 Coulter Collaborations
Coulter Collaborations
The Coulter Translational Research Partners II Program at the University of Pittsburgh
program is led and administered by the Swanson School of Engineering’s Department of Bioengineering in partnership with the University of Pittsburgh School of Medicine and the University of Pittsburgh Innovation Institute.
The program’s mission is to identify, select, develop, and commercialize promising projects undertaken together by bioengineers and clinical faculty that address unmet clinical needs and better patient care worldwide. This effort requires a multidisciplinary team involving many schools, centers, and institutes within the University of Pittsburgh, as well as entities outside the University with whom we are engaging in partnership to achieve these objectives.
During the pursuit of these goals, the University envisions directly impacting regional economic prosperity, and that these translational research activities will positively contribute to and complement the traditional world-class research and educational outcomes for which the University is well-known.
Programs funded involving McGowan Faculty include:
MagWire: Kirschner Wires as Early Application for ResMag Technology
Prashant Kumta, PhD, Scientific PI
Professor of Bioengineering & Material Science
Engineering Director, Center for Craniofacial Regeneration
Founding Director, CCEMM
Email: pkumta@pitt.edu
Phone: (412) 648-0223
http://www.pitt.edu/~pkumta
Vijay Gorantla, MD, PhD, Clinical PI
Associate Professor of Plastic Surgery
Administrative Medical Director, Pittsburgh Reconstructive Transplant Program
Email: vsg2@pitt.edu
Phone: (412) 383-6881
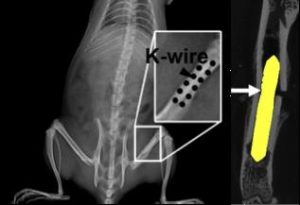 Every year about 320,000 procedures to fix bone fractures are performed using Kirschner wires (K-wires) to hold bone fragments in place during healing, an annual market of about $210 million dollars. Because K-wires are typically left to protrude through the skin during healing, for easy removal, their use typically has a high complication rate (up to 42% has been reported). Complications include infection at the insertion site, loosening, migration and severe infection of the bone. These products also require a secondary procedure for removal once healing is complete, which itself costs money and increases recovery time. MagWire, a bioresorbable magnesium alloy version of these devices, will have sufficient mechanical strength to directly replace traditional stainless steel K-wires, but will resorb over time. This feature eliminates the need to remove the wires, thereby reducing risk of infection and associated complications. Thus, MagWire has the potential to significantly increase patient outcomes and decrease healthcare costs associated with treating bone fractures.
Every year about 320,000 procedures to fix bone fractures are performed using Kirschner wires (K-wires) to hold bone fragments in place during healing, an annual market of about $210 million dollars. Because K-wires are typically left to protrude through the skin during healing, for easy removal, their use typically has a high complication rate (up to 42% has been reported). Complications include infection at the insertion site, loosening, migration and severe infection of the bone. These products also require a secondary procedure for removal once healing is complete, which itself costs money and increases recovery time. MagWire, a bioresorbable magnesium alloy version of these devices, will have sufficient mechanical strength to directly replace traditional stainless steel K-wires, but will resorb over time. This feature eliminates the need to remove the wires, thereby reducing risk of infection and associated complications. Thus, MagWire has the potential to significantly increase patient outcomes and decrease healthcare costs associated with treating bone fractures.
NeuroGel: Injectable Hydrogel for Peripheral Nerve Reconstruction
Paul Gardner, MD, Clinical PI
Associate Professor of Neurological Surgery
Email: pagst28@pitt.edu
Phone: (412) 802-8259
Bryan Brown, PhD, Scientific PI
Assistant Professor of Bioengineering
Email:bnb9@pitt.edu
Phone: (412) 624-5273
Travis Prest, MS, PhD, Graduate Student
Email: tap56@pitt.edu
Phone: (412) 648-7875
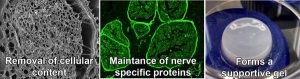 In the U.S., more than 900,000 procedures per year are performed to address traumatic and surgical nerve injuries, representing a $1.68 billion market. While there are currently a variety of devices such as conduits, wraps, and sponges that are used to try to bridge transected nerves, most have shown only limited benefit. As a result, surgeons prefer to use nerve autografts where possible, despite the potential for permanent loss of sensation at the donor site. But even these grafts have shown limited success. NeuroGel provides a new regenerative matrix that promotes healing of the original nerve tissue. Neurogel is composed of a solution of nerve-specific structural and functional components that are present in normal healthy nerves, and obtained through a proprietary decellularization and enzymatic treatment process. In pre-clinical studies, injection of NeuroGel at the site of a transected nerve has been shown to speed recovery and improve nerve regeneration. To-date, three preclinical animal studies have been performed that have demonstrated that NeuroGel is more effective than traditional approaches in a number of nerve injury scenarios, including end-to-end nerve repair, nerve gap repair, and nerve graft support.
In the U.S., more than 900,000 procedures per year are performed to address traumatic and surgical nerve injuries, representing a $1.68 billion market. While there are currently a variety of devices such as conduits, wraps, and sponges that are used to try to bridge transected nerves, most have shown only limited benefit. As a result, surgeons prefer to use nerve autografts where possible, despite the potential for permanent loss of sensation at the donor site. But even these grafts have shown limited success. NeuroGel provides a new regenerative matrix that promotes healing of the original nerve tissue. Neurogel is composed of a solution of nerve-specific structural and functional components that are present in normal healthy nerves, and obtained through a proprietary decellularization and enzymatic treatment process. In pre-clinical studies, injection of NeuroGel at the site of a transected nerve has been shown to speed recovery and improve nerve regeneration. To-date, three preclinical animal studies have been performed that have demonstrated that NeuroGel is more effective than traditional approaches in a number of nerve injury scenarios, including end-to-end nerve repair, nerve gap repair, and nerve graft support.
LigaMend: Resorbable Magnesium Ring for ACL Reconstruction
Savio L-Y Woo, PhD, DSc, Deng, Scientific PI
Professor of Bioengineering
Founder/Director of Musculoskeletal Research Center
Email: slyw@pitt.edu
Phone: (412) 648-2000
http://www.pitt.edu/~msrc
Patrick J. McMahon, MD
Associate Professor of Bioengineering
Founder of McMahon Orthopedics & Rehabilitation
Email: mcmahonp@upmc.edu
Phone: (412) 431-7342
Katie Farraro, PhD
Postdoctoral Fellow
Email: kff@pitt.edu
Phone: (412) 648-2000
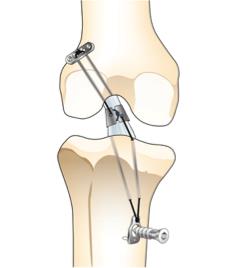 Over 200,000 people each year in the US sustain an anterior cruciate ligament (ACL) injury during sports-related activities. More than two-thirds of patients with ACL injuries undergo surgical ACL reconstruction to re-stabilize their knee, representing a market of over $3 billion. However, complications following ACL reconstruction are common, including residual pain and knee instability. Furthermore, about 10% of such patients experience a procedural failure that requires a revision surgery and there is a three-fold increase in the risk of the early onset of osteoarthritis of the knee following reconstructive surgery. LigaMend is a novel alternative option to ACL reconstruction that stabilizes the torn ACL following surgery and promotes regeneration of connective tissue. Comprised of a bioresorbable magnesium ring, combined with FDA-approved extracellular bioscaffolds (ECM), LigaMend has been shown in pre-clinical trials to fully restore the natural ligament. This technology has the potential to significantly decrease the time and cost of ACL surgery, while improving the outcome for patients.
Over 200,000 people each year in the US sustain an anterior cruciate ligament (ACL) injury during sports-related activities. More than two-thirds of patients with ACL injuries undergo surgical ACL reconstruction to re-stabilize their knee, representing a market of over $3 billion. However, complications following ACL reconstruction are common, including residual pain and knee instability. Furthermore, about 10% of such patients experience a procedural failure that requires a revision surgery and there is a three-fold increase in the risk of the early onset of osteoarthritis of the knee following reconstructive surgery. LigaMend is a novel alternative option to ACL reconstruction that stabilizes the torn ACL following surgery and promotes regeneration of connective tissue. Comprised of a bioresorbable magnesium ring, combined with FDA-approved extracellular bioscaffolds (ECM), LigaMend has been shown in pre-clinical trials to fully restore the natural ligament. This technology has the potential to significantly decrease the time and cost of ACL surgery, while improving the outcome for patients.
SoliDrop: Long-lasting Intraocular Drug Delivery Initially for Glaucoma
Morgan Fedorchak, PhD, Scientific PI
Assistant Professor of Ophthalmology
Email: mod8@pitt.edu
Phone: (412) 624-8625
Ian Conner, MD, Clinical PI
Assistant Professor of Ophthalmology & Bioengineering
Director, UPMC Eye Center
Email: connerip@gmail.com
Phone: (412) 647-2200
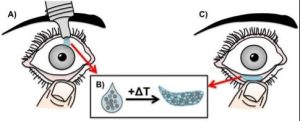 Glaucoma is a chronic condition currently affecting 2.71 million Americans with prevalence expected to increase 28% by the year 2020. Current US health expenditure for glaucoma is over $2.5 billion. Due to limited bioavailability and poor retention time, many glaucoma drugs require multiple doses per day. This results in only 30% patient adherence level. SoliDrop is a drug delivery system for ocular therapeutics, reducing frequency from 4x per day to only 1x per month and having fewer systemic side effects. Fewer doses typically means greater compliance, which means less risk of increased medical costs due to glaucoma-related vision loss for insurance companies and patients.
Glaucoma is a chronic condition currently affecting 2.71 million Americans with prevalence expected to increase 28% by the year 2020. Current US health expenditure for glaucoma is over $2.5 billion. Due to limited bioavailability and poor retention time, many glaucoma drugs require multiple doses per day. This results in only 30% patient adherence level. SoliDrop is a drug delivery system for ocular therapeutics, reducing frequency from 4x per day to only 1x per month and having fewer systemic side effects. Fewer doses typically means greater compliance, which means less risk of increased medical costs due to glaucoma-related vision loss for insurance companies and patients.
