April 2021 | VOL. 20, NO. 4| www.McGowan.pitt.edu
Pitt Innovation Awards
In a April 22nd virtual presentation, the University of Pittsburgh Innovation Institute has recognized the achievements of three McGowan affiliated faculty members:
Marlin Mickel Outstanding Innovator Award to Dr. William Federspiel
 The Outstanding Innovator Award recognizes Pitt faculty who have demonstrated an extraordinary commitment to achieving impact through the commercialization of their research. It is named in honor of the late Marlin Mickle, who was one of the most prolific inventors in the history of the university, and a pioneer of radio frequency identification technology.
The Outstanding Innovator Award recognizes Pitt faculty who have demonstrated an extraordinary commitment to achieving impact through the commercialization of their research. It is named in honor of the late Marlin Mickle, who was one of the most prolific inventors in the history of the university, and a pioneer of radio frequency identification technology.
In his time at Pitt, Dr. Federspiel has submitted 32 invention disclosures and has been issued 14 patents, with many more pending. He has had his work licensed 11 times.
Not long after arriving at Pitt, Dr. Federspiel licensed his work on an artificial lung device to form Alung Technologies. Dr. Federspiel serves as the head of the company’s scientific advisory board.
This time last year, Alung was in multiple clinical trials for its Hemolung Respiratory Assist System to treat chronic obstructive pulmonary Disease. That work was interrupted by the onset of the SARS CoV-2 pandemic. The company recognized a role for the device in treating critically ill COVID patients and applied for emergency use permission from the FDA, which it quickly received.
Since then, nearly 100 patients have used the device successfully. This data may help the company finally obtain full U.S. regulatory approval by this summer. Read more.
Emerging Innovator Award to Dr. Cecelia Yates
 This award recognizes an early-to-mid-career Pitt faculty member who has demonstrated an extraordinary commitment to innovation commercialization.
This award recognizes an early-to-mid-career Pitt faculty member who has demonstrated an extraordinary commitment to innovation commercialization.
Dr. Yates has developed the first cellular and molecular laboratory fully equipped for basic, translational, and clinical research located within the School of Nursing. Her tissue repair laboratory investigates the chronic and fibrotic responses to injury.
Dr. Yates has submitted 11 invention disclosures to the Innovation Institute that have thus far resulted in 6 issued U.S. patents, and three licenses of her work, including the formation of a startup company four years ago, Ocugenix, aimed at treating macular degeneration.
Most recently, Dr. Yates has received a $250,000 grant from CSL Behring, a global biotech company focused on rare diseases, to further develop peptides that imitate the action of proteins that target the underlying causes of fibrosis.
Finally, Dr. Yates has demonstrated a willingness to help mentor and train the next generation of Pitt innovators. She is the program co-director for the Clinical and Translational Science Fellowship, which equips researchers with the skills to advance the translation of discoveries into improved patient outcomes. Read more.
License of the Year: Dr. George Gittes/Genprex
 Dr. Gittes’ research has demonstrated that by delivering select genes to the pancreas via a virus vector, the genes will express proteins that transform alpha cells in the pancreas, which do not produce insulin, into functional insulin-producing beta cells. These cells are distinct enough from beta cells to avoid the attention of the immune system.
Dr. Gittes’ research has demonstrated that by delivering select genes to the pancreas via a virus vector, the genes will express proteins that transform alpha cells in the pancreas, which do not produce insulin, into functional insulin-producing beta cells. These cells are distinct enough from beta cells to avoid the attention of the immune system.
Last July, Dr. Gittes was awarded a grant of $2.59 million from the National Institutes of Health to build upon his groundbreaking gene therapy work toward finding a cure for diabetes.
Genprex, based in Austin, Texas, was founded in 2009 as an immunogene cancer therapy company. The company raised $40 million in capital last year in preparation for two cancer clinical trials it expects to begin this year. The company recognized in Dr. Gittes’ research the opportunity to expand its gene therapy platform to diabetes. Read more.
RESOURCES AT THE MCGOWAN INSTITUTE
May Histology Special – Verhoeff-Van Gieson (VVG) Staining
Elastin is a protein in connective tissue that allows many tissues in the body to resume their shape after stretching or contracting. Elastin helps skin to return to its original position when it is poked or pinched.
Elastin serves an important function in arteries as a medium for pressure wave propagation to help blood flow and is particularly abundant in large elastic blood vessels such as the aorta. Elastin is also very important in the lungs, elastic ligaments, the skin, and the bladder, elastic cartilage. It is present in all vertebrates above the jawless fish.
Verhoeff’s stain forms a variety of cationic, anionic and non-ionic bonds with elastin, the main constituent of elastic fiber tissue. Elastin has a strong affinity for the iron-hematoxylin complex formed by the reagents in the stain and will hence retain dye longer than other tissue elements, which allows elastin to remain stained while other tissue elements are decolorized. Elastic fibers and cell nuclei are stained black, collagen fibers are stained red, and other tissue elements including cytoplasm are stained yellow.
You will receive 25% off VVG Staining for the entire month of May when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SCIENTIFIC ADVANCES
Pitt Skin Patch Vaccine Partners with Cambridge COVID-19 Biotech Startup

The ultimate mission at Helix Nanotechnologies, Inc. (HelixNano) is to build technologies that unlock a future where freedom from cancer is a fundamental human right. But right now, the fight against a global pandemic needs all scientific hands on-deck. In response to this existential challenge, the HelixNano team is applying its expertise to deploy a novel vaccination approach against COVID-19. McGowan Institute for Regenerative Medicine affiliated faculty member Louis Falo, Jr., MD, PhD, Professor and Chairman of the Department of Dermatology at the University of Pittsburgh School of Medicine with faculty appointments in the Department of Bioengineering of the University of Pittsburgh Swanson School of Engineering, the University of Pittsburgh UPMC Hillman Cancer Institute, and the Pittsburgh Clinical and Translational Science Institute, and his team are partnered with HelixNano to help deliver its vaccine to patients.
HelixNano’s cancer drug would have mRNA deliver a message to kill cancers cells and attract immune cells to the tumor. Its cancer vaccine would use mRNA to deliver a message to make a cancer cell more visible to the human body’s immune system so that it could attack the cancer.
The idea behind that cancer vaccine is the same as HelixNano’s COVID-19 vaccine, except that the vaccine against SARS-CoV-2 goes after the virus instead of cancer cells, says Hannu Rajaniemi, PhD, co-Founder and CEO of HelixNano, Cambridge, MA. The mRNA delivers a message to make parts of the novel coronavirus visible to the immune system.
Beyond its own vaccine technology innovations, HelixNano is collaborating with Dr. Falo’s lab to make a vaccine technology that can be applied to the skin, rather than by a shot, which therefore can be self-administered.
“The mRNA platform has proven to be effective for vaccination but does have limitations including the requirement for very low temperatures (cold-chain) across the storage, delivery, and deployment process,” says Dr. Falo. “We imagine an mRNA vaccine that is stable at room temperature and can therefore be readily deployed in global vaccination campaigns the same way that one would distribute and apply Band-Aids.”
Separately, Dr. Falo’s lab has its own skin application vaccine called PittCoVacc, which has submitted preclinical data to the U.S. Food and Drug Administration as a Pre-Investigational New Drug Application.
Illustration: UPMC.
Read more…
Unlocking Richer Intracellular Recordings

Behind every heartbeat and brain signal is a massive orchestra of electrical activity. While current electrophysiology observation techniques have been mostly limited to extracellular recordings, a forward-thinking group of researchers from Carnegie Mellon University (CMU) and Istituto Italiano di Tecnologia has identified a flexible, low-cost, and biocompatible platform for enabling richer intracellular recordings.
The group’s unique “across the ocean” partnership started two years ago at the Bioelectronics Winter School (BioEl) with libations and a bar napkin sketch. It has evolved into research published in Science Advances, detailing a novel microelectrode platform that leverages three-dimensional fuzzy graphene (3DFG) to enable richer intracellular recordings of cardiac action potentials with high signal to noise ratio. This advancement could revolutionize ongoing research related to neurodegenerative and cardiac diseases, as well as the development of new therapeutic strategies.
A key leader in this work, McGowan Institute for Regenerative Medicine affiliated faculty member Tzahi Cohen-Karni, PhD, associate professor of biomedical engineering and materials science and engineering at CMU, has studied the properties, effects, and potential applications of graphene throughout his entire career. Now, he is taking a collaborative step in a different direction, using a vertically grown orientation of the extraordinary carbon-based material (3DFG) to access the intracellular compartment of the cell and record intracellular electrical activity.
Due to its unique electrical properties, graphene stands out as a promising candidate for carbon-based biosensing devices. Recent studies have shown the successful deployment of graphene biosensors for monitoring the electrical activity of cardiomyocytes, or heart cells, outside of the cells, or in other words, extracellular recordings of action potentials. Intracellular recordings, on the other hand, have remained limited due to ineffective tools…until now.
“Our aim is to record the whole orchestra—to see all the ionic currents that cross the cell membrane—not just the subset of the orchestra shown by extracellular recordings,” explains Dr. Cohen-Karni. “Adding the dynamic dimension of intracellular recordings is fundamentally important for drug screening and toxicity assay, but this is just one important aspect of our work.”
“The rest is the technology advancement,” Dr. Cohen-Karni continues. “3DFG is cheap, flexible, and an all-carbon platform; no metals involved. We can generate wafer-sized electrodes of this material to enable multi-site intracellular recordings in a matter of seconds, which is a significant enhancement from an existing tool, like a patch clamp, which requires hours of time and expertise.”
So, how does it work? Leveraging a technique developed by Michele Dipalo, PhD, and Francesco De Angelis, PhD, researchers at Istituto Italiano di Tecnologia, an ultra-fast laser is used to access the cell membrane. By shining short pulses of laser onto the 3DFG electrode, an area of the cell membrane becomes porous in a way, allowing for electrical activity within the cell be recorded. Then, the cardiomyocytes, are cultured to further investigate interactions between the cells.
Interestingly, 3DFG is black and absorbs most of the light, resulting in unique optical properties. Combined with its foam-like structure and enormous exposed surface area, 3DFG has many desirable traits that are needed to make small biosensors.
“We have developed a smarter electrode; an electrode that allows us better access,” emphasizes Dr. Cohen-Karni. “The biggest advantage from my end is that we can have access to this signal richness, to be able to look into processes of intracellular importance. Having a tool like this will revolutionize the way we can investigate effects of therapeutics on terminal organs, such as the heart.”
Additional contributions to the project were made by McGowan Institute affiliated faculty member Adam Feinberg, PhD, professor of biomedical engineering and materials science and engineering at CMU, and Sheng Shen, PhD, professor of mechanical engineering at CMU. Dr. Feinberg and his lab team assisted with pieces of the cardiomyocyte research done at CMU, while Dr. Shen’s lab team focused on the characterization of graphene’s optical properties.
As this work moves forward, the team plans to apply its learnings in large-scale cell/tissue interfaces, to better understand tissue development and toxicity of chemical compounds (e.g., drug toxicity).
Federal Policy to Reduce Sepsis Deaths Mostly Ineffective

The first large-scale, multi-hospital evaluation of an “all or none” federal policy intended to improve outcomes in sepsis patients finds that the guidelines are a wash—on average they neither helped nor hurt despite significant investments in their implementation, according to an analysis by University of Pittsburgh School of Medicine (UPMC) clinician-scientists of nearly a dozen hospitals in one academic health system. McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, Executive Vice President, and Chief Healthcare Innovation Officer, UPMC; Associate Vice Chancellor for Healthcare Innovation, University of Pittsburgh Schools of the Health Sciences; Distinguished Professor and Mitchell P. Fink Endowed Chair, Department of Critical Care Medicine, University of Pittsburgh and UPMC; Senior editor, JAMA, is a co-author on the study.
The findings, reported in the Annals of Internal Medicine, indicate ways the guidelines, known as Severe Sepsis and Septic Shock: Management Bundle, or “SEP-1,” could be built upon and potentially improved.
“We can’t just stop with SEP-1 as it currently exists. Our data suggest the need for ongoing efforts to refine and update SEP-1 so that it benefits more patients,” said lead author Ian Barbash, MD, MS, a UPMC intensivist and assistant professor in Pitt’s Division of Pulmonary, Allergy and Critical Care Medicine. “It’s a big deal to move a health system to install the infrastructure to respond to this kind of measure, so if you’re going to do it, you want to be pretty convinced it will improve patient outcomes.”
Sepsis occurs when a person’s organs cease to function properly as the result of an out-of-control immune response to infection. It is responsible for 1 in 5 deaths worldwide. In the U.S., at least 1.7 million adults develop sepsis each year and 1 in 3 patients who died in a hospital had sepsis, according to the U.S. Centers for Disease Control and Prevention.
In response to these staggering numbers, the Centers for Medicare & Medicaid Services (CMS) implemented SEP-1 in October 2015. For hospitals to be considered compliant, patients must receive a bundle of treatments, including blood cultures, early antibiotics, regular lab tests and IV fluids, and hospitals must collect and report data on their adherence.
Dr. Barbash and his team looked at electronic health records data on 54,225 visits by adult patients at 11 hospitals of varying sizes in the UPMC system, which served urban, suburban, or rural populations. UPMC responded to SEP-1 with several strategies common to hospitals across the U.S., including sepsis alerts, electronic order sets and clinical documentation reminders.
The researchers compared data from two years before and two years after SEP-1 implementation.
The most significant change across the study period was that clinicians increased their ordering of lactate measurement, which is a test to measure lactic acid in a patient’s blood to determine if they are experiencing low blood flow or low blood oxygen. But the increased testing did not translate to other changes in care delivery or to less deaths overall.
“It’s not that the bundle elements aren’t good for patients—we know that early sepsis treatment saves lives,” said senior author Jeremy Kahn, MD, MS, professor of critical care medicine and health policy and management at Pitt. “The issue is whether SEP-1, as it currently exists, was sufficient to move the needle.
“Tests like lactate are useful when they give you information that you can act on to improve patient outcomes,” Dr. Kahn continued. “But testing for the sake of reporting that you did the test is not helpful unless you also do other things.”
Overall, SEP-1 wasn’t associated with clinically meaningful patient outcomes. Deaths from sepsis were decreasing before the policy was implemented and the trend continued as would have been expected afterward. Moreover, a different study found that one academic hospital was investing more than $150,000 per month in responding to SEP-1.
“Sepsis is deadly, but it can be treated,” Dr. Barbash said. “I suspect that simplifying SEP-1 and focusing on what works—such as early administration of appropriate antibiotics to the patients who need them—will lead to improvements.
“That said, a limitation of our study was that it included hospitals all in one academic health system—UPMC—which has long been working to improve sepsis outcomes in its patients and currently is working with CMS to improve the measure,” continued Dr. Barbash. “It is possible UPMC already had achieved the improvements that SEP-1 might induce at other hospitals.”
Breast Cancer Treatment in Those Over 70 Can Be Reduced

Oncologists faced with treating older women with breast cancer often must decide if the treatment may be more detrimental than the cancer. A study published in JAMA Network Open by researchers at UPMC Hillman Cancer Center and the University of Pittsburgh School of Medicine sheds new light on this choice and suggests the rate of cancer recurrence or survival may be no different in treated vs. untreated elderly patients diagnosed in the early stages of the cancer diagnosed most commonly in women. McGowan Institute for Regenerative Medicine affiliated faculty member Steffi Oesterreich, PhD, the Shear Family Foundation Chair in Breast Cancer Research Professor and Vice-Chair Department of Pharmacology and Chemical Biology, University of Pittsburgh; Co-Leader Cancer Biology Program, UPMC Hillman Cancer Center (UPMC HCC); and Co-Director Women’s Cancer Research Center, UPMC HCC and Magee Womens Research Institute, is a co-author on the study.
“As a breast surgeon, I want to give my patients the best chance of survival with the best quality of life,” said senior author Priscilla McAuliffe, MD, PhD, surgical oncologist at UPMC HCC and attending surgeon in the Department of Surgery at Pitt. “However, we have found that overtreatment of early-stage breast cancer in older patients may actually cause harm while not improving recurrence or survival rates.”
Despite guidelines recommending the de-escalation of treatment among older women with early-stage cancer, there has been no definitive study of this patient cohort, who often are excluded in clinical trials because of their age.
To determine whether patients were being overtreated, the researchers analyzed deidentified data from more than 3,000 women over the age of 70 who were diagnosed with ER-positive, HER2-negative breast cancer from 2010 to 2018. Age 70 was used because clinical trials in breast cancer don’t often include women in this age category. The data, collected at 15 UPMC Hillman Cancer Center community and academic sites, were evaluated with the UPMC Network Cancer Registry, which tracks cancer patients using UPMC’s electronic health record system.
The study looked at two procedures: sentinel lymph node biopsy (SLNB), which is a surgical procedure to remove the lymph nodes from the underarm to determine if the cancer has spread beyond the breast, and radiotherapy (RT), which is standard treatment after breast-conserving surgery, given in multiple doses to control or kill any residual tumor. Side effects including nerve pain, lymphedema and skin irritation can sometimes occur after these procedures, which may be more difficult for the older patient to overcome.
The researchers found that the number of SLNB and RT procedures among patients over age 70 remained high at 65.3% and 54.4% respectively. The rates of SLNB also steadily increased by 1% per year, while rates of RT declined by 3.4% per year over the study period.
Most importantly, they found that the rates of disease recurrence or survival remained the same, whether the carefully selected patients received SLNB or RT, or not—no matter their comorbidities or tumor grade.
“This study is an example of how we can use big data to deliver on the promise of precision medicine—getting the right treatment to the right patient at the right time,” said Adrian Lee, PhD, investigator at the Women’s Cancer Research Center at UPMC HCC and Magee Womens Research Institute, and director of the UPMC/Pitt Institute of Precision Medicine. “Sometimes—as it happens to be in this case—that could mean deciding not to provide a certain treatment to ensure better care for the patient.”
The American Society of Breast Surgeons has released its second list of specific tests or procedures that clinicians and patients should question as part of Choosing Wisely®, an initiative of the ABIM Foundation. Dr. Lee hopes this study will further validate that a de-escalation of treatment for certain breast cancers will not affect the quality of life or outcome for patients and that SLNB and RT can safely be omitted.
“Our study is unique in that we were able to look at very granular patient data, thanks to the size of our health system and the availability of electronic medical records, which makes the findings more robust than any other such study to date,” said Neil Carleton, lead author and an MD/PhD student at Pitt. “This is the result of an incredible collaborative of researchers, oncologists and clinical analytics experts.”
COVID-19 Treatment Reduces Risk of Hospitalization, Death

Monoclonal antibodies, a COVID-19 treatment given early after coronavirus infection, cut the risk of hospitalization and death by nearly 70% in those most likely to suffer complications of the disease, according to a preliminary analysis of UPMC patients who received the medication compared to similar patients who did not.
In an effort to share crucial information and save lives, UPMC and University of Pittsburgh School of Medicine physician-scientists published the findings in medRxiv, a preprint journal, and announced the results ahead of peer-reviewed publication. McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, Executive Vice President, and Chief Healthcare Innovation Officer, UPMC; Associate Vice Chancellor for Healthcare Innovation, University of Pittsburgh Schools of the Health Sciences; Distinguished Professor and Mitchell P. Fink Endowed Chair, Department of Critical Care Medicine, University of Pittsburgh and UPMC; Senior editor, JAMA, is a co-author on the study.
“A one-time monoclonal antibody treatment has helped keep our patients with COVID-19 out of the hospital,” said Erin McCreary, PharmD, an infectious diseases pharmacist at UPMC and clinical assistant professor in Pitt’s School of Medicine. “If given early to high-risk patients, this treatment works to prevent COVID-19-related complications. We look forward to research with next-generation monoclonal antibodies and hope to continue to find safe and effective treatments for our patients.”
Monoclonal—“mono” means “one” and “clonal” means “copy”—antibodies are a type of medication that seeks out the COVID-19 virus in a person’s body and blocks it from infecting their cells and replicating. Since late 2020, the U.S. Food & Drug Administration has granted Emergency Use Authorization to three monoclonal antibody treatments—one from Regeneron and two from Eli Lilly—which are given through a one-time IV infusion. This is the same type of emergency authorization given to the COVID-19 vaccines being administered in the U.S.
Federal and UPMC guidelines require the antibodies be administered within 10 days of COVID-19 symptom onset and diagnosis for patients at high risk of a poor outcome, including patients of advanced age, who are obese or those with conditions such as diabetes or lung disease.
UPMC has given monoclonal antibody infusions to more than 1,000 qualifying patients in the past three months. The researchers analyzed data on approximately the first half of those patients to learn how they’re faring since their infusions. They compared antibody-treated patients’ data to that of a matched set of patients of similar age and health status who had contracted COVID-19 and were eligible for the treatment but did not receive it.
The strongest effect was seen in older patients. Those age 65 and older who received monoclonal antibodies from UPMC were nearly three times less likely to be hospitalized or die in the following month, compared to their untreated counterparts. The results were less pronounced in younger populations, but overall, more positive results were seen in those who received monoclonal antibody infusions than in those who did not.
UPMC’s data also showed a stronger positive effect the earlier patients received the treatment after contracting the virus, and a very low rate of adverse reactions to the infusion, all of which were mild.
“If there’s one key take-away that we’re seeing in our data, it’s this: If you get COVID-19 and are at higher risk for severe illness, ask your doctor about monoclonal antibodies,” said Graham Snyder, MD, MS, medical director of infection prevention and hospital epidemiology at UPMC and associate professor in Pitt’s School of Medicine. “Don’t hesitate. Early treatment, while your symptoms are still mild, may be essential.”
UPMC offers monoclonal antibody infusions at 16 sites in Pennsylvania and New York, as well as home infusion services, if needed. Patients and providers can find out more about monoclonal antibody treatment at UPMC by visiting upmc.com/AntibodyTreatment or calling 866-804-5251.
Brown Lab’s Marissa Behun Receives CTS Predoctoral Fellowship

Marissa Behun, a first-year PhD student in bioengineering at the University of Pittsburgh, received a Predoctoral Clinical and Translational Science Fellowship. This competitive award equips researchers with the skills to advance the translation of discoveries into improved patient outcomes and health policy.
Ms. Behun works in the Brown Lab, which is led by Bryan Brown, PhD, a member of the McGowan Institute for Regenerative Medicine and associate professor of bioengineering at Pitt’s Swanson School of Engineering. The group’s research seeks to couple a mechanistic understanding of the host inflammatory response with the development of biomaterials for regenerative medicine.
Ms. Behun’s research examines the correlation between aging, skeletal muscle repair and immune cell populations. She works to address defects in aging skeletal muscle, such as sarcopenia – a condition characterized by loss of skeletal muscle mass and function that affects 10 percent of individuals over 65 years old.
“Sarcopenia is a chronic, debilitating condition with many unsatisfactory treatment plans,” Ms. Behun explained. “This condition is often present in elderly patients who experience adverse outcomes, morbidity and mortality during surgery. Aging and sarcopenia are also associated with a reduced capacity to heal muscle injury, contributing to the incidence of incisional hernias.”
While defects in extracellular matrix composition and/or cellular response to injury are deemed explanations for this condition, both have been poorly tested.
“The cellular response to injury has been well characterized in young animals, and evidence has shown that old cells placed on young matrix have a youthful phenotype,” Ms. Behun said. “However, recent evidence from our lab has shown that defective repair in aging is at least partially attributed to defects in immune cell recruitment, not polarization.”
Ms. Behun uses special biomaterials in a well-established body wall defect to assess tissue remodeling and compare young animals to aged ones.
“The main things we want to evaluate are the types of cells that respond to injury, the effect of different bioactive molecules delivered via biomaterials on infiltrating immune cell types, and the contribution these cells have on constructive repair of injured tissues,” she said.
Understanding these bioactive materials may improve development, which could ultimately help clinicians more effectively address defects in aging skeletal muscle in humans and improve surgical outcomes.
Illustration: Behun (Institute for Clinical Research Education)/Brown (McGowan Institute for Regenerative Medicine)
DIY Device Climbs to the Top of the Charts

Michael Behrens, a student in the lab of McGowan Institute for Regenerative Medicine affiliated faculty member Warren Ruder, PhD, associate professor and William Kepler Whiteford Faculty Fellow of Bioengineering at Pitt, created a side project to optimize his lab work. This side project piqued the interest of the global scientific community, putting it in the top 10 chemistry papers published in Scientific Reports in 2020.
Working as a bioengineering PhD student at Pitt’s Swanson School of Engineering, Mr. Behrens’ synthetic biology research at the University of Pittsburgh requires the use of microfluidic devices, which allow researchers to rapidly perform biology or chemistry experiments on a small scale.
Doing this work on a small scale helps save time and precious research dollars by allowing investigators to stretch resources, but Mr. Behrens saw more room for improvement. The peripheral equipment often required for microfluidic experiments adds to the cost and complexity, so he decided to innovate a solution to this problem.
Mr. Behrens’ open-source, 3D-printed tool is not only cheaper, but it also adds a level of flexibility for tech-savvy researchers to fully harness these transformative devices.
“Peristaltic pumps for microfluidic devices already exist, but I wanted a simple, reliable version that could carry small volumes of liquid,” said Mr. Behrens. “It’s cheaper to build it yourself, but we also added a level of flexibility by incorporating programmable microcontrollers that allow for custom flow profiles.”
Microfluidic devices have a wide range of applications, including point-of-care diagnostics – much like the testing we have witnessed for the COVID-19 pandemic. This type of technology, also known as lab-on-a-chip, can quickly deliver much needed results and has transformed testing – particularly in times of emergency, at-home care, or in places that lack clinical infrastructure.
“Since our pump is relatively cheap and easy to build and use, it could enable places with resource constraints to still have advanced diagnostics,” he said. “This pump could allow clinicians to run reagents past cells grown in a microfluidic device and do quick on-site testing or allow high school labs to experiment with modern chemistry and biology research techniques.”
For Mr. Behrens, the tool has helped advance his biorobotics research in the Synthetic Biology and Biomimetics Lab led by Dr. Ruder. He believes this tool could help fellow engineers stretch their budgets and more effectively utilize microfluidic technologies.
“I think there an unmet need to develop cheap tools to make microfluidics more accessible, especially for researchers,” he said. “The success of the paper shows me that a lot of people are interested in microfluidics and providing open-source tools to help enable those technologies seems like a useful thing to do.”
Congratulations, Mr. Behrens!
Illustration: Behrens (Ruder Lab)/Ruder (McGowan Institute for Regenerative Medicine)
Certain Cancer Patients at Risk of COVID-19 Vaccine Failure

People with cancer that affects the blood, bone marrow or lymph nodes are at elevated risk of COVID-19 vaccine failure, particularly those with chronic lymphocytic leukemia, according to new results from an analysis of UPMC Hillman Cancer Center patients. McGowan Institute for Regenerative Medicine faculty member Alan Wells, MD, DMSc, medical director of UPMC Clinical Laboratories and the Thomas Gill III Professor of Pathology in Pitt’s School of Medicine, is a co-author of these results.
The finding prompted University of Pittsburgh School of Medicine and UPMC clinician-scientists to issue a cautionary statement in the preprint journal medRxiv, urging such patients and those who interact with them to take the COVID-19 vaccines available, but to continue wearing masks and practicing social distancing, even after full vaccination. They simultaneously are pursuing peer-reviewed publication of the findings.
“As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” said senior author Ghady Haidar, MD, UPMC transplant infectious diseases physician and assistant professor in Pitt’s Department of Infectious Diseases. “Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip.”
Dr. Haidar cautioned that a negative antibody test does not necessarily mean that the patient lacks protection from the virus. At this time, UPMC and the U.S. Centers for Disease Control and Protection do not recommend repeat or booster vaccinations for previously vaccinated people, even if they test negative for antibodies.
Hematologic malignancies are a classification of non-solid tumor cancers, including leukemias, myelomas and lymphomas. These patients have a greater than 30% risk of death if they contract COVID-19 and often receive antibody-depleting therapies, which means they should be prioritized for COVID-19 vaccination. However, they were excluded from COVID-19 mRNA vaccine trials, so data on the vaccines’ effectiveness are nonexistent.
Approximately three weeks after their final vaccination, 67 patients with hematologic malignancies who had been vaccinated with either the Pfizer or Moderna COVID-19 two-dose vaccines had their blood tested. Dr. Haidar and his colleagues found that more than 46% of the participants had not produced antibodies against SARS-CoV-2.
Moreover, only three in 13 patients with chronic lymphocytic leukemia (CLL)—a slowly progressing cancer of the blood and bone marrow—produced measurable antibodies, even though 70% of them weren’t undergoing any form of cancer therapy.
“This lack of response was strikingly low,” said Mounzer Agha, MD, the study’s lead author and a hematologist at UPMC Hillman Cancer Center. “We’re still working to determine why people with hematologic malignancies—particularly those with CLL—have a lower antibody response and if this low response also extends to patients with solid tumors.”
The team did not find a link between cancer therapy and antibody levels to indicate why some of the patients did not mount an adequate immune response to the vaccine. As expected, however, older patients were less likely to produce antibodies compared to younger patients.
“It’s critically important for these patients to be aware of their continued risk and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha added. “They may benefit from outpatient treatments, such as monoclonal antibodies, before the illness becomes severe.”
Studying the Mechanism Behind Metastatic Breast Cancer

University of Pittsburgh bioengineer Partha Roy, PhD, Associate Professor of Bioengineering, Cell Biology, and Pathology at the University of Pittsburgh, received awards from the National Cancer Institute (NCI) of the National Institute of Health and the Magee Women’s Cancer Research and Education Funding Committee to investigate the role of actin-binding protein profilin1 in metastatic breast cancer — the second most common cancer among women in the United States. In 2021, an estimated 281,550 new cases of invasive breast cancer will be diagnosed in U.S. women, and around 15 percent of those cases are expected to be fatal.
“Metastatic cancer is the cause of the majority of breast cancer deaths,” said Dr. Roy, who leads the Cell Migration Lab at Pitt’s Swanson School of Engineering. “During metastasis, nests of cells escape from the primary tumor and spread to other parts of the body. Treating these metastatic growths only temporizes the lethal outcome, so my group will investigate the mechanism that leads to metastatic dissemination and growth.”
Dr. Roy’s lab previously found that profilin1 has contrasting effects on early vs. late stage of breast cancer metastasis. While reduced level of profilin1 in cancer cells makes these cells more migratory and competent in dissemination from the primary tumor, cancer cells are dependent on profilin1’s action for metastatic colonization.
As part of the NCI-R01 grant, Dr. Roy’s lab will study how profilin1 controls lipid signaling and the downstream processes during dissemination of breast cancer cells. This study will be in collaboration with Pitt’s Gerry Hammond, PhD, assistant professor of cell biology, and Beth Roman, PhD, associate professor of human genetics and member of the Vascular Medicine Institute.
The pilot grant from the Magee Women’s Cancer Research will have two foci. They will conduct preclinical proof-of-concept studies to determine whether novel small molecules targeting the profilin1-actin interaction suppress metastatic colonization of breast cancer cells. They will also examine the mechanistic understanding of how profilin1’s interaction with actin activates certain signaling pathways to regulate dormancy-to-emergence behavior of cancer cells.
These studies could pave the way for novel therapeutic directions in metastatic breast cancer.
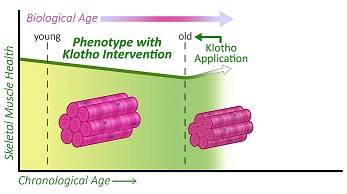
Study Reveals Roadmap of Muscle Decline with Age
Scientists have produced a comprehensive roadmap of muscle aging in mice that could be used to find treatments that prevent decline in muscle mobility and function, according to a report published in eLife. McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, MPT, Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh with secondary appointments in the Departments of Bioengineering, Physical Therapy, Orthopaedic Surgery, Microbiology & Molecular Genetics, and Environmental & Occupational Health, is the senior author on the work.
The study reveals which molecules in the muscle are most significantly altered at different life stages, and shows that a molecule called Klotho, when administered to mice in old, but not very old age, was able to improve muscle strength.
Age-related loss of skeletal muscle mass and function – called sarcopenia – is associated with loss of mobility and increased risk of falls. Yet, although scientists know how sarcopenia affects the appearance and behavior of muscle tissues, the underlying molecular mechanisms for sarcopenia remain poorly understood. Current treatments for sarcopenia largely involve prescribing physical activity or dietary modifications, and these have shown moderate success.
“Although there are no proven treatments for sarcopenia yet, there are some pharmaceutical treatments entering clinical trials. Interestingly, many of these act on mechanisms that also involve a protein called Klotho,” says co-first author Zachary Clemens, doctoral student at the Department of Environmental and Occupational Health, University of Pittsburgh. “Evidence suggests that Klotho levels gradually decline with age, and so we wanted to test whether supplementation with Klotho may attenuate the development of sarcopenia.”
The team first characterized and compared changes in the structure, function, and gene activity in skeletal muscle across the lifespan in mice. They grouped mice into four age categories – young, middle-aged, old, and oldest-old – and looked at muscle weight, type of muscle fibers, whether the muscles had accumulated fat, and skeletal muscle function. Although old mice displayed mild sarcopenia, the common clinical features of sarcopenia were only present in the oldest-old mice.
Next, they looked at changes in muscle gene activity and found a progressive disruption in genes known to be associated with the hallmarks of aging from the young to the oldest-old mice.
“To date, most studies in skeletal muscle have focused on the identification of specific pathways that are associated with sarcopenia to identify a molecular mechanism linked to the condition,” explains co-first author Sruthi Sivakumar, doctoral student at the Department of Bioengineering, University of Pittsburgh. “We employed an integrative approach, where we created a network by converting gene expression levels to protein-protein interactions, and then we studied how this interaction network changed over time.”
From this network, the team determined the ‘network entropy’ of the muscle cells as a means to estimate the loss of molecular order within the system over time. They found the greatest difference in order between the young and old age groups (at which point it reached maximal entropy), with little difference between the old and oldest-old mice. Additionally, when they looked at human muscle gene data from different age groups, they saw that entropy reached its lowest level in the fourth decade of life, after which time entropy escalated. This was of interest to the team as the fourth decade of life is the time point when sarcopenia often starts to develop.
Next, they looked at whether administering Klotho to mice would have beneficial effects on the muscle healing after injury. They found that applying Klotho after muscle injury reduced scarring and increased structures associated with force production in the animals. Injured mice that received Klotho also had better muscle function – such as muscle twitch and force production – and their whole-body endurance improved two-fold.
Finally, the team looked at whether giving the mice Klotho could reverse age-related declines in muscle quality and function. They found that Klotho administration led to some improvements in the old mice: force production was improved by 17% and endurance when supporting whole body weight was 60% greater compared to mice without treatment. But this was only seen in the old mice, and not in the oldest-old animals. Further investigation showed that Klotho affected genes associated with the hallmarks of aging in all age groups, but that the oldest-old mice showed a dysregulated gene response.
“Our data suggest that treatment with Klotho may be more effective in slowing the progression of sarcopenia at an earlier time point, rather than rescuing advanced age-related disease, by which time the gene responses seem to be more random,” concludes Dr. Ambrosio. “It will be interesting in future studies to determine whether boosting Klotho levels at a younger age could prevent muscle declines into old, and even oldest-old, age.”
Illustration: Image extracted from graphical abstract, “The biphasic and age-dependent impact of Klotho on hallmarks of aging and skeletal muscle function.” eLife 2021;10:e61138.
PNA-Based Technique an Essential Part of the Gene Editing Toolkit

With their article published in Nature, the National Institutes of Health’s Somatic Cell Gene Editing Consortium provided a detailed update on the progress of their nationwide effort to develop safer and more effective methods to edit the genomes of disease-relevant somatic cells and reduce the burden of disease caused by genetic changes. McGowan Institute for Regenerative Medicine affiliated faculty member Samira Kiani, MD, Associate Professor, Liver Research Center, Department of Pathology, School of Medicine, University of Pittsburgh, is a co-author of this work.
Gene editing allows scientists to modify sections of an organism’s DNA and is considered a promising treatment for a number of genetic diseases. There have been numerous advances in the laboratory over the last few decades, but there are still many challenges to overcome before gene editing can be widely used in the patient population. Launched in 2018, the Somatic Cell Gene Editing Consortium (SCGE) has brought together some of the leading researchers in the field to advance discovery and accelerate the translation of somatic gene editing advances in the lab to the clinical setting.
Over six years, the NIH will allocate approximately $190 million to SCGE to realize gene editing’s potential. The end result will be a freely available toolkit that will provide the biomedical research community with rigorously evaluated information about genome editors and methods for delivering and tracking gene editing molecules.
“NIH realized it was important for all of us who are investigating gene editing to work together toward a common goal,” said Carnegie Mellon University Professor of Chemistry Danith Ly, PhD, who joined the consortium in 2019. “We’re designing molecules that can go into the cell and we’re cataloging each and every one. What we’ll end up with is a very valuable, rigorously evaluated resource for those who want to bring gene editing to patients.”
While much of the consortium’s work focuses on CRISPER-Cas related systems, the SCGE points out that it’s important to continue to develop other systems. They specifically single out the peptide nucleic acid-based gene editing technique developed by Carnegie Mellon’s Dr. Ly and Yale University’s Peter Glazer, MD, PhD.
“Although there is a significant focus on CRISPR-Cas related systems within the SCGE, it is crucial to continue to explore alternate systems, in part because they may differ in both their potential for delivery and their biological or immunological responses,” the consortium wrote in Nature.
While CRISPR-Cas edits genes in cells that have been removed from the body, Dr. Ly and Glazer’s peptide nucleic acid (PNA) system is administered intravenously and edits cells in vivo. Using nanoparticles, a PNA molecule paired with a donor strand of DNA is delivered directly to a malfunctioning gene. Dr. Ly, a leading researcher in synthetic nucleic acid technology, has programmed PNA molecules to open double stranded DNA at the site of a targeted mutation. The donor DNA from the complex binds to the cell’s faulty DNA and triggers the DNA’s innate repair mechanisms to edit the gene. The team has used the technique to cure beta thalassemia in adult mice and in fetal mice in utero.
The PNA gene editing system doesn’t have the high-yield of CRISPER-Cas systems, but it does have the advantage of being less likely to make off-target modifications. According to Dr. Ly, that means their technique might be better for genetic diseases that only need to have a small percentage of cells corrected to make a therapeutic difference. For example, in the beta thalassemia studies, Drs. Ly and Glazer found that editing only six to seven percent of cells was curative.
Drs. Ly and Glazer plan to further refine and improve their technique through their participation in SCGE, and they look forward to sharing their results with the consortium and the greater biomedical community.
Stem Cells Derived from Fat Show Promise as a Treatment for Mass Radiation Exposure

Nuclear power offers an efficient, reliable way to provide energy to large populations – as long as all goes well. Accidents involving nuclear reactors such as those that took place in 1986 at Chernobyl and at Fukushima Daiichi after the March 2011 tsunami raise major concerns about what happens if the worst occurs and large numbers of people are simultaneously exposed to high levels of radiation. Currently, there are no effective, safe therapies for total body irradiation (TBI) – a condition known as acute radiation syndrome (ARS). That could change, in the future based on new research published in Stem Cells Translational Medicine.
Researchers at the University of Pittsburgh Medical Center (UPMC) demonstrated, for the first time, how allogeneic adipose-derived stem cells (ASCs) can mitigate TBI-induced ARS. This would allow for the stockpiling of these cells to be used in case of a radioactive emergency.
“In nearly all instances of TBI exposure, the primary life-threatening damage is inflicted on the hematopoietic system, which primarily consists of the bone marrow, spleen, tonsils and lymph nodes involved in the production of blood. High doses of radiation can cause irreparable damage to the bone marrow, affecting the immune system and potentially causing inflammation and infection,” said the study’s co-author, Asim Ejaz, PhD, from the UPMC Department of Plastic Surgery.
A matched hematopoietic stem cell transplant is the current therapy of choice. Patients are either able to donate their own stem cells for transplantation – called an “autologous” transplant – or a donor whose stem cells are a good match is found. The odds of finding an adequate match are low at about 30 percent and for some populations the odds are even less.
“That fact leads to the major issue with relying on this type of therapy: In a mass population exposure scenario involving several hundred to millions of individuals, hematopoietic stem cell transfusion is an impossible way forward as there is just no way to treat massive numbers of people who are exposed to radiation at the same time. And unfortunately, such a delay in treatment for a large number of individuals would most certainly lead to an increase in their mortality rate,” Dr. Ejaz said.
Several drugs are currently being looked at as potential therapies, but none provides complete protection, and all have unwanted side effects. This leaves the need for alternative therapies. Previous studies have reported that mesenchymal stem cells derived from bone marrow, placenta or Wharton’s jelly can mitigate the effects of ARS. However, low yield and difficulty in harvest and mass production of these cells for stockpiling are some of their drawbacks.
“There is one attractive cell-based candidate we’re looking at,” said McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, chair of the Department of Plastic Surgery at UPMC and a co-author of the study, “and that is adipose-derived allogeneic stem cells (ASCs). These are mesenchymal stem cells derived from adipose tissue (fat), which can easily be harvested in vast quantities from donors via liposuction.
“Adipose-derived ASCs have been proven to be safe, they have self-renewal capacity and can undergo differentiation to mature cells,” he added. “They also are very easy to propagate in cell culture compared to other cell types, which means they can be stockpiled for application at mass levels in case of radiation accidents.”
The team’s study focused on how treating mice that were exposed to high levels of radiation with injections of allogeneic ASCs measured up to treating them with autologous ASCs. In particular, they wanted to see if the injections would improve the animals’ survival rates and repair damage to their hematopoietic systems.
The injections were given 24 hours after the mice were exposed to the radiation. When, 35 days later, the researchers examined the results, they found that the allogeneic ASC-treated groups performed equally to the autologous cells in improving the animals’ survival and recovery rates vs. the non-treated control group.
“The ASCs had migrated to the bone marrow and facilitated repair by secreting several factors known to reduce oxidative stress and rescue damaged bone marrow cells from apoptosis,” Dr. Ejaz said.
Added Dr. Rubin, “This suggests that allogeneic ASCs therapy may be beneficial for clinical adaptation to treat TBI-induced toxicities. Further studies will help to advocate the scale-up and adaptation of allogeneic ASCs as the radiation countermeasure.”
“The data from this pre-clinical study suggesting the ability of fat-derived stem cells as a therapy to effectively mitigate acute radiation syndrome is interesting and potentially useful,” said Anthony Atala, MD, Editor-in-Chief of Stem Cells Translational Medicine and Director of the Wake Forest Institute for Regenerative Medicine. “Currently, there are no effective and safe approaches to treat mass population exposure to acute radiation syndrome; we welcome future studies that will enable the clinical adaptation of this type of therapy.”
A Super Researcher

As reported by Amerigo Allegretto of Pittwire, of all people, it was Superman who changed the course of the professional life of McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD.
In 2002, the chemist was attending a tissue engineering conference in downtown Pittsburgh. One of the featured speakers was actor and disability advocate Christopher Reeve, who Dr. Marra recalls saying, “Whatever you’re doing, think about what you’re working on and how that can translate to spinal cord injury.”
The day after Mr. Reeve’s speech, Dr. Marra turned her attention to just that.
Today, she is a professor of plastic surgery in the University of Pittsburgh School of Medicine and bioengineering in Pitt’s Swanson School of Engineering, as well as vice chair of research for the Department of Plastic Surgery. Her research focuses on a biodegradable tube that repairs severe peripheral nerve injuries, such as loss of feeling in limbs due to spinal cord injury like Mr. Reeve had. The tube releases a protein that repairs affected tissue in wounds while slowly disappearing to prevent infection or dislodging. Prior research in animals has demonstrated that the nerve guide device could restore up to 80% of nerve function.
The device could have applications for people injured in car accidents, machinery accidents, newborn nerve injuries incurred during delivery, nerve damage due to tumor removal, and diabetic neuropathy. Soldiers, too, could benefit.
If it is approved by the U.S. Food and Drug Administration, the project will then enter human clinical trials.
“Over half of our injured soldiers have a nerve injury. As a daughter of a Marine and a Vietnam vet who fought on the front lines, I found that highly motivational,” Dr. Marra said. “To turn my research efforts into something that can help our brave soldiers and veterans completely motivated me.”
Dr. Marra has been attending military medicine meetings and working with colleagues at the Walter Reed National Military Medical Center in Maryland. She has also met with soldiers affected by nerve injuries.
To help advance her research, Dr. Marra has been entering competitions and filed an invention disclosure with Pitt’s Innovation Institute for her research. In 2018, she created her own startup company, Nerve Repair Technologies. In June 2020, Dr. Marra received the People’s Choice award at Equalize2020, a competition for female academic entrepreneurs. Shortly after that, she was named a senior member of the National Academy of Inventors.
“I tell my students to try to find something in life that you’re going to be passionate about. That’s what got me to where I am today,” she said. “I knew I wanted to be a scientist since I was 10. I didn’t even realize I could do this kind of work for the military without enlisting.”
AWARDS AND RECOGNITION
Controlled Release Society to Present Dr. Steven Little with Distinguished Service Award

The Controlled Release Society (CRS) has announced that McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, will receive its Distinguished Service Award at its virtual annual meeting this July 25-29. Dr. Little, the William Kepler Whiteford Endowed Professor and Chair of the Department of Chemical and Petroleum Engineering at the University of Pittsburgh’s Swanson School of Engineering, is internationally recognized for his research in drug delivery systems that mimic the body’s own mechanisms of healing and resolving inflammation.
This is Dr. Little’s third honor from CRS; in 2018 he received the society’s Young Investigator Award, and in 2020 was elected to its College of Fellows for “outstanding and sustained contributions to the field of delivery science and technology over a minimum of ten years.”
“Dr. Little’s leadership of the focus groups of the Controlled Release Society has been transformational for the society as a whole,” said nominator Justin Hanes, PhD, the Lewis J. Ort Professor of Ophthalmology at the Johns Hopkins University School of Medicine. “I have never seen the young rising superstars of our field so engaged in the CRS, and their engagement is key to the long-term success of this remarkable scientific society. Dr. Little has also been a highly valued member of the CRS board of directors. He is a visionary and a natural leader. We are so grateful to him.”
Rather than traditional drug treatments that are distributed throughout the entire body, Dr. Little’s controlled release research focuses on time-released microcapsules that target specific cells on site. In 2020, Dr. Little published a groundbreaking discovery of a new immunotherapy system that mimics how cancer cells invade the human immune system and thereby reduces the risk of transplant rejection. He has also made advancements to the fundamentals of delivery science with predictive models enabling rational design of drug delivery systems, leading to the founding of Qrono Inc., a specialty pharma company in Pittsburgh.
“The CRS is a tremendous organization, and I am extremely humbled by this recognition. A large number of people sacrificed so much of their time to achieve the positive changes that this award is recognizing. I am very confident that I speak for all of these people when I say how rewarding it is for all of us to see the next generation of scientists and engineers being recognized for what they do and having a way to exercise their own leadership in this world-class organization,” said Dr. Little.
Congratulations, Dr. Little!
McGowan Institute Faculty Support Student Winners at iGEM

McGowan Institute for Regenerative Medicine affiliated faculty members Jason Shoemaker, PhD, assistant professor of chemical and petroleum engineering, and Sanjeev Shroff, PhD, Distinguished Professor and Gerald E. McGinnis Chair of Bioengineering, were among the group of faculty advisors who supported a team of University of Pittsburgh undergraduates in the 2020 International Genetically Engineered Machine (iGEM) competition. This contest is an annual synthetic biology research competition in which teams from around the world design and carry out projects to solve an open research or societal problem. More than 250 teams participated in the organization’s first Virtual Giant Jamboree, and the Pitt undergraduate group received a gold medal for their project titled “Bluetooth Bacteria.” This year’s group was also one of three teams that were nominated for “Best Foundational Advance Project.” This is the first time a Pitt iGEM team has been nominated for an award at the iGEM competition.
The team included one Swanson School of Engineering student: Lia Franco, a chemical engineering junior. Other members included Sabrina Catalano, a senior molecular biology student; Dara Czernikowski, a senior biological sciences student; Victor So, a senior microbiology and English literature student; and Chenming (Angel) Zheng, a junior molecular biology student.
“This sort of non-invasive technology could be used for timed drug release, synthetic organ and neuron stimulation, or even industrial applications,” Ms. Czernikowski said. “We first considered optogenetics, which uses light to manipulate cell behavior, but this strategy cannot target deep tissue without risky invasive methods, so we needed to change our approach.”
The team ultimately decided to attach magnetic nanoparticles to the surface of bacteria and stimulate them with an alternating magnetic field (AMF). The nanoparticles react to the AMF stimulation and dissipate heat, causing the temperature of the bacterium’s cytoplasm to rise. They then used a protein dimer to act as a “bio-switch” to control gene expression.
“At lower temperatures, the protein dimers bind to a target DNA sequence and turn off gene expression, but at higher temperatures, heat causes the proteins to un-dimerize,” Ms. Catalano explained. “In its un-dimerized state, it can no longer inhibit gene expression, turning the system on. The change in temperature is controlled by the stimulation of magnetic nanoparticles with AMF, allowing wireless control of gene expression in bacteria.”
The team hopes that there is therapeutic potential for their design but recognizes that they need to improve spatial control in order to match techniques like optogenetics. They would like to improve their design to use localized heating that could selectively target one bacterium or a specific region of the cytoplasm. They plan to continue development during the upcoming semester.
Another unique aspect of their project is the “Bluetooth Bacteria Podcast” – a casual and conversational podcast that seeks to educate the general population on topics and current developments in synthetic biology.
“One of our main project goals was effective science communication,” said Ms. Catalano. “Because COVID-19 limited our ability to teach synthetic biology in person, we thought it would be fun to make a podcast as it is accessible to a wide audience. It gave us the opportunity to hear from iGEM teams all over the world, including France, London, and India.”
The team published two episodes every week, and they are available on Apple Podcast or Spotify. Listen to one of their episodes here.
Congratulations, all!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#220 –– Mr. Keith Kaufman discusses his accident and participation in a tissue engineering clinical trial.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Zachary Clemens, Sruthi Sivakumar, Abish Pius, Amrita Sahu, Sunita Shinde, Hikaru Mamiya, Nathaniel Luketich, Jian Cui, Purushottam Dixit, Joerg D Hoeck, Sebastian Kreuz, Michael Franti, Aaron Barchowsky, Fabrisia Ambrosio
Title: The biphasic and age-dependent impact of Klotho on hallmarks of aging and skeletal muscle function
Summary: Aging is accompanied by disrupted information flow, resulting from accumulation of molecular mistakes. These mistakes ultimately give rise to debilitating disorders including skeletal muscle wasting, or sarcopenia. To derive a global metric of growing ‘disorderliness’ of aging muscle, we employed a statistical physics approach to estimate the state parameter, entropy, as a function of genes associated with hallmarks of aging. Escalating network entropy reached an inflection point at old age, while structural and functional alterations progressed into oldest-old age. To probe the potential for restoration of molecular ‘order’ and reversal of the sarcopenic phenotype, we systemically overexpressed the longevity protein, Klotho, via AAV. Klotho overexpression modulated genes representing all hallmarks of aging in old and oldest-old mice, but pathway enrichment revealed directions of changes were, for many genes, age-dependent. Functional improvements were also age-dependent. Klotho improved strength in old mice but failed to induce benefits beyond the entropic tipping point.
Source: eLife 2021;10:e61138 DOI: 10.7554/eLife.61138
GRANT OF THE MONTH
PI: Louis Falo
Title: Engineering the Skin Immune System to Induce Systemic Immune Responses
Description: The skin functions as a potent immune organ, rendering the readily accessible cutaneous microenvironment an attractive target that can be locally engineered to induce and regulate systemic immune responses. Increasing age is associated with changes in immune function (immunosenescence) that result in increased susceptibility to diseases and poor vaccine responses in older adults. This proposal is designed to bring together very recent and significant advances in dermatology and skin biology to address critical problems in human health. Here, we specifically focus on the skin microenvironment and strategies for cutaneous immunomodulation. The overall approach is to utilize a clinically applicable dissolvable microneedle array delivery platform we have developed to locally engineer the highly adaptive and immunoresponsive skin microenvironment. Specifically, we propose to modulate local immune regulatory circuits in the skin to enable the induction and regulation of systemic immune responses. The studies we propose will investigate the effect of locally delivered immune modulators on the mechanisms underlying cutaneous immune regulation in young and aged skin. Importantly, our studies include translational studies using living human skin to enable the rapid advancement of novel cutaneous immunomodulation strategies into clinical trials. The proposed studies will result in a better understanding of skin immunobiology specifically relevant to the development of safe and predictable skin immunoengineering strategies to regulate systemic immune responses across the lifespan.
Source: National Institute of Arthritis and Musculoskeletal and Skin Diseases
Term: March 4, 2021 – January 31, 2026
Amount: $652,360 (one year)
