June 2014 | VOL. 13, NO. 6 | www.McGowan.pitt.edu
Malfunction in Molecular ‘Proofreader’ Prevents Repair of UV-Induced DNA Damage
 Malfunctions in the molecular “proofreading” machinery, which repairs structural errors in DNA caused by ultraviolet (UV) light damage, help explain why people who have the disease xeroderma pigmentosum (XP) are at an extremely high risk for developing skin cancer, according to McGowan Institute for Regenerative Medicine affiliated faculty member Simon Watkins, PhD, founder and director of the Center for Biologic Imaging and a professor and vice chairman within the Department of Cell Biology at the University of Pittsburgh, and researchers at the University of Pittsburgh School of Medicine and the University of Pittsburgh Cancer Institute (UPCI). Their findings are published in the online version of the Proceedings of the National Academy of Sciences.
Malfunctions in the molecular “proofreading” machinery, which repairs structural errors in DNA caused by ultraviolet (UV) light damage, help explain why people who have the disease xeroderma pigmentosum (XP) are at an extremely high risk for developing skin cancer, according to McGowan Institute for Regenerative Medicine affiliated faculty member Simon Watkins, PhD, founder and director of the Center for Biologic Imaging and a professor and vice chairman within the Department of Cell Biology at the University of Pittsburgh, and researchers at the University of Pittsburgh School of Medicine and the University of Pittsburgh Cancer Institute (UPCI). Their findings are published in the online version of the Proceedings of the National Academy of Sciences.
Previous research has shown that a DNA-repair protein called human UV-damaged DNA-binding protein, or UV-DDB, signals for a repair when two UV-DDB molecules bind to the site of the problem, said senior investigator Bennett Van Houten, PhD, the Richard M. Cyert professor of molecular oncology, Pitt School of Medicine, and co-leader of UPCI’s Molecular and Cell Biology Program.
“Our new study shows UV-DDB makes stops along the DNA strand and transiently attaches to it, causing a proofreading change in the protein’s conformation, or shape. If the DNA is damaged the protein stays, if the DNA is not damaged the protein leaves,” Dr. Van Houten said. “When it comes to a spot that has been damaged by UV radiation, two molecules of UV-DDB converge and stay tightly bound to the site, essentially flagging it for the attention of repair machinery.”
The researchers followed the trail of single molecules of UV-DDB by tagging them with light-emitting quantum dots, enabling them to watch the molecules jump from place to place in real time on both normal and UV-exposed DNA strands.
They also tracked a mutant UV-DDB protein associated with XP, an inherited, incurable disease of light sensitivity that affects about 1 in 250,000 people. They found that the mutant UV-DDB molecules are still capable of binding to DNA, but continued to slide along the DNA rather than staying put to signal where the fix was needed.
“Without this important damage control, UV-induced errors could accumulate to cause cell alterations that foster cancer development,” Dr. Van Houten said. “Like a bus with no brakes, the XP-associated UV-DDB complex stays on the road and sees possible passengers, but keeps going past the stop.”
RESOURCES AT THE MCGOWAN INSTITUTE
July Special at the Histology Lab
Mucins are a family of high molecular weight, heavily glycosylated proteins (glycoconjugates) produced by epithelial tissues in most metazoans. Mucins’ key characteristic is their ability to form gels; therefore they are a key component in most gel-like secretions, serving functions from lubrication to cell signalling to forming chemical barriers. They often take an inhibitory role. Some mucins are associated with controlling mineralization, and bone formation in vertebrates. They bind to pathogens as part of the immune system. Overexpression of the mucin proteins, especially MUC1, is associated with many types of cancer.
Alcian Blue/PAS
The combination of the alcian blue and the PAS techniques can be used as a means of distinguishing neutral mucins from acid mucins. In most protocols, sections are stained with the standard alcian blue (pH 2.5) method followed by the PAS technique. The alcian blue at a pH of 2.5 will stain all acid mucins deep blue but will not color the neutral mucins. The subsequent application of the PAS technique will stain the neutral mucins bright magenta. Tissues or cells that contain both neutral and acidic mucins may demonstrate a dark blue or purple coloration. The combined application of alcian blue and PAS is useful for several reasons. Changes in the distribution or pattern of expression of neutral and acid mucins are indicative of certain pathological conditions. In addition, the combined alcian blue/PAS technique is perhaps the most sensitive or comprehensive means for detection of mucins as all mucins should react regardless of the charge nature of the mucin.
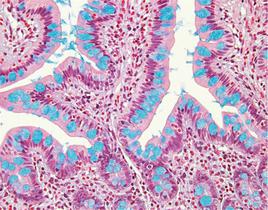
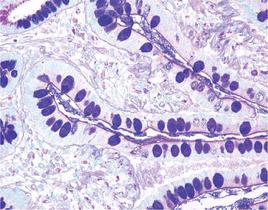
Colon stained with Alcian Blue (left) and Colon stained with Alcian Blue/PAS (right)
You’ll receive 30% off your Alcian Blue or PAS staining or both when you mention this article any time in July. Contact Lori at the McGowan Core Histology Lab and ask about our Staining specials. As always, you will receive the highest quality histology in the quickest turn-around time.
Contact Lori at the McGowan Core Histology Lab and ask about our VVG specials. Email perezl@upmc.edu or call 412-624-5265
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Winner announced for May Flow Cytometry Contest
This past May and June, the McGowan Institute Flow Cytometry Facility held two contests. The first, for researchers using eight colors or less, was won by the Badylak lab – Tim Keane, assisted by Aaron DeWard. The prize is 1hour of free time on the BDFACSAria. Unfortunately, the second contest, for researchers using eight colors or more, had no participants. So that prize, 2hours of free time on the BDFACSAria, will go unclaimed. Prizes must be used by Thursday, August 14, 2014.
Thank you all for your participation. If there is enough interest, we may do this again in the future. Please stop by and visit our facility. We would love to discuss how flow cytometry can contribute to your research projects. Consultations are free of charge.
For more information about our services, visit our website.
SCIENTIFIC ADVANCES
Novel Biological Sources for Battery Materials
 The squirmy marine cuttlefish may be the next best source of electrode materials for batteries to power edible medical devices.
The squirmy marine cuttlefish may be the next best source of electrode materials for batteries to power edible medical devices.
McGowan Institute for Regenerative Medicine affiliated faculty member Christopher Bettinger, PhD, an assistant professor in the Departments of Materials Science and Engineering (MSE) and Biomedical Engineering, Carnegie Mellon University (CMU), and his CMU colleague Jay Whitacre, PhD, have found that ink from the cuttlefish, a close relative of the squid, provides the perfect chemistry and nanostructure to power tiny electronic devices that can be either ingested or implanted into the body for applications ranging from biosensing to drug delivery.
“We found that the melanin pigments in cuttlefish ink make it a perfect fit for use in battery electrodes that would ultimately be used in devices that operate in close proximity to sensitive living tissue,” said Dr. Bettinger. Melanin is the pigment responsible for the dark color of skin, hair, scales, and is also found in animals.
In a paper appearing in The Proceedings of the National Academy of Sciences, CMU researchers show that naturally occurring melanins exhibit higher charge storage capacity compared to other synthetic melanin derivatives when used as anode materials. Pigment-based anodes are an important component in sodium-ion batteries, a battery technology that has been pioneered by Dr. Whitacre, an associate professor of materials science and engineering and engineering and public policy.
“Using natural materials in energy storage devices increases the likelihood for use in powering devices that operate in sensitive environments such as the human body,” Dr. Bettinger said.
At present, high-performance energy storage systems for medical devices are designed to supply power to semi-permanent devices that are often encapsulated. These scenarios permit the use of potentially toxic electrode materials and electrolytes. Electronically active medical devices that are either biodegradable or ingestible require new energy storage materials that are benign and can operate in hydrated environments. Melanin-based electrodes represent a step closer to this goal.
“Our research shows that alternative systems that use biocompatible electrode materials with aqueous sodium-ion batteries could provide onboard energy sources for a variety of temporary medical devices including biodegradable electronic implants and ingestible systems,” Dr. Bettinger said.
Earlier, Drs. Bettinger and Whitacre reported that they were creating edible power sources for medical devices using materials found in a daily diet. Their initial design involved a flexible polymer electrode and a sodium ion electrochemical cell, which allows them to fold the mechanism into an edible pill that temporarily encapsulates the device.
Dr. Juan Carlos Puyana to Share $1.6M Grant
 McGowan Institute for Regenerative Medicine affiliated faculty member Juan Carlos Puyana, MD, associate professor of surgery and critical care, University of Pittsburgh, and trauma surgeon, director of Surgical ICU, and director of the Surgical Critical Care Program at the Presbyterian Hospital of the University of Pittsburgh Medical Center, is the principal investigator of a grant received from the National Institutes of Health (NIH) in its first round of funding from Fogarty’s Global Health Research and Research Training eCapacity Initiative. In an effort to increase the use of information and communication technology (ICT) in global health interventions and address technical expertise gaps among scientists in developing countries, this new NIH initiative has awarded $1.6 million over 3 years to 5 institutions. Monies will support the efforts of former and current grantees to establish education programs designed to teach trainees to incorporate ICT resources into their research and research training activities.
McGowan Institute for Regenerative Medicine affiliated faculty member Juan Carlos Puyana, MD, associate professor of surgery and critical care, University of Pittsburgh, and trauma surgeon, director of Surgical ICU, and director of the Surgical Critical Care Program at the Presbyterian Hospital of the University of Pittsburgh Medical Center, is the principal investigator of a grant received from the National Institutes of Health (NIH) in its first round of funding from Fogarty’s Global Health Research and Research Training eCapacity Initiative. In an effort to increase the use of information and communication technology (ICT) in global health interventions and address technical expertise gaps among scientists in developing countries, this new NIH initiative has awarded $1.6 million over 3 years to 5 institutions. Monies will support the efforts of former and current grantees to establish education programs designed to teach trainees to incorporate ICT resources into their research and research training activities.
Dr. Puyana’s project entitled “eCapicity for Trauma Information Systems and Research Education (eCATIS)” includes a collaboration of investigators from Pitt, the University of British Columbia Department of Surgery (Canada), Fundacion Meditech (Neiva, Colombia), Centro de Informatica e Investigacion Clinica [CIIC] (Rosario, Argentina), and Oregon Health & Science University [OHSU] (Portland, Oregon). Colombia, Paraguay, and Guatemala are the countries targeted for this research training program.
Developing a trauma information system for regions in the world where injury is the greatest burden of disease, as injuries kill more patients than AIDS, malaria, and tuberculosis all combined, has the extraordinary potential of changing a paradigm in global public health. eCATIS for low and middle income countries (LMICs) is a fundamental and critically needed step towards understanding the magnitude of injury and the burden of disease. Such information is pivotal to design and implement public health interventions, allocate resources, and answer research questions relevant to the environment in LMICs. An additive benefit of this project will be the delivery of trauma information systems capable of gathering data relevant to the local epidemiology of injury and providing the basis for designing injury prevention initiatives, quality improvement programs, and facilitating research endeavors to meet LMICs’ most pressing questions regarding trauma/injury care.
Fogarty’s Global Health Research and Research Training eCapacity Initiative aims to support innovative research education programs to teach researchers at LMIC institutions the knowledge and skills necessary to incorporate ICT into global health research and research training. Funding from this effort was also received by Cayetano Heredia University (Lima, Peru), Johns Hopkins University, Tulane University, and University of Washington.
Lifestyle Interventions Can Prevent Major Depression in Older Black and White Adults with Mild Symptoms
 Discussions with a dietary coach to learn about healthy eating were as effective as meeting with a counselor for problem-solving or “talk” therapy in preventing major depression among older black and white adults with mild symptoms of the mood disorder, according to McGowan Institute for Regenerative Medicine affiliated faculty member Mary Amanda Dew, PhD, Professor of Psychiatry, Psychology, Epidemiology, and Biostatistics at the University of Pittsburgh and also a Professor at the Clinical and Translational Science Institute, and researchers at the University of Pittsburgh and the University of Maryland. Their findings were published online recently in Psychiatric Services.
Discussions with a dietary coach to learn about healthy eating were as effective as meeting with a counselor for problem-solving or “talk” therapy in preventing major depression among older black and white adults with mild symptoms of the mood disorder, according to McGowan Institute for Regenerative Medicine affiliated faculty member Mary Amanda Dew, PhD, Professor of Psychiatry, Psychology, Epidemiology, and Biostatistics at the University of Pittsburgh and also a Professor at the Clinical and Translational Science Institute, and researchers at the University of Pittsburgh and the University of Maryland. Their findings were published online recently in Psychiatric Services.
Depression is common and treatments often don’t completely resolve the disability that attends the illness, said senior author Charles F. Reynolds III, MD, UPMC Endowed Professor of Geriatric Psychiatry, University of Pittsburgh School of Medicine. Sadness, fatigue, and disinterest in activities that used to bring pleasure can leave patients isolated and unable to care for themselves.
“That’s why we’re very interested in finding ways to prevent the disease in those we know are particularly vulnerable,” he said. “Avoiding episodes of major depression can help people stay happy and engaged in their communities, as well as reduce health care costs.”
The team assessed whether problem-solving therapy for primary care (PST-PC), a scientifically proven seven-step approach delivered by non-mental-health professionals to help patients resolve difficulties and thus improve coping skills and confidence, could prevent elderly adults who have mild symptoms of depression from developing full-blown disease. Instead of comparing the PST-PC participants to those who received “usual care,” which would most likely mean receiving no intervention, the team took the novel approach of comparing the PST-PC group to participants who underwent a program of dietary coaching at a similar visit interval for the same number of hours.
“Because racial minorities are at greater risk for depression, in part due to socioeconomic disadvantages, lower educational attainment, and a greater likelihood of other medical problems, we established a foundation of trust working through churches and community-based organizations in black communities,” said Dr. Quinn. Of the 244 participants, 90, or more than a third, were African-American.
“This project tells us that interventions in which people actively engage in managing their own life problems, such as financial or health issues, tend to have a positive effect on well-being and a protective effect against the onset of depression.”
“We suspect we had a higher than usual proportion of black participants because community leaders championed the project, no medication was prescribed, and treatment could be delivered at home or at other non-clinical settings,” said Dr. Thomas. “Lifestyle interventions, such as dietary coaching, may be more culturally appropriate and acceptable in racial-ethnic minority communities.”
Dr. Dew serves as the Co-Director of the NIMH Center for Late Life Depression Prevention and Intervention, and the Director of the Center’s Research Methodology and Biostatistics Core, both positions within Research for the Study of Late Life Mood Disorders, Western Psychiatric Institute and Clinic. She is the Director of the Clinical Epidemiology Program, Western Psychiatric Institute and Clinic, and the Director of the Quality of Life Research, Artificial Heart Program, Adult Cardiothoracic Transplantation, at UPMC.
Determining Acute Kidney Injury
 McGowan Institute for Regenerative Medicine affiliated faculty member John Kellum, MD, a professor of critical care medicine at the University of Pittsburgh, a transplant physician in anesthesiology at the Thomas E. Starzl Transplantation Institute, and co-director at the Mechanisms and Novel Therapies for Resuscitation and Acute Illness (MANTRA) Lab, discussed the pathogenesis of acute kidney injury (AKI) as a member of an international panel of critical care experts at the event, “Early AKI Assessment: Are New Biomarkers Rising to the Challenge,” held in conjunction with the Society of Critical Care Medicine (SCCM) 43rd Critical Care Congress in San Francisco, California. The panel discussed how new biomarkers for risk assessment of AKI could affect the care of critically ill patients. Dr. Kellum was joined with experts from the Mayo Clinic, the University of Michigan Health System, and the University of Florida.
McGowan Institute for Regenerative Medicine affiliated faculty member John Kellum, MD, a professor of critical care medicine at the University of Pittsburgh, a transplant physician in anesthesiology at the Thomas E. Starzl Transplantation Institute, and co-director at the Mechanisms and Novel Therapies for Resuscitation and Acute Illness (MANTRA) Lab, discussed the pathogenesis of acute kidney injury (AKI) as a member of an international panel of critical care experts at the event, “Early AKI Assessment: Are New Biomarkers Rising to the Challenge,” held in conjunction with the Society of Critical Care Medicine (SCCM) 43rd Critical Care Congress in San Francisco, California. The panel discussed how new biomarkers for risk assessment of AKI could affect the care of critically ill patients. Dr. Kellum was joined with experts from the Mayo Clinic, the University of Michigan Health System, and the University of Florida.
AKI is a complex and increasingly prevalent condition associated with high mortality and morbidity in critically ill patients. Failure to recognize and manage AKI in the early stages can lead to devastating outcomes for patients and increased costs to the healthcare system. Because AKI develops quickly (over the course of hours) and current tools available to physicians for risk assessment are inadequate, the condition often goes undetected until it is too late to help the patient.
AKI risk is difficult to determine because the condition is typically caused by something outside of the kidney, including sepsis, nephrotoxins, and oxygen deprivation. An international, multi-center study led by UPMC researchers aimed to identify early markers in order to detect AKI when interventions could provide benefit. Researchers collected blood and urine samples of more than 1,000 critically ill patients in North America and Europe. The biomarkers, known as TIMP-2 and IGFB7, signal that the kidneys are stressed and not functioning properly but may still recover. They are indicators of cell damage, a key component in the onset of AKI.
“These biomarkers send an early ‘alarm’ from the site of the injury and offer a better chance at treatment that can prevent further damage,” Dr. Kellum said.
Turing’s Theory of Morphogenesis Validated 60 Years After His Death
 British mathematician Alan Turing’s accomplishments in computer science are well known—he’s the man who cracked the German Enigma code, expediting the Allies’ victory in World War II. He also had a tremendous impact on biology and chemistry. In his only paper in biology, Turing proposed a theory of morphogenesis, or how identical copies of a single cell differentiate, for example, into an organism with arms and legs, a head and tail.
British mathematician Alan Turing’s accomplishments in computer science are well known—he’s the man who cracked the German Enigma code, expediting the Allies’ victory in World War II. He also had a tremendous impact on biology and chemistry. In his only paper in biology, Turing proposed a theory of morphogenesis, or how identical copies of a single cell differentiate, for example, into an organism with arms and legs, a head and tail.
Now, 60 years after Turing’s death, McGowan Institute for Regenerative Medicine affiliated faculty member G. Bard Ermentrout, PhD, University Professor of Computational Biology and Professor of Mathematics in the Kenneth P. Dietrich School of Arts and Sciences, University of Pittsburgh, and researchers from Brandeis University have provided the first experimental evidence that validates Turing’s theory in cell-like structures.
Turing, in 1952, was the first to offer an explanation of morphogenesis through chemistry. He theorized that identical biological cells differentiate and change shape through a process called intercellular reaction-diffusion. In this model, chemicals react with each other and diffuse across space—say between cells in an embryo. These chemical reactions are managed by the interaction of inhibitory and excitatory agents. When this interaction plays out across an embryo, it creates patterns of chemically different cells. Turing predicted six different patterns could arise from this model.
At Brandeis, Seth Fraden, PhD, professor of physics, and Irv Epstein, PhD, professor of chemistry, created rings of synthetic, cell-like structures with activating and inhibiting chemical reactions to test Turing’s model. Dr. Ermentrout undertook mathematical analysis of the experiments.
The researchers observed all six patterns plus a seventh unpredicted by Turing.
In addition, they noticed that, as Turing theorized in the 1950s, the once identical cell-like structures—now chemically different—also began to change in size due to osmosis. This may explain how some cells, further down the development assembly line, become large egg cells or tiny sperm cells.
The research “tells you how a zebra gets its stripes,” said Dr. Ermentrout. Turing’s theory underlies pattern formation in every biological area from pigmentation of seashells to the shapes of flowers and leaves and to the geometric structures seen in drug-induced hallucinations, he adds. Thus, validating Turing’s theory could have an impact on future research in fields ranging from embryology to neurology to cardiology. This research could impact not only the study of biological development but the study of materials science as well. For example, Turing’s model could help grow soft robots with certain patterns and shapes.
AWARDS AND RECOGNITIONS
Medical Device Technology Receives Gold Medical Design Excellence Award
 ALung Technologies is a Pittsburgh-based medical start-up company which is the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure. Based on technology developed by McGowan Institute of Regenerative Medicine faculty members William Federspiel, PhD, W.K. Whiteford professor of bioengineering, chemical engineering, and critical care medicine, and the late Brack Hattler, MD, as a spin-out company of the University of Pittsburgh in 1997, ALung has developed the Hemolung Respiratory Assist System (RAS) as a dialysis-like alternative or supplement to mechanical ventilation. ALung began commercial operations in 2001 with work focusing on the development of Dr. Hattler’s intravenous oxygenator known as the Hattler Catheter. Development of the Hemolung RAS began in 2005 and human clinical trials began in 2010. The device received CE-mark approval in 2013. (CE marking indicates the compliance with European Union (EU) legislation of a product.)
ALung Technologies is a Pittsburgh-based medical start-up company which is the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure. Based on technology developed by McGowan Institute of Regenerative Medicine faculty members William Federspiel, PhD, W.K. Whiteford professor of bioengineering, chemical engineering, and critical care medicine, and the late Brack Hattler, MD, as a spin-out company of the University of Pittsburgh in 1997, ALung has developed the Hemolung Respiratory Assist System (RAS) as a dialysis-like alternative or supplement to mechanical ventilation. ALung began commercial operations in 2001 with work focusing on the development of Dr. Hattler’s intravenous oxygenator known as the Hattler Catheter. Development of the Hemolung RAS began in 2005 and human clinical trials began in 2010. The device received CE-mark approval in 2013. (CE marking indicates the compliance with European Union (EU) legislation of a product.)
ALung announced recently that the Hemolung RAS won the Gold Award in the Critical-Care and Emergency Medicine Category of the 17th Annual Medical Design Excellence Awards (MDEA) competition. Winners were announced at a ceremony in conjunction with the Medical Design & Manufacturing (MD&M) East Conference in New York.
The Hemolung RAS provides Respiratory Dialysis®, a simple, minimally invasive form of extracorporeal carbon dioxide removal which serves as an alternative or supplement to mechanical ventilation for critically ill patients suffering from acute respiratory failure due to conditions like acute respiratory distress syndrome (ARDS) and chronic obstructive pulmonary disease (COPD). Unlike mechanical ventilation, the Hemolung RAS is minimally invasive and works by removing carbon dioxide and delivering oxygen directly to the blood, allowing the lungs to rest and heal. The device is currently approved for use in 29 countries across Europe, the Middle East, and Asia-Pacific.
“The Hemolung RAS is a truly innovative medical device that provides a much needed alternative treatment option for patients who may require mechanical ventilation, an invasive procedure associated with significant side effects,” said Peter DeComo, Chairman and Chief Executive Officer of ALung. “Receiving the Gold Award represents a culmination of more than 10 years of research and development behind the Hemolung RAS. It showcases the efforts of both our company’s academic founders who conceptualized this device, as well as our outstanding product development team that has made this technology a clinical reality.”
The Medical Design Excellence Awards (MDEA) is the medtech industry’s premier design competition committed to searching worldwide for the highest caliber finished medical devices, products, systems, or packaging available on the market. The awards program celebrates the achievements of the medical device manufacturers, their suppliers, and the many people behind the scenes — engineers, scientists, designers, and clinicians — who are responsible for the cutting-edge products that are saving lives, improving patient healthcare, and transforming medtech — one innovation at a time. A total of 54 finalists in 11 medical product categories
 Regenerative Medicine Podcast Update
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#136 –– Dr. Frederick Schoen is a Senior Pathologist, Brigham and Women’s Hospital; Professor of Pathology and Health Sciences and Technology, Harvard Medical School; and Executive Vice Chairman, Department of Pathology, Brigham and Women’s Hospital. Dr. Schoen discusses his research in the tissue engineering of heart valves.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
Picture of the Month
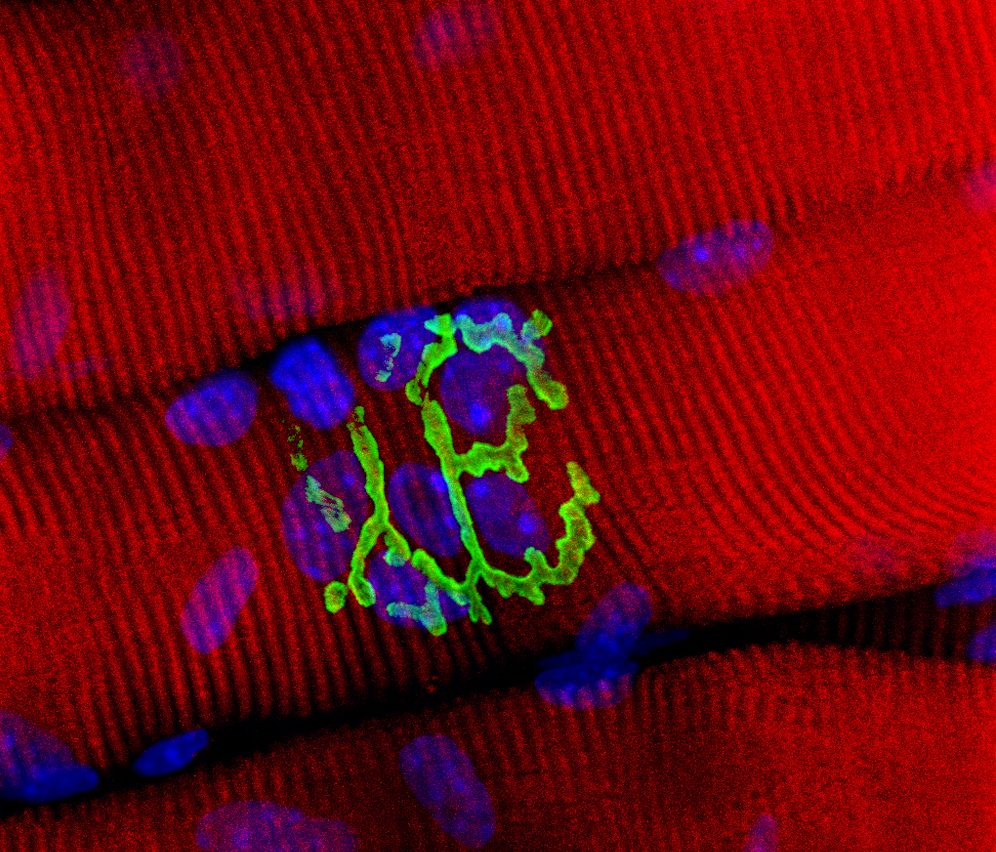
Neuromuscular Junction, stained with alpha bungarotoxin (green), on rhodamine-phalloidin stained muscle tissue. Photomicrograph by Donna Stolz. This was from this published study:
Turner, NJ, AJ Yates, Jr., DJ Weber, IR Qureshi, DB Stolz, TW Gilbert, SF Badylak. Xenogenic extracellular matrix as an inductive niche for regeneration of a functioning musculotendinous junction. Tissue Engineering, Part A. 16(11):3309-3317. 2010. PMID 20528669.
