November 2023 | VOL. 22, NO. 11 | www.McGowan.pitt.edu
Swanson School of Engineering Celebrates 10 Years of an “Alliance of Innovation”

In 2013, the University of Pittsburgh and the Lubrizol Corporation began a partnership to foster the future of chemistry and engineering science.
The Lubrizol Corporation, founded in 1928, has 8,800 employees and more than 100 facilities worldwide. The company focuses on vehicle needs, including energy efficiency and reducing carbon emissions, as well as products that facilitate safe drinking water and materials that are used in healthcare, wellness-related applications, and medical, beauty, and home care.
In an article for the Swanson School of Engineering, Steven R. Little, PhD (pictured), McGowan faculty, notes, “When we began this partnership [with Lubrizol], our faculty saw it as an opportunity to apply their expertise to industry and find solutions for projects that were relatively small but impactful.”
Dr. Little, who is a Distinguished Professor and Department Chair of Chemical and Petroleum Engineering at Pitt continues, “Additionally, it presented an opportunity to engage students in industry research and ideation. As our alliance strengthened, however, this synergy and excitement expanded to where we were able to help Lubrizol create game-changing industry processes and innovations.”
A large component of the alliance between Pitt and Lubrizol is faculty lab research. Among other initiatives, faculty and students at the Swanson School have developed systems that have allowed Lubrizol to take a more sustainable approach to manufacturing, including a circular economy where processes are streamlined, waste is reduced, and a product can be reused, recycled, or repurposed.
Another important component of the alliance is training the next generation of chemical engineers.
“Industries often evolve through innovation or attrition, and at Lubrizol we believe innovation is most successful when fostering the future of chemistry and science and creating healthy talent pathways,” says Glenn Cormack, Lubrizol’s Global Process Innovation Manager and Technical Fellow. “By using the knowledge gained in labs and creating new courses that give students new paths into research, we help to develop a more holistic chemical engineer.”
Part of becoming a more holistic chemical engineer is focusing not just on the “quality of the chemistry,” but on quality of life. The alliance between Pitt and Lubrizol is additionally focused on research and growth in medical polymers, medical devices, and pharmaceuticals.
“Both chemistry and pharmacology are evolving from an ‘everything and the kitchen sink’ approach to manufacturing and treatment to a more ‘quality by design’ ethos,” Dr. Little explains. “Today, we have a greater understanding of particle design and function as well as microfluidic systems. For example, rather than treating conditions from tooth decay to eye diseases and cancer by flooding the body with medication, we can design particles that are stimulus responsive and go into the body and treat one area or organ.”
Sanjeev Shroff, PhD, McGowan faculty and Interim U.S. Steel Dean of Engineering, notes, “One of the greatest attributes that has grown out of this alliance is the excitement between our faculty and Lubrizol scientists when they discover that solving a relatively small problem results in a greater innovation.”
“The other benefit,” Dr. Shroff continues, “is that it has provided the template for the Swanson School and Pitt to develop similar partnerships in other departments which are also bearing fruit. I look forward to seeing what the next decade may bring.”
The original four-year, $1.4 million alliance received a three-year, $1 million renewal from Lubrizol in 2022. In the past decade, the alliance has yielded a multitude of advancements in manufacturing processes and sustainability efforts, as well as $9 million in external funding and support for nearly 30 graduate and postdoctoral students.
RESOURCES AT THE MCGOWAN INSTITUTE
December Histology Special – Herovici Staining
Let it snow! At the McGowan Histology Core, we are cutting frozen sections and offering 25% off your entire frozen project bill.*
We offer a high degree of technical expertise and can accommodate even the more specialized service needs.
You’ll receive 25% off your frozen project in December when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedure: Please contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
* 25% off applies to routine sample preparation (processing, embedding, cutting, and staining). It does not apply to specialized services such as immunohistochemistry workups and IHC staining.
UPCOMING EVENT
10th Annual International Symposium on Regenerative Rehabilitation

The 10th Annual International Symposium on Regenerative Rehabilitation will be held on March 7-9, 2024 in Boston, MA. This symposium is the largest medical and scientific conference specific to Regenerative Rehabilitation in the world and brings together renowned experts in the fields of regenerative medicine and rehabilitation. This year’s keynote speakers include Ana Rodríguez Castillo, Artist and Art Historian, who will speak “On Originality of Ideas: Breaking Rigid Concepts of Authorship from the Perspective of the Art World;” and Alan J. Grodzinsky, ScD, Professor of Biological, Electrical and Mechanical Engineering Emeritus at MIT, who will speak on “Human Osteochondral-Synovium Co-Culture Model of Cartilage Degradation & Therapeutic Intervention: Relevance to Post-Traumatic Osteoarthritis on Earth and in Space.”
The symposium will highlight the state-of-the-science for regenerative medicine technologies (at basic, pre-clinical, and clinical stages) and will provide clinical examples of the Regenerative Rehabilitation paradigm. Emphasis will be placed on opportunities, obstacles, and challenges encountered in the clinical translation of these novel approaches. The session will conclude with an interactive and moderated discussion between panel members and the audience so as to help map a strategic plan for advancing the field. Cutting-Edge Research, Trainee Opportunities, Travel Awards, Diversity Grants, Networking and More!
View the agenda and register here.
SCIENTIFIC ADVANCES
Resiliency and Tele-rehabilitation in the Russo-Ukraine War: The Department of Defense Funds Dr. Poropatich to Set up Telemedicine Training Program

Since the Russian invasion of Ukraine in February 2022, much of Ukraine’s healthcare infrastructure has been strained or destroyed. However, recent World Health Organization (WHO) findings point to resiliency, in part aided by telemedicine.
An average of 79% of primary health care (PHC) facilities across Ukraine have conducted training in the provision of remote healthcare. Approximately one third of all the facilities surveyed now provide electronic or tele-prescriptions and have shifted clinical encounters to digital platforms (teleconsultations). Around one quarter of doctor consultations are conducted via video link, and many doctors and nurses now monitor their patients remotely.
Advancements in technology, such as telemedicine, are changing the landscape of medical care in warzones.
However, it is not enough to simply send technology to the frontline or to the outlying cities and communities that need it. Training local medical professionals on how to employ new technologies is critical.
McGowan affiliated faculty Ron Poropatich, MD (pictured center), who is also the Director of the Center for Military Medicine Research (CMMR) at the University of Pittsburgh, was recently sponsored by the Department of Defense (DoD) to travel to Poland to help stand up a new battlefield telemedicine training program for Ukrainian doctors and nurses.
Dr. Poropatich, who served 30 years on active duty in the U.S. Army and retired with the rank of Colonel, led the U.S. Army effort from 1992 to 2012 in the development and deployment of telemedicine capability across 22 time zones for remote consultation for overseas locations, including in Afghanistan, Iraq, Somalia, Kuwait, Haiti, and Croatia, as well as stateside U.S. Army locations.
Following his military service, Dr. Poropatich became the Director of the CMMR, where he works closely with clinical providers at military hospitals to address the military’s current needs as well as anticipate future needs. The CMMR focuses on trauma, emergency, and critical care, specifically on developing autonomous systems to enhance the care of the warfighter on the battlefield.
“When I left the Army in 2012 (a mandatory retirement) after 30 years,” states Dr. Poropatich, “we were doing about 5,000 telemedicine consults a month across 22 time zones, which at this time was significant.”
Today in Ukraine, tens of thousands of telemedicine visits have been completed. Because many of those visits involve only text between clinicians and patients¾meaning that live audio and visual are not required – only minimal internet connectivity is needed, making telemedicine a resilient form of healthcare.
For Dr. Poropatich, much of his work has centered around patient care and systems that allow for the resiliency and effectiveness in the delivery of healthcare. He notes that his key research goals are “improving access to care, improving and utilizing technologies, and improving outcomes and hospital care.”
His trip to Warsaw focused specifically on the potential of tele-rehabilitation and artificial intelligence to positively impact rehabilitative care for patients. Tele-rehabilitation encompasses rehabilitation services such as assessment, prevention, treatment, education, and counseling and can include speech-language pathology, occupational therapy, and physical therapy.
Currently, according to WHO, thousands of people in Ukraine have complex conflict-related injuries that require rehabilitation services. At the same time, the need for rehabilitation not related to the conflict continues, including for those with impairments due to aging, accidents, and noncommunicable diseases. The war has simultaneously increased rehabilitation needs as well as the difficulty in accessing rehabilitation services.
As the humanitarian crisis persists, telemedicine and tele-rehabilitation continue to help enable the resiliency of Ukraine’s healthcare system. And the work of Dr. Poropatich, the CMMR, and other clinicians, researchers, and volunteers around the world are helping to save the lives and facilitate the medical work of people in Ukraine.
Can a Stem Cell “Paste” Cure Corneal Blindness?

More than 12.7 million people globally suffer from corneal blindness, many of whom live in low- and middle-income countries. Corneal blindness can be caused by infection due to bacteria, viruses, and fungi; from contact lens usage; or from the use of steroid medications. Trauma, dry eye disease, and diseases such as trachoma and keratoconus can also cause corneal blindness.
Currently, corneal transplants are the standard treatment for restoring vision to those experiencing corneal blindness. However, only 1 in 70 people with corneal blindness receive a transplant, and those that do are often faced with post-operative complications, graft rejection, and scarcity of donors.
Hin Fai (Gary) Yam, PhD (pictured), McGowan affiliated faculty and Research Associate Professor of Ophthalmology, and Yen-Michael S. Hsu, MD, PhD, Associate Professor of Medicine at the University of Pittsburgh and Laboratory Director of the Immunology Monitoring and Cellular Products Laboratory (IMCPL) are collaborating on a project that involves preparing corneal cells for clinical trials in order to treat patients who can no longer see due to corneal scarring.
They are the principle investigators on a National Institutes of Health-funded Cooperative Agreement study titled “Cell Therapy Program with Scale-up cGMP Manufacturing of Human Corneal Stromal Stem Cells.”
Dr. Yam and Dr. Hsu are working on a “paste” of restorative stem cells that can be applied to a patient’s eye to repair the scarring on the cornea. This would eliminate the need for a corneal transplant while also providing an option for patients who live in countries where a transplant is unavailable.
Their team has effectively reduced scarring in animal models, and their project seeks to establish manufacturing procedures that confirm the safety and the efficacy of the stem cells, which would ultimately lead to future clinical trials.
Drs. Church and Yazdani Awarded Grants from the Competitive Medical Research Fund

Joseph T. Church, MD (pictured top), and Hamza Yazdani, MD (pictured bottom), both McGowan affiliated faculty, have been awarded grants from the Competitive Medical Research Fund (CMRF).
The CMRF was established by the University of Pittsburgh in 1985 to “provide […] research support for projects across the broad range of biomedical sciences. These funds are used to support new investigators as they conduct the preliminary studies necessary to develop the hypotheses, preliminary data, and methods that support submission of highly competitive applications to extramural funding sources.”
In the last five years, the application success rate has averaged just over 35%, with researchers receiving standard funding of between $30,000 and $50,000.
Dr. Church is a pediatric surgeon and is board-certified in general surgery and in pediatric general and thoracic surgery by the American Board of Surgery. He is an Assistant Professor of Surgery at the University of Pittsburgh School of Medicine.
His laboratory research seeks to discover new prenatal therapies for congenital diaphragmatic hernia, and he is a member of the American Pediatric Surgical Association (APSA) and the American College of Surgeons (ACS).
Dr. Yazdani is a Research Assistant Professor in the Department of Surgery at the University of Pittsburgh. His research, clinical, and/or academic interests include liver transplant, liver ischemia-reperfusion, sterile inflammation; surgical stress in regulating tumor growth, metastasis, and the tumor microenvironment; the interaction between innate and adaptive immunity in the setting of surgical stress; exercise training in surgical stress and metastasis; and exercise in training innate and adaptive immunity.
Weinbaum Lab Student Ande Marini Receives NIH F31

University of Pittsburgh Graduate student Ande Marini (pictured) – who works in the Vascular Bioengineering Lab under PI Justin Weinbaum, PhD, Research Assistant Professor in the Departments of Bioengineering and Pathology at the University of Pittsburgh – received an F31 award from the National Heart, Lung, and Blood Institute (NHLBI).
Ms. Marini received funding for her project, “Extracellular Vesicle Delivery System for Treatment of Abdominal Aortic Aneurysm.” The project aims to study abdominal aortic aneurysm, which is an enlargement of the aorta caused by loss of elastic fiber integrity in the vascular wall. This enlargement, once it reaches the threshold of 5.5 cm, increases the risk of rupture, which can be life-threatening. At this point, surgical intervention is necessary.
However, smaller AAAs still rupture approximately 13% of the time. Ms Marini’s project aims to provide “regenerative treatment that inhibits the degradation of elastic fibers and increases elastogenesis and collagen synthesis may stabilize the vessel mechanically and slow aneurysm progression,” per the abstract.
In her Public Health Relevance Statement, Ms. Marini states, “There are only surgical options for treating large AAAs that are at the highest risk of rupture, and currently no treatments for small AAA, which will become a high risk if ignored. The proposed research will investigate how a localized delivery system of stem cell secretions can stimulate restoration of structural proteins to pave the way for a new, non-invasive treatment of small AAAs.”
Ms. Marini encourages those who will apply for a F31 fellowship in the future. When asked about the honor, she replied, “Even if you don’t feel like you’re completely ready to apply for a fellowship, you always receive helpful feedback about how to improve it for the next time.”
“If you don’t get it the first time or even the second, that’s fine – just make use of the reviewer comments.”
A F31 fellowship aims to provide promising predoctoral students in the biomedical, behavioral, or clinical sciences the opportunity to develop into a productive, independent research scientist while obtaining mentored research training and conducting dissertation research.
Congratulations, Ms. Marini!
AWARDS AND RECOGNITION
Dr. Whitcomb Presented with Lifetime Achievement Award from the National Pancreas Foundation

David Whitcomb, MD, PhD (pictured), McGowan affiliated faculty, has been awarded the Legacy Award from the National Pancreas Foundation (NPF).
The NPF, established in 1997, is the only foundation dedicated to patients who experience any form of pancreatic disease, including pancreatic cancer, acute pancreatitis, chronic pancreatitis, and pediatric pancreatitis. The foundation also participates on the advisory council for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKD), the National Cancer Institute Gastrointestinal Steering Committee, and as Department of Defense Consumer Reviewers.
The NPF’s Legacy Award, created in 2021, honors the career achievements of clinicians working in the field of pancreatic disease.
Dr. Whitcomb received the Legacy Award on October 6, 2023, at the NFP’s Courage for a Cure Gala held at PNC Park. Dr. Whitcomb leads active research teams focusing on pancreatic diseases and provides an information service on pancreas-related issues to patients, physicians, and scientist through participation in Pancreas.org and with a quarterly patient newsletter, Pancreas Education and Research Letter (PEaRL).
Dr. Whitcomb is the Giant Eagle Foundation Professor of Cancer Genetics and a tenured Professor of Medicine, Cell Biology and Physiology, and Human Genetics at the University of Pittsburgh, as well as the Director of the Precision Medicine Service at UPMC. He is editor-in-chief of Clinical and Translational Gastroenterology, the top-ranked open-access gastroenterology and hepatology journal.
The NPF recognized Dr. Whitcomb for his lifetime contributions to pancreas research.
Dr. Edington Named Director of New Skin Cancer Center
Howard D. Edington, MD (pictured), McGowan affiliated faculty, is the Director of Allegheny Health Network’s (AHN) new skin cancer center.
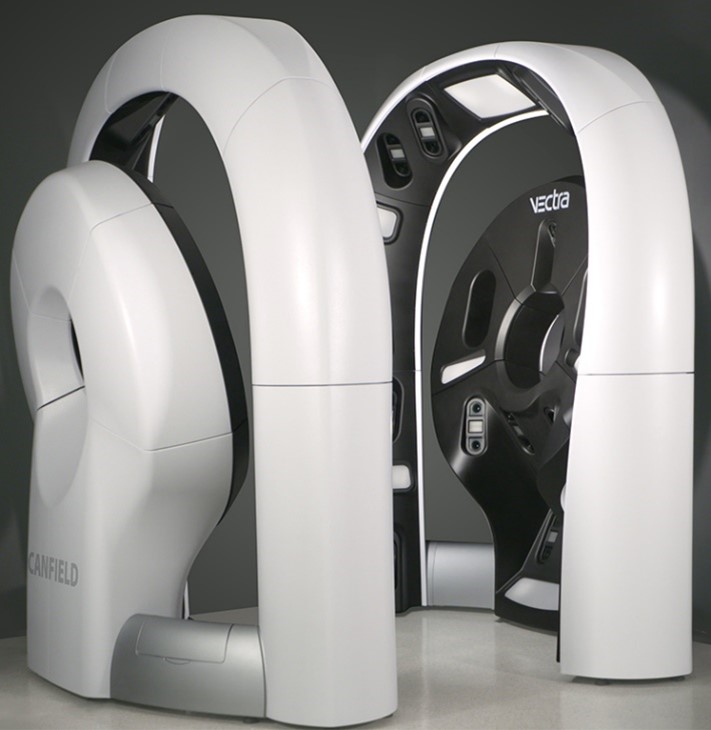
The AHN Skin Cancer Center is a 9,000 square-foot-facility that will treat dermatology and cancer patients. It is also home to the VECTRA WB360 Whole Body Imaging System. Through the use of 92 cameras and special lighting, the imaging system is able to capture the entire skin surface of a patient, creating a 3D “avatar” that allows clinicians to map and monitor moles, skin lesions, and other abnormalities. The VECTRA WB360 is one of only 15 machines in operation in the U.S.
Diagnostic technologies, including the VECTRA WB360, will enable physicians to discover cancer at earlier stages. The new skin cancer center will also focus on researching medical therapies and identifying novel biomarkers that can be used to develop precision medicine. In addition to researching new therapies, the institute offers various treatments for skin cancer patients.
In an interview for OncLive, a website that specializes in oncology news, Dr. Edington states, “Dermatology and skin cancer medicine are visual fields – having better images means better care for our patients, and more timely detection of suspicious abnormalities. By combining artificial intelligence with the clinical expertise of our physicians, we can provide more thorough preventive care.”
Dr. Edington is also the System Chairman, Department of Surgery, and the Chief of Surgical Oncology at West Penn Allegheny Health System. His research interests include anti-tumor immunity, tissue engineering and gene therapy approach to wound healing, effects of cleft lip and palate repair on craniofacial growth and development, and skin cancer.
Image of the VECTRA WB360 courtesy of Canfield Scientific, Inc.
2023 PInCh® Winning Teams Include McGowan Institute Affiliated Faculty Members

The Pitt Innovation Challenge (PInCh®) is an annual program, with 2023 being the 10th year of competition, supported by the Clinical and Translational Science Institute (CTSI) at the University of Pittsburgh. It is designed to generate innovative solutions to challenging health problems by mitigating risk and providing financial and administrative support to move ideas forward. The PInCh program stimulates the translation of novel problem-focused research into the community by giving researchers a venue to be creative, develop new ideas, and work with people beyond their usual sphere of collaborators.
Up to $555,000 in total awards are available for funding each year. This year, Bonus Awards were granted to anyone who engaged with the Makerspaces at the Pitt Swanson School of Engineering Innovation & Entrepreneurship program. Nine awardee teams proposed creative solutions to address important health problems, including diagnostics, treatments, interventions, and programs. These solutions, if successfully brought into clinical practice, could significantly improve health outcomes for many and create healthier communities.
The PInCh program consisted of three rounds:
- Teams entering Round One must submit a video describing their idea.
- Teams selected for Round Two must submit a written document expanding on their idea.
- Teams selected for Round Three must submit a response to reviewers, revisions to their written document, and pitch their idea to a panel of judges at the final event.
The winning teams with members from the McGowan Institute include:
$100,000 Awardee:
AneuRisk: An artificial intelligence-based tool to assess the risk of abdominal aortic aneurysm patients that can reduce imaging costs, radiation, and prevent adverse outcomes. Timothy Chung, PhD; David Vorp, PhD; Nathan Liang, MD, MS.
$50,000 Awardee + $15,000 Bonus Award:
A-SIDE: A bioengineered Autologous Serum Ocular Insert for Dry Eye Disease, using a patient’s own blood serum proteins to treat dry eye disease with convenience, comfort, and efficacy. Morgan DiLeo, PhD; Vishal Jhanji, MD.
Congratulations, all!
Illustration: PInCH logo.
Brown Lab Shanae Butler Student Awarded the McGinnis Fellowship

Shanae Butler (pictured), a third-year PhD student in bioengineering at the University of Pittsburgh, received the McGinnis Fellowship, was awarded an internal fellowship in the Swanson School of Engineering. This fellowship supports PhD students who have a strong interest in developing innovative medical technologies with significant near-term clinical translational potential.
Ms. Butler works in the Brown Lab, led by Bryan Brown, PhD, a member of the McGowan Institute for Regenerative Medicine and Associate Professor of Bioengineering at the University of Pittsburgh. Ms. Butler was honored for her work in using microspheres to heal skin wounds and minimize scars.
Ms. Butler views this as an incredible opportunity, both for providing a framework to develop innovative medical products and for its availability to international students.
“I’m a Jamaican international student, and that provides me a really unique perspective in the United States, but what it doesn’t do is provide me with a lot of opportunities to access resources in the academic and research world,” Butler said, emphasizing that a lot of funding for performing research is government-based and is only available to U.S. citizens. “But I was actually eligible to apply for this, which offers this amazing balance between scientific research and progress and how that can directly impact somebody in real life.”
The fellowship will last for two years while Ms. Butler works towards her PhD.
“Being able to have a group of people as a support system devoted and dedicated to you feeling capable even as you fail is amazing,” Ms. Butler added, “and this fellowship is just one more instance of me testing my capabilities.”
Congratulations, Ms. Butler!
Brown Lab Student Named the Bioengineering 2023 Wesley C. Pickard Fellow

Marissa Behun (pictured), a fourth-year bioengineering graduate student working in the laboratory of McGowan faculty member Bryan Brown, PhD, Associate Professor of Bioengineering at the University of Pittsburgh, was named the 2023 Wesley C. Pickard Fellow by the Department of Bioengineering. Recipients of this award are selected by the department chair and chosen based on academic merit. The fellowship also gives Ms. Behun the opportunity to meet Mr. Pickard, a Pitt alumnus and former CFO of Synergy Inc.
Ms. Behun’s research focuses on nerve reinnervation – specifically on healing drastic nerve injuries, such as volumetric muscle loss, where the body lacks the necessary pre-existing nerve structures required to function properly.
Ms. Behun, who hopes to specialize in military medicine, was inspired to pursue research by her own autoimmune disorder.
“When I was younger [my disorder] bounced me around from doctor to doctor — that’s what really pushed me into research,” she said. “And I’ve always wanted to work with military veterans and military medicine.”
Congratulations Ms. Behun!
The McGowan Institute Welcomes New Finance Team Members

The McGowan Institute for Regenerative Medicine welcomes four new members to its finance team from Indiana University.
Mrs. Kim Burns (pictured top left) is the new Director for Finance and Operations. Prior to joining the University of Pittsburgh, Mrs. Burns held a number of positions at Indiana University and has over 15 years of finance, research administration, and operations experience.
Mrs. Roma Palmer (pictured top right) is an Assistant Director of Finance and Operations. Prior to joining the University of Pittsburgh, Mrs. Palmer held a variety of positions at Indiana University School of Medicine. Mrs. Palmer is an experienced administrative professional with expertise in research administration, clinical trials, administrative and fiscal affairs, auditing, and reconciliation of fiscal processes. She is a Certified Research Administrator (CRA); the certification is administered by the Research Administrators Certification Council.
Ms. Rachel Maloy (pictured bottom left) is a Financial Analysts Manager. Her contract and grants experience has spanned over 15 years in medical/university research administration, housing, and federal grants.
Ms. Jessa Johnson (pictured bottom right) is also a Financial Analysts Manager. She has worked in research administration for more than 5 years, most recently with Indiana University School of Medicine and the National Center for Atmospheric Research.
All four new employees will initially be working remotely.
Welcome all!
PUBLICATION OF THE MONTH
Author: Michael J Buckenmeyer, Meena Sukhwani, Aimon Iftikhar, Alexis L Nolfi, Ziyu Xian, Srujan Dadi, Zachary W Case, Sarah R Steimer, Antonio D’Amore, Kyle E Orwig, Bryan N Brown
Title: A bioengineered in situ ovary (ISO) supports follicle engraftment and live-births post-chemotherapy
Summary: Female cancer patients who have undergone chemotherapy have an elevated risk of developing ovarian dysfunction and failure. Experimental approaches to treat iatrogenic infertility are evolving rapidly; however, challenges and risks remain that hinder clinical translation. Biomaterials have improved in vitro follicle maturation and in vivo transplantation in mice, but there has only been marginal success for early-stage human follicles. Here, we developed methods to obtain an ovarian-specific extracellular matrix hydrogel to facilitate follicle delivery and establish an in situ ovary (ISO), which offers a permissive environment to enhance follicle survival. We demonstrate sustainable follicle engraftment, natural pregnancy, and the birth of healthy pups after intraovarian microinjection of isolated exogenous follicles into chemotherapy-treated (CTx) mice. Our results confirm that hydrogel-based follicle microinjection could offer a minimally invasive delivery platform to enhance follicle integration for patients post-chemotherapy.
Source: J Tissue Eng. 2023 Nov 17:14:20417314231197282. doi: 10.1177/20417314231197282. eCollection 2023 Jan-Dec.
GRANT OF THE MONTH
PI: Alejandro Jose Almarza
Title: Collaborative for REsearch to Advance TMD Evidence (CREATE)
Description: We propose to finally bridge the gap in our understanding of the relationship between temporomandibular disorder (TMD) pain and underlying mechanisms, which will provide new diagnostics and therapeutics for TMD patients. We envision that the TMD Collaborative for Improving Patient-Centered Translational Research (TMD IMPACT) will address the need for: TMD prevention, the development of effective and personalized TMD therapies, and expanding the current work force of those researching and managing TMD pain by taking a bidirectional translational approach. Our vision for TMD IMPACT involves five key components: 1) a Clinical Phenotyping Core responsible for Clinical Centers across the US, to coordinate patient assessment and analyses, ensure clinical studies include whole-person evaluations of individuals with a TMD, the collection of phenotypic data that will be used to identify TMD subtypes and biomarkers predictive of underlying mechanisms, and patient outcomes and response to therapeutic interventions; 2) a Preclinical Core responsible for the validation of preclinical models, establishing causal links between mechanisms and pain phenotypes, and for the initial efficacy, specificity, and safety testing of therapeutic interventions; 3) a Data and Biospecimen Management Core that will coordinate the integration of different data types and storage of biospecimens, such that TMD IMPACT resources follow FAIR principles and provide long-lasting impacts on TMD research into the future; 4) an Education and Outreach Core to build scientific and clinical workforce capacity, thereby increasing the number of programs pursuing preclinical and clinical TMD research, expanding the availability of evidence-based care for people living with a TMD, and promoting dissemination and implementation of advances made by the Collaborative; and 5) a Stakeholder Group consisting of patients/patient advocates, payers, and industry representatives who will provide feedback on all Collaborative efforts. During the proposed planning phase, our Collaborative for REsearch to Advance TMD Evidence (CREATE) team will work with the other selected teams to lay the groundwork needed to establish the TMD-Collaborative and achieve a shared vision for TMD-IMPACT. Our general strategy will be to engage with funded sites and centers chosen for the TMD-IMPACT planning phase, as they will have expertise and infrastructure relevant to the four core components of the Collaborative.
Source: National Institute of Dental and Craniofacial Research
Term: September 5, 2023 – August 31, 2024
Amount: $272,152
