In its July 2021 edition, Healthcare Radius e-magazine covered the acellular biologic scaffold treatment for volumetric muscle loss (VML) research led by McGowan Institute for Regenerative Medicine Deputy Director Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery and Director of the Center for Pre-Clinical Tissue Engineering within the Institute. The article (pages 30-34) explained how VML is a severe and debilitating clinical medical problem and how Dr. Badylak’s team is helping to solve that problem.
The current standard of care does not address underlying muscle strength deficits, nor does it address restoring muscle function. However, participants in Dr. Badylak’s clinical trial have shown significant improvement in strength and range of motion and evidence for skeletal muscle regeneration.
“Previously, there was no effective treatment for these patients, but this approach holds significant promise,” said Dr. Badylak. “This approach could be a game-changer and not just an incremental advance.”
For the Muscle Tendon Tissue Unit Repair and Reinforcement Reconstructive (MTURR) Reconstructive Surgery Research Study, which was sponsored by the U.S. Department of Defense, 11 men and 2 women who had lost at least 25 percent of leg or arm muscle volume and function first underwent a customized regimen of physical therapy for 4 to 16 weeks.
Lead study surgeon, McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh, then surgically implanted a “quilt” of compressed ECM sheets designed to fill in their injury sites. Within 48 hours of the operation, the participants resumed physical therapy for up to 24 additional weeks.
By 6 months after implantation, patients showed an average improvement of 37.3 percent in strength and 27.1 percent in range of motion tasks compared with pre-operative performance numbers. CT or MRI imaging also showed an increase in post-operative soft tissue formation in all 13 patients.
“For well-selected patients with this type of loss, we now have a treatment available to help improve their function,” Dr. Rubin said.
The new data builds upon a previous Pitt study that showed damaged leg muscles grew stronger and showed signs of regeneration in three out of five men whose old injuries were surgically implanted with ECM derived from pig bladder. Those patients also underwent similar pre- and post-operative physical therapy.
The recent results included more patients with varying limb injuries; used three different types of pig tissues for ECM bioscaffolds; investigated neurogenic cells as a component of the functional remodeling process; and included CT and MRI imaging to evaluate the remodeled muscle tissue.
“The three different types of matrix materials used all worked the same, which is significant because it means this is a generic property of these materials and gives the surgeons a choice for using whichever tissue they like,” Dr. Badylak said. “Prior to the surgery, each patient went through physical therapy focused on getting them to the point where they couldn’t get any better. We then started active rehab within 48 hours after surgery, which proved to be critically important for these patients.”
This work was published in npj Regenerative Medicine.
In another study published in the Journal of Immunology and Regenerative Medicine, Dr. Badylak and his team showed that cellular communication occurs using nanovesicles, extremely tiny fluid-filled sacs that bud off from a cell’s outer surface and allow cells to communicate by transferring proteins. Researchers had yet to identify them in solid body tissues.
“We always thought exosomes are free floating, but recently wondered if they are also present in the solid ECM and might facility the cellular communication that is critical to regenerative processes,” said Dr. Badylak. To explore this possibility, his team used specialized proteins to break up the ECM.
Researchers then exposed two different cell types—immune cells and neuronal stem cells—to isolated matrix-bound vesicles, and found that they caused both cells types to mimic their normal regrowth behaviors.
“Sure enough, we found that vesicles are embedded within the ECM. In fact, these bioscaffolds are loaded with these vesicles. This study showed us that the matrix-bound vesicles are active, can influence cellular behavior, and are possibly the primary mechanism by which bioscaffolds cause tissue regrowth in the body,” Dr. Badylak explained.
Other McGowan Institute affiliated faculty members involved in these works include George Hussey, PhD, Hēth Turnquist, PhD, Fabrisia Ambrosio, PhD, MPT, Neill Turner, PhD, and Michael Boninger, MD.
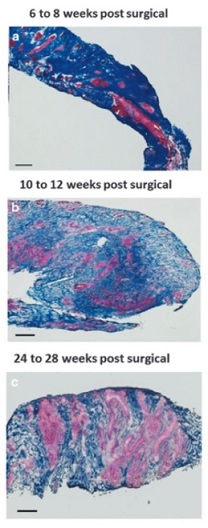
Illustration: Massons trichrome staining of human muscle biopsies shows islands of skeletal muscle present at 6-8 weeks, 10-12 weeks, and 24-28 weeks post-surgery, respectively. (Figure 2, npj Regenerative Medicine; 1, Article number: 16008 (2016).)
Read more…
Article (Healthcare Radius, July 2021, Vol. 9, Issue 10, Pages 30-34.)
ClinicalTrials.gov: Musculotendinous Tissue Repair Unit and Reinforcement (MTURR)
Abstract (Matrix bound nanovesicle-associated IL-33 activates a pro-remodeling macrophage phenotype via a non-canonical, ST2-independent pathway. George S Hussey, Jenna L Dziki, Yoojin C Lee, Joseph G Bartolacci, Marissa Behun, Hēth R Turnquist, Stephen F Badylak. Journal of Immunology and Regenerative Medicine, 2019 Mar;3:26-35.)
Abstract (An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. Jenna Dziki, Stephen Badylak, Mohammad Yabroudi, Brian Sicari, Fabrisia Ambrosio, Kristen Stearns, Neill Turner, Aaron Wyse, Michael L Boninger, Elke H. P. Brown and J. Peter Rubin. npj Regenerative Medicine; 1, Article number: 16008 (2016).)
