffold fabrication: Bioinspired control of macro-scale morphology, mechanics and micro-structure. D’Amore A, Luketich SK, Raffa GM, Olia S, Menallo G, Mazzola A, D’Accardi F, Grunberg T, Gu X, Pilato M, Kameneva MV, Badhwar V, Wagner WR. Biomaterials; 2018 Jan;150:25-37. Epub 2017 Oct 6. Link to pdf.
Nitro-oleic acid (NO2OA) release enhances regional angiogenesis in a rat abdominal wall defect model. Dr. Antonio D’Amore, Dr. Marco Fazzari, Dr. Hongbin Jiang, Mr. Samuel K. Luketich, Mr. Michael E. Luketich, Mr. Richard F. Hoff, Mr. Daniel L. Jacobs, Dr. Xinzhu Gu, Dr. Stephen F. Badylak, Dr. Bruce A. Freeman, and Dr. William R. Wagner. Tissue Engineering Part A; November 29, 2017, ahead of print. Link to abstract. Link to video: Representative multi-photon image stack showing a three components design approach combining polymeric fibrous matrix (blue); dermal ECM component (red), and; PLGA drug loaded microparticles (green). Courtesy Antonio D’Amore, PhD.
Congratulations, Dr. D’Amore!
Dr. Rocky Tuan Named a 2017 National Academy of Inventors Fellow

Dr. Tuan is the Director, Center for Cellular and Molecular Engineering, the Arthur J. Rooney, Sr. Professor and Executive Vice Chair, Department of Orthopaedic Surgery, the Director, Center for Military Medicine Research, and a Professor in the Departments of Bioengineering and Mechanical Engineering & Materials Science, University of Pittsburgh, Pittsburgh, Pennsylvania. Recently, the Council of The Chinese University of Hong Kong (CUHK) unanimously approved the appointment of Dr. Tuan as the eighth Vice-Chancellor and President of CUHK for a period of 6 years from 1 January 2018.
Dr. Tuan directs a multidisciplinary research program which focuses on orthopaedic research as a study of the biological activities that are important for the development, growth, function, and health of musculoskeletal tissues, and the utilization of this knowledge to develop technologies that will regenerate and/or restore function to diseased and damaged skeletal tissues. Ongoing research projects are directed towards multiple aspects of skeletal and related biology, including skeletal development, stem cells, growth factor signaling, bone-biomaterial interaction, extracellular matrix and cell-matrix interaction, nanotechnology, biomaterials, 3D printing, mechanobiology, regenerative medicine, and tissue engineering, utilizing an integrated experimental approach combining contemporary technologies of biochemistry, cell and molecular biology, embryology and development, cellular imaging, and engineering.
Dr. Tuan joins McGowan Institute affiliated faculty members William Wagner, PhD, Rory Cooper, PhD, and Krzysztof Matyjaszewski, PhD, who are also NAI Fellows.
With the election of the 2017 class there are now 912 NAI Fellows, representing over 250 research universities and governmental and non-profit research institutes. The 2017 Fellows are named inventors on nearly 6,000 issued U.S. patents, bringing the collective patents held by all NAI Fellows to more than 32,000 issued U.S. patents.
Included among all NAI Fellows are more than 100 presidents and senior leaders of research universities and non-profit research institutes; 439 members of the National Academies of Sciences, Engineering, and Medicine; 36 inductees of the National Inventors Hall of Fame; 52 recipients of the U.S. National Medal of Technology and Innovation and U.S. National Medal of Science; 29 Nobel Laureates; 261 AAAS Fellows; 168 IEEE Fellows; and 142 Fellows of the American Academy of Arts & Sciences, among other awards and distinctions.
On 5 April 2018, the 2017 NAI Fellows will be inducted as part of the Seventh Annual NAI Conference of the National Academy of Inventors at the Mayflower Hotel, Autograph Collection in Washington, D.C. Andrew H. Hirshfeld, U.S. Commissioner for Patents, will provide the keynote address for the induction ceremony.
Congratulations, Dr. Tuan!
Pitt’s Fall 2017 First Gear Cohort Honors

Through the University of Pittsburgh’s Innovation Institute, the First Gear program helps shape Pitt inventions originating from University research from early-stage discovery to products and services that can be taken to market. The program offers hands-on guidance and mentorship that takes an inventor through the necessary steps in creating a go-to-market plan that can result in the creation of a new enterprise or licensing agreement for the technology.
Nitrix, which is aiming for a less expensive solution for treating chronic wounds, received $5,000 from the Chancellor Innovation Commercialization Funds at the most recent Pitt Ventures First Gear Demo Day. Nitrix is one of the latest products of the McGowan Institute for Regenerative Medicine in the laboratory of principal investigator (PI) Bryan Brown, PhD, Assistant Professor of Bioengineering. Dr. Brown’s graduate student researcher, Samuel LoPresti, the project’s entrepreneurial lead (EL), said First Gear helped in engaging clinical experts to learn what needs they have in wound care and in proving that the technology his team is developing is an advance over current standards via small animal studies.
The Nitrix project was advised by volunteer mentors (M) Leon Perez and Peter Vercilla.
Other McGowan Institute affiliated teams participating in the fall 2017 First Gear cohort included:
Vital-Dent is developing a system to improve root canal procedures to prevent bacterial infection leading to root infection.
PI: Juan Taboas, PhD, Herbert Ray, DMD
EL: Jingming Chen, Adam Chin
M: Denny Wist, Kerry Hanahan
Flash non-interference tool for reducing length of cardiac surgery is a surgical tool that improves the operating experience and reduces time for cardiac surgeries.
PI: Youngjae Chun, PhD
EL: Seth Stern
M: Kuang Zhang
Congratulations, all!
Illustration: Pitt Ventures First Gear Fall 2017 Cohort Demo Day winners included the Nitrix team represented by student entrepreneurial lead Sam LoPresti (left) and business mentor Leon Perez (right).
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#178 –– Dr. Antonio D’Amore is a Research Assistant Professor in the Departments of Surgery and Bioengineering at the University of Pittsburgh. Dr. D’Amore discusses his research on artificial heart valves and cardiac patches.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: D’Amore A, Fazzari M, Jiang H, Luketich SK, Luketich ME, Hoff RF, Jacobs DL, Gu X, Badylak SF, Freeman BA, Wagner WR
Title: Nitro-oleic acid (NO2OA) release enhances regional angiogenesis in a rat abdominal wall defect model.
Summary: Ventral hernia is often addressed surgically by the placement of prosthetic materials, either synthetic or from allogeneic and xenogeneic biologic sources. Despite advances in surgical approaches and device design, a number of postsurgical limitations remain, including hernia recurrence, mesh encapsulation, and reduced vascularity of the implanted volume. The in situ controlled release of angiogenic factors from a scaffold facilitating abdominal wall repair might address some of these issues associated with sub-optimal tissue reconstruction. Furthermore, a biocomposite material that combines the favorable mechanical properties achievable with synthetic materials and the bioactivity associated with xenogeneic tissue sources would be desirable. In this report an abdominal wall repair scaffold has been designed based on a micro-fibrous, elastomeric poly(ester carbonate)urethane urea matrix integrated with a hydrogel derived from decellularized porcine dermis (ECM gel) and poly(lactic-co-glycolic acid) (PLGA) microspheres loaded with nitro-oleic acid (NO2-OA). NO2-OA is an electrophilic fatty acid nitro-alkene derivative that, under hypoxic conditions, induces angiogenesis. This scaffold was utilized to repair a rat abdominal wall partial thickness defect, hypothesizing that the nitro-fatty acid release would facilitate increased angiogenesis at the 8-week end-point. The quantification of neovascularization was conducted by novel methodologies to assess vessel morphology and spatial distribution. The repaired abdominal wall defects were evaluated by histopathologic methods including quantification of the foreign body response and cellular ingrowth. The results showed that NO2-OA release was associated with significantly improved regional angiogenesis. The combined biohybrid scaffold and NO2-OA controlled release strategy also reduced scaffold encapsulation, increased wall thickness, and enhanced cellular infiltration. More broadly, the three components of the composite scaffold design (ECM gel, polymeric fibers and PLGA microparticles) enable the tuning of performance characteristics including scaffold bioactivity, degradation, mechanics and drug release profile, all decisive factors to better address current limitations in abdominal wall repair or other soft tissue augmentation procedures.
Source: Tissue Eng Part A. 2017 Nov 29. doi: 10.1089/ten.TEA.2017.0349. [Epub ahead of print]
GRANT OF THE MONTH
PI: Warren C. Ruder
Title: Creating Smart Biomaterials using Engineered Bacteria that Cooperatively Reprogram Mammalian Cells
Description: An ability to reprogram the function of mammalian cells is critical for exploring the scientific underpinnings of cell behavior as well as improving human health. This effort will result in the development of smart biomaterials and experimental systems made up of microparticles carrying engineered bacteria. These hybrid, living-nonliving biomaterials will be used as a tool to reprogram mammalian cell signaling. One advantage of an extendable synthetic biomaterials approach that uses engineered bacteria to regulate mammalian calcium signaling could be the abrogation of the need to directly alter mammalian cells to reprogram their behavior. Thus, the need for vectors such as viruses or the creation of transgenic animal models could be reduced. For this effort, bacteria-bound microparticles will be developed that can deliver synthetic genetic components to mammalian cells and reprogram calcium signaling. Calcium signaling is a ubiquitous system that effects cells ranging from nerves to the cells that line the gut, and controls both slow and fast cellular processes. Next, these particles will be made into “smart” biomaterials as their living component – the bacteria – will be engineered with an ability to collaborate and collectively determine when to transmit genetic components to mammalian cells. Finally, mathematical modeling and computational simulation will be used to explore calcium signaling dynamics in mammalian cells in order to determine when alterations will cause the most significant changes in cell signaling dynamics. The smart biomaterials will then be used to reprogram this signaling. In addition, this effort will be integrated with the development of multiple teaching modules for a bioengineering summer camp, and the final curriculum will be widely disseminated to the broader scientific and educational community.
Source: DMR, National Science Foundation
Term: 8/1/2017 – 7/31/2020
Amount: $338,414/year
